Abstract
Background
In this study, we investigate the correlation between reduced global longitudinal peak systolic strain (GLPSS) and the SYNTAX score (SS) in patients undergoing coronary angiography.
Methods
We examined 71 patients undergoing both echocardiogram and coronary angiography within 15 days. All patients had normal global and/or regional wall motion on resting echocardiogram. We calculated GLPSS using two-dimensional speckle-tracking echocardiography. SS was calculated for each group of patients based on the presence and/or the severity of coronary artery disease (CAD): no CAD on angiogram (n=10, control group), low SS (n=36, SS<22) and high SS (n=25, SS≥22). We hypothesised that GLPSS at rest is inversely correlated with the angiographically derived SS.
Results
Age, sex and most of the risk factors were equally distributed among the groups. There was a significant inverse correlation between GLPSS and SS values (r2=0.3869, P<0.001). This correlation was weaker in the low-SS group (r2=0.1332, P<0.05), whereas it was lost in the high-SS group (r2=0.0002, P=NS). Receiver operating characteristic curve analysis identified that the optimal cut-off for the detection of high-SS patients was 13.95% (sensitivity=71%, specificity=90%, P<0.001).
Conclusions
The results of our study suggest that GLPSS might be promising for the detection of patients with high SYNTAX score on coronary angiogram. There is an inverse correlation between resting GLPSS and SS as assessed by coronary angiography. In patients with the highest SS, however, the correlation with GLPSS was less significant.
Keywords: speckle-tracking echocardiography, coronary artery disease
Introduction
Percutaneous coronary intervention with TAXUS and cardiac surgery known as SYNTAX score (SS) is an angiographic tool used to determine the severity of coronary artery disease (CAD) in patients undergoing coronary angiography. Two-dimensional strain echocardiography may be able to detect early changes in cardiac function caused by ischaemia and predict the extent of coronary lesions (1) in the shape of SS. The echocardiographic diagnosis of CAD mostly relies on the visual detection of LV wall motion abnormalities of radial myocardial shortening and thickening and assessment of LV ejection fraction (EF). However, LV wall motions are usually normal at rest in these patients, unless there is a history of myocardial infarction (2). The contribution of longitudinal myocardial deformation is largely neglected. Myocardial strain represents the magnitude of myocardial deformation, which is an energy-requiring process. Longitudinal mechanics predominate in the ischaemia-vulnerable subendocardium, and abnormalities of myocardial deformation in the longitudinal axis are seen in the development of many pathophysiologic states, including CAD and myocardial infarction (3, 4, 5, 6, 7, 8, 9, 10).
Purpose
In this study, we hypothesised that there is an inverse relationship between SYNTAX score and global longitudinal peak systolic strain (GLPSS) and that a reduced GLPSS may be able to predict a high SS in patients undergoing coronary angiography, with no history of myocardial infarction.
Methods
Study subjects
We prospectively recruited a total of 85 patients, 15 of whom were excluded due to suboptimal image quality on echocardiography. The inclusion criteria were as follows: evaluation of patients with suspected CAD, no global and/or regional wall motion abnormalities on resting echocardiogram with an EF of at least >50%, no conduction abnormalities on ECG, no more than mild valvular disease, no more than mild left ventricular hypertrophy and no evidence of cardiomyopathy. All patients were referred by primary or secondary care physicians, with chest pain suggestive of CAD for angiogram. All patients consented before their participation in this study. A total of 71 patients were finally recruited who underwent both echocardiogram and coronary angiography within 15 days. The study was approved by the local ethics committee.
