Abstract
Objective:
To determine the preventive effects of local administration of simvastatin for postoperative intra-abdominal adhesion formation in animal model of rat.
Methods:
In this experimental study, 32 Wistar albino rats as the animal model of intra-abdominal adhesion formation were included. Adhesions were induced in all the animals via abrasion of the peritoneal and intestinal surface during laparotomy. Afterwards, the rats were randomly assigned to receive simvastatin (30 mg/kg body weight) as a single intraperitoneal dose at the time of laparotomy (n=16) or normal saline in same volume at the same time (n=16). At the day 21, animals were euthanized and the adhesions were quantified clinically (via repeated laparotomy) and pathologically and compared between the two groups.
Results:
The baseline characteristics of the animals were comparable between two study groups. Clinically, in simvastatin group, 10 rats (62.5%) did not develop any adhesion and 6 (37.5%) had first-grade adhesion; whereas in the control group, 11 (68.8%) rats had first- and 5 (31.2%) had second-grade adhesions (p<0.001). Pathologically, in simvastatin group, 6 rats (37.5%) had first-grade adhesion, while in control group, 11 rats (68.8%) had first- and 5 (31.2%) had second-grade adhesions (p<0.001).
Conclusion:
Our findings suggest that intraperitoneal administration of simvastatin is an effective method for prevention of postoperative intra-abdominal adhesion formation in animal model of rat.
Key Words: Intra-abdominal adhesion, Simvastatin, Laparotomy, Rat, Prevention
Introduction
Postoperative intra-abdominal adhesion formation is considered the most common complication of abdominal or pelvic surgery. Unlike other postoperative complications, such as wound infection or anastomotic leakage, the consequences of adhesion formation comprise a lifelong risk for various clinical entities [1,2]. Peritoneal adhesions are reported as the cause of 32% of acute intestinal obstruction and 65-75% of all small bowel obstructions. It is estimated that peritoneal adhesions develop after 93-100% of upper abdominal laparotomies and after 67-93% of lower abdominal laparotomies. Nevertheless, only 15-18% of these adhesions require surgical re-intervention [3]. In addition, autopsy findings show that about 10.4% of the patients suffer intra-abdominal adhesions without any history of abdominal surgery [4]. In a 14-year study in Sweden, the total cost for treatment of the abdominal adhesion-related problems was estimated to be 39.9-59.9 million euros which is equal to the annual cost for gastric cancer treatment in Sweden [5]. Current lines of evidence suggest that optimal surgical technique defined as less abdominal tissue manipulation and less peritoneal irritation, is associated with reduced rates of intra-abdominal adhesion formation [1]. Given the importance of intestinal adhesion and toiling for surgeons [6], so far, many studies have been conducted to prevent and reduce intestine adhesions after the surgery [6-8]. Barrier methods have been previously described for prevention of the intra-abdominal adhesion formation with different results [1,2,6,7]. Simvastatin, a strong fibrinolytic agent in mesothelial human cells in inflammatory conditions has been previously used as oral agent without effects on intra-peritoneal adhesion formation [9]. The previous studies showed simvastatin can increases the level of tissue-type plasminogen activator (t-PA) and decreases the level of plasminogen activator inhibitor (pai-1) [9,10]. Although the oral simvastatin has not been shown to be effective in reducing the intra-peritoneal adhesion formation [9], local administration has not been previously studied. The aim of the current study is to determine the preventive effects of local administration of simvastatin for postoperative intra-abdominal adhesion formation in animal model of rat.
Materials and Method
Animals This was an experimental study on 32 male Wistar albino rats weighing 250-300 g with the age of 5-6 months. They were divided into two equal groups of case and control. Study animals were handled in conformity with the guidelines for the care and handling of laboratory animals provided by Shahid Beheshti Laboratory Animals Center in accordance with global standards for laboratory biosafety guidelines. The rats were housed in the animal nest of the Department of Immunology in School of Medicine. The study was approved by the institutional review board and the Ethics Committee of Shahid Beheshti University of Medical Sciences, Iran.
Induction of adhesionsThe rats were anesthetized via injection of ketamine (60 mg per kg body weight) and xylazine (6 mg per kg body weight). Then, laparotomy was performed using a standard midline incision measuring 4 cm in length. All surgical procedures were performed by the same surgeon. In both groups, during the operation, powder-free gloves were used; then, using a blue needle, in the parietal peritoneum and cecum levels, 3 scratches with 2 cm of length were created. At the end, in the case group, before closing the abdominal wall layers, 2 mL solution of simvastatin with a dosage of 30 mg per kg body weight and in the control group, 2 mL of normal saline was administered. Then, the layers of the abdominal wall were restored with 3-0 nylon thread and the skin was restored with 3-0 nylon suture.