Echocardiographic examination
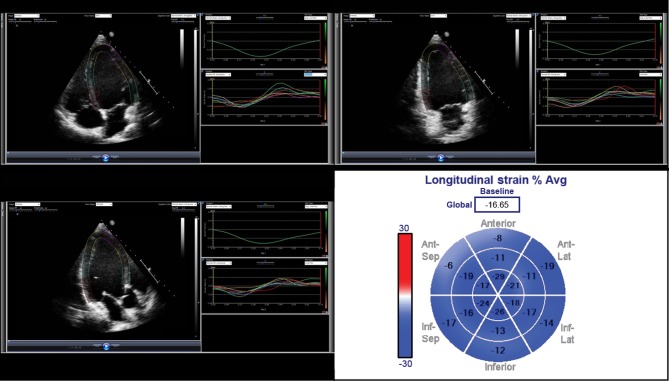
Conventional 2D echocardiographic examination was performed in each patient using an iE33, ultrasound system (Philips Healthcare) and Vivid E9 (GE Healthcare; Medical Diagnostics). Two-dimensional greyscale harmonic images were obtained in the apical long axis four-chamber and two-chamber views for the global analysis with a frame rate of a minimum of 40Hz. Strain analysis was performed off-line by one investigator who was unaware of the angiographic results using the vendor non-specific software EchoInsight Suit (Research Tool; Epsilon Imaging, Ann Arbor, MI, USA). GLPSS was obtained from the apical four-chamber, three-chamber and two-chamber views, after manually tracing the endocardium and epicardium of the left ventricle, beginning from the annulus of the mitral valve for all the three views. Necessary adjustments were made in order to include the entire myocardial layer with the best possible quality throughout the cardiac cycle. The software provided an average value of GLPSS for a total of 16 segments of the left ventricle (Fig. 1). The LV end-systolic volume, LV end-diastolic volume, the LV EF and fractional shortening were measured as described previously (11).
Figure 1.
Measurement of GLPSS in a patient with low SS. From top to bottom and left to right: apical four-chamber, two-chamber and three-chamber strain analysis. In the last image, segmental strain is represented as ‘bull’s eye’ display along with the GLPSS.
Coronary angiography
Two experienced interventional cardiologists assessed the coronary angiography results and independently provided the SS for each patient. Patients were grouped according to their SS as follows: (i) no CAD, (ii) low SS (SS<22) and (iii) high SS (SS≥22).
Statistical analysis
Normality was assessed with Shapiro–Wilk test, which showed that strain values were normally distributed (P > 0.05). The results are presented as mean±s.d. or as frequency. Student’s t-test was used to compare the means of continuous variables. Mean scores of more than two groups were compared using analysis of variance (ANOVA). Categorical variables are expressed as absolute values and frequency percentages and are compared using χ2 tests. Receiver operating characteristic (ROC) curve analysis was used to identify parameters that best predicted the presence of high-risk CAD. Pearson’s r was used to measure the linear correlation between SS and %GLPSS giving a value between +1 and −1, where 1 is total positive correlation, 0 is no correlation and −1 is total negative correlation. P<0.05 was regarded as denoting a statistically significant difference. Computations were performed using SPSS version 23.0 (SPSS).
Reproducibility between observers for grading SS was assessed based on their scores on 15 multi-vessel angiograms. Inter-observer agreement was determined using the Fleiss k statistic values. Good reproducibility of the SS measurements (k=0.82; 95% CI (0.72, 1.00)) between the two investigators was observed.
Results
GLPSS was successfully measured in 71 of the 85 (84%) patients and is presented as absolute values. Patients’ clinical characteristics are shown in Table 1. Their mean age was 62±11 and 49 (69%) were men. There were no significant differences between the groups for most of the risk factors. Patients in the low-SS group were older than those in the control group.
Table 1.
Characteristics of the study population.
| Characteristics | CAD (+) group | No CAD group (n=10) | Total (n=71) | P-value | |
|---|---|---|---|---|---|
| High-SS group (n=25) | Low-SS group (n=36) | ||||
| Male (n%) | 19 (76) | 22 (67) | 6 (60) | 49 (69) | NS |
| Age (years) | 61±12 | 64±10* | 55±13 | 62±11 | 0.05 |
| Obesity (n%) | 4 (16) | 2 (6) | 1 (10) | 7 (10) | NS |
| Diabetes (n%) | 9 (36) | 15 (42) | 