Determination of Intra-abdominal Adhesion FormationThe rats were maintained for three weeks in the same conditions without the use of antibiotics. Then, they were euthanized using carbon dioxide and repeated laparotomy was performed. The severity of adhesions in the two study groups were determined based on the following clinical and pathological criteria. The severity of clinical adhesions at the abdominal cavity was evaluated using an established scoring system as: 0: No adhesion, 1: One adhesion band, no vessel, easily separated, 2: Two thin adhesion bands, no vessel, easily separated, 3: Three thin adhesion bands, no vessel, easily separated, and 4: More than three thin adhesion bands, easily separated with no vessel or diffuse adhesion bands with vessels [11]. The degree of pathological adhesion was scaled as: No adhesion, Fat, Fat and fibrosis, and Fibrosis.Statistical analysisUsing sample size formula, with confidence interval of 95%, test power of 80%, standard deviation of 0.59 for both case and control groups based on the similar studies [9], mean of 2.93 for control and 1.85 for case groups, and the correction coefficient of 0.2 for small population, the sample size was calculated as 16 for each group. The differences between the two groups were evaluated using statistical package for social sciences (SPSS Inc., Chicago, IL, United States) version 20.0. The Kruskal-Wallis test was used to assess the differences in the adhesion score between the groups. A two-sided p-value of less than 0.05 was considered statistically significant.
Results
Totally, 32 male Wistar albino rats weighing 250-300 g with the age of 5-6 months underwent were included in the study. The duration of each surgery was 15 minutes. No mortality was recorded. The amount of intraoperative bleeding was comparable between groups. We found that the clinical grading of the intra-abdominal adhesions was significantly lower in thosewho received intra-peritoneal simvastatin. In the control group, all the rats had adhesions; 11 cases had first-degree adhesions and were easily released, 3 had second-degree adhesions, and 2 had third-degree adhesion (Figure 1). But in the simvastatin group, 10 rats had no adhesion and 6 had first-degree adhesion (Table 1). Histopathological examination revealed that in the control group, 11 rats had fat and fibrosis (second-degree adhesion) and 5 had third-grade adhesions. But in the simvastatin group, no fibrosis adhesion was observed (Table 2); and only in 6 rats, fat changes (Figure 2). The pathologic grade of intra-abdominal adhesions was significantly lower in those who received intraperitoneal administration of simvastatin (p<0.001).
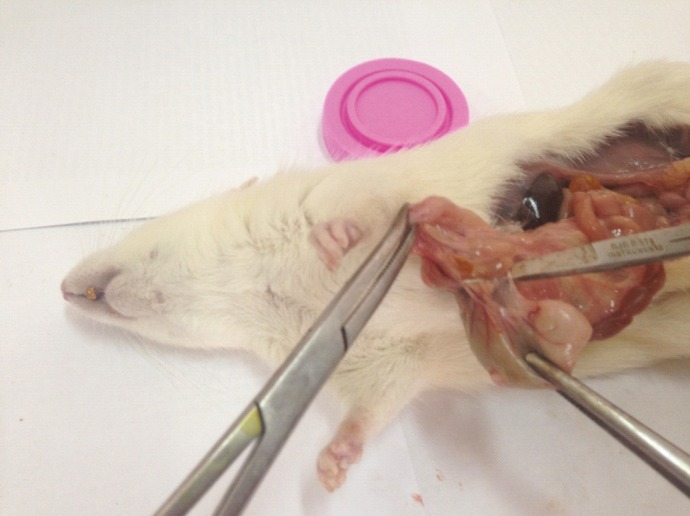
Fig. 1.
Third-degree intra-abdominal adhesion in a rat receiving normal saline (control group). Note the intraperitoneal adhesion between the colon and the mesothelium of small intestine. Three thin adhesion bands between the two tissues with no vessels are seen which cannot be easily separated
Table 1.
Degree of clinical intra-abdominal adhesions in 32 rats receiving simvastatin (n=16) or normal saline (n=16).
| The degree of clinical adhesion | Simvastatin (n=16) | Normal Saline (n=16) | p -value |
|---|---|---|---|
| 0: No adhesion | 10 (62.5%) | 0 (0.0%) | <0.001 |
| 1: One adhesion band, no vessel, easily separated | 6 (37.5%) | 11 (68.8%) | |
| 2: Two thin adhesion bands, no vessel, easily separated | 0 (0.0%) | 3 (18.7%) | |
| 3: Three thin adhesion bands, no vessel, easily separated | 0 (0.0%) | 2 (12.5%) | |
| 4: More than three thin adhesion bands, easily separated with no vessel or diffuse adhesion bands with vessels | 0 (0.0%) | 0 (0.0%) |
Table 2.
Degree of pathological intra-abdominal adhesions in 32 rats receiving simvastatin (n=16) or normal saline (n=16).
| The degree of pathological adhesion | Simvastatin (n=16) | Normal Saline (n=16) | p -value |
|---|---|---|---|
| 0: No adhesion | 10 (62.5%) | 0 (0.0%) | <0.001 |
| 1: Fat | 6 (37.5%) | 0 (0.0%) | |
| 2: Fat and fibrosis | 0 (0.0%) | 11 (68.8%) | |
| 3: Fibrosis | 0 (0.0%) | 5 (31.2%) |
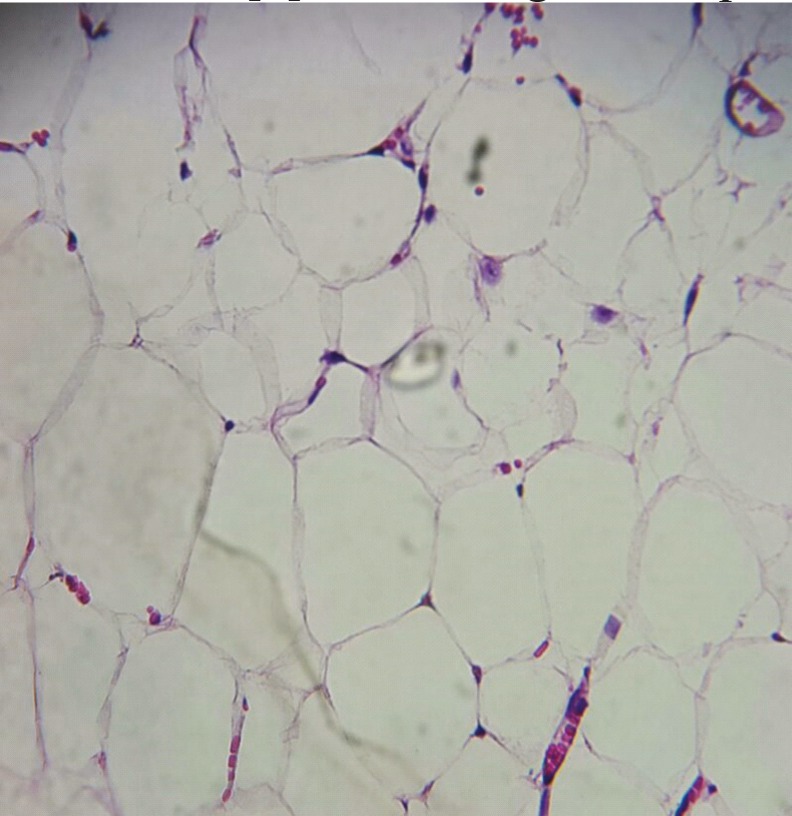
Fig. 2.
Histological examination of intra-abdominal adhesions in a rat receiving simvastatin. Note the pathological fat changes in favor of grade 1 adhesion. Only fat cells are seen with no vessels or fibrosis (H&E staining; Í40
Discussion
Intestinal adhesion after laparotomy is the most common complications of abdomen and pelivic surgeries associated with further complications including small intestine obstruction, chronic pelvic pain, infertility, and with high social and economic burden [1,2]. In the current study, we assessed that effect of intraperitoneal administration of simvastatin for prevention of postoperative intra-abdominal adhesion formation in animal model of rat. We found that in local administration of simvastatin in the peritoneal cavity after laparotomy is associated with decreased rate and severity of intra-abdominal adhesion formation both clinically and pathologically. Yildiz et al., [10] performed an experimental study to determine the effects of statins on prevention of intra-abdominal adhesion formation and wound healing. Oral and topical atorvastatin with the dose of 30 mg/kg was administered and the results were compared. They found that oral statin is not associated with decreased intra-abdominal adhesion formation while topical administration of atorvastatin was associated with incidence and severity of postoperative adhesion formation [9]. These results are in concordance with ours results demonstrating the beneficial effects of local statins in prevention of intra-abdominal adhesion formation. Pathophysiology of intestinal adhesion is based on micro-trauma to the peritoneum and subsequent release of inflammatory mediators. These factors cause fibrin deposition in the abdomen, and by persistent inflammation and the migration of fibroblasts, this fibrin is organized and adhesive bands are created [7]. According to this process, an approach to use fibrinolytic drugs has been utilized in patients undergoing laparotomy. Statins encompass antioxidant and anti-inflammatory fibrinolytic effects [7,9]. A suggested mechanism for preventing effect of simvastatin on the formation of intra-abdominal adhesion bands is increased level of t-PA and reduced level of PAI-1. Increasing of this proportion can accelerate the fibrinolysis process and decrease the adhesion [2,11-13]. Kucuk et al. administrated 0.57 mg/kg of intraperitoneal simvastatin and measured t-PA in the abdomen; they showed that reduced adhesion was associated with the increase of t-PA level in the abdominal cavity [11]. In-vitro studies on mesothelial cells showed that simvastatin stimulated the fibrinolytic activity with increasing the level of t-PA and decreasing the level of PAI-1 [12]. Another hypothesis for the mechanism of statins in decreasing intra-abdominal adhesion is restraining leukocyte function antigen (LFA-1), as an inflammatory factor inducing adhesion [14]. Davis et al., [15] in a survey on 8 rats showed that oral simvastatin can reduce fibrosis in rotator cuff tear of case group compared to controls; this can support the potential of simvastatin in reducing adhesion bands [15]. In a similar study, administration of oral fluvastatin in 48 female rats tended to significant reduction in the severity of adhesion [16]. Lalountas et al., [17] demonstrated that post-laparotomy administration of atorvastatin solution is effective in reducing intra-abdominal adhesion as well as hyaluronate/carboxy methyl cellulose [17]. Although the hyaluronate/carboxy methyl cellulose is an effective drug in reducing postsurgical adhesions, it can increase the risk of anastomotic leakage [6]. In a human study on 419 patients admitted with intraperitoneal adhesion, it was observed that the history of using statins significantly reduced the need to reoperation [18]. Although the results of this study were consistent with other studies and confirmed the effectiveness of local simvastatin in preventing intestinal adhesions after the laparotomy; this study was limited to animal model and a short-time period. However, we suppose further studies to assess the prolonged use of the drug and its long-term effects in animal models as well as the effect of the drug on humans.In conclusion, our findings suggest that intraperitoneal administration of simvastatin is an effective method for prevention of postoperative intra-abdominal adhesion formation in animal model of rat.
Acknowledgment
This article has been extracted from the thesis written by Marjan Kafaei (Registration No.: m 211) Department of surgery, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Conflict of Interest: None declared.
Note
Please cite this paper as: Javaherzadeh M, Shekarchizadeh A, Kafaei M, Mirafshrieh A, Mosaffa N, Sabet B. Effects of Intraperitoneal Administration of Simvastatin in Prevention of Postoperative Intra-Abdominal Adhesion Formation in Animal Model of Rat. Bull Emerg Trauma. 2016;4(3):156-160.
References
- 1.Fortin CN, Saed GM, Diamond MP. Predisposing factors to post-operative adhesion development. Hum Reprod Update. 2015;21(4):536–51. doi: 10.1093/humupd/dmv021. [DOI] [PubMed] [Google Scholar]
- 2.Aarons CB, Cohen PA, Gower A, Reed KL, Leeman SE, Stucchi AF, et al. Statins (HMG-CoA reductase inhibitors) decrease postoperative adhesions by increasing peritoneal fibrinolytic activity. Ann Surg. 2007;245(2):176–84. doi: 10.1097/01.sla.0000236627.07927.7c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ouaissi M, Gaujoux S, Veyrie N, Deneve E, Brigand C, Castel B, et al. Post-operative adhesions after digestive surgery: their incidence and prevention: review of the literature. J Visc Surg. 2012;149(2):e104–14. doi: 10.1016/j.jviscsurg.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 4.Ellis H. Postoperative intra-abdominal adhesions: a personal view. Colorectal Dis. 2007;9 (Suppl 2):3–8. doi: 10.1111/j.1463-1318.2007.01344.x. [DOI] [PubMed] [Google Scholar]
- 5.Tingstedt B, Isaksson J, Andersson R. Long-term follow-up and cost analysis following surgery for small bowel obstruction caused by intra-abdominal adhesions. Br J Surg. 2007;94(6):743–8. doi: 10.1002/bjs.5634. [DOI] [PubMed] [Google Scholar]
- 6.Tahmasebi S, Tahamtan M, Tahamtan Y. Prevention by rat amniotic fluid of adhesions after laparatomy in a rat model. Int J Surg. 2012;10(1):16–9. doi: 10.1016/j.ijsu.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 7.Nikeghbalian S, Vafaei H, Moradian F, Kazemi K, Tanideh N, Shayan L, et al. Administration of Intravenous Inf liximab for Prevention of Peritoneal Adhesions Formation in Rats. Bull Emerg Trauma. 2015;3(3):97–103. [PMC free article] [PubMed] [Google Scholar]
- 8.Karaca T, Gozalan AU, Yoldas O, Bilgin BC, Tezer A. Effects of tamoxifen citrate on postoperative intra-abdominal adhesion in a rat model. Int J Surg. 2013;11(1):68–72. doi: 10.1016/j.ijsu.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 9.Yildiz MK, Okan I, Dursun N, Bas G, Alimoglu O, Kaya B, et al. Effect of orally administered simvastatin on prevention of postoperative adhesion in rats. Int J Clin Exp Med. 2014;7(2):405–10. [PMC free article] [PubMed] [Google Scholar]
- 10.Canbaz MA, Ustun C, Kocak I, Yanik FF. The comparison of gonadotropin-releasing hormone agonist therapy and intraperitoneal Ringer's lactate solution in prevention of postoperative adhesion formation in rat models. Eur J Obstet Gynecol Reprod Biol. 1999;82(2):219–22. doi: 10.1016/s0301-2115(98)00230-9. [DOI] [PubMed] [Google Scholar]
- 11.Kucuk HF, Kaptanoglu L, Kurt N, Uzun H, Eser M, Bingul S, et al. The role of simvastatin on postoperative peritoneal adhesion formation in an animal model. Eur Surg Res. 2007;39(2):98–102. doi: 10.1159/000099156. [DOI] [PubMed] [Google Scholar]
- 12.Gharibzadeh S, Hoseini SS. Does TGF beta suppressing effect of simvastatin lead to protection against surgical adhesion band formation? Iran J Med Hypotheses Ideas. 2008;2:1–4. [Google Scholar]
- 13.Arslan E, Talih T, Oz B, Halaclar B, Caglayan K, Sipahi M. Comparison of lovastatin and hyaluronic acid/carboxymethyl cellulose on experimental created peritoneal adhesion model in rats. Int J Surg. 2014;12(2):120–4. doi: 10.1016/j.ijsu.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 14.Gajendragadkar PR, Cooper DG, Walsh SR, Tang TY, Boyle JR, Hayes PD. Novel uses for statins in surgical patients. Int J Surg. 2009;7(4):285–90. doi: 10.1016/j.ijsu.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 15.Davis ME, Korn MA, Gumucio JP, Harning JA, Saripalli AL, Bedi A, et al. Simvastatin reduces fibrosis and protects against muscle weakness after massive rotator cuff tear. J Shoulder Elbow Surg. 2015;24(2):280–7. doi: 10.1016/j.jse.2014.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoscan Y, Karabulut Z, Hoscan MB, Arikan S, Ogus E, Muderrisoglu H. Oral fluvastatin reduces the severity of peritoneal adhesions in rats. Acta Chir Belg. 2010;110(1):66–70. doi: 10.1080/00015458.2010.11680568. [DOI] [PubMed] [Google Scholar]
- 17.Lalountas MA, Ballas KD, Skouras C, Asteriou C, Kontoulis T, Pissas D, et al. Preventing intraperitoneal adhesions with atorvastatin and sodium hyaluronate/carboxymethylcellulose: a comparative study in rats. Am J Surg. 2010;200(1):118–23. doi: 10.1016/j.amjsurg.2009.06.026. [DOI] [PubMed] [Google Scholar]
- 18.Srinivasa S, Kahokehr AA, Sammour T, Yu TC, Abbas SM, Hill AG. Use of statins in adhesive small bowel obstruction. J Surg Res. 2010;162(1):17–21. doi: 10.1016/j.jss.2010.02.028. [DOI] [PubMed] [Google Scholar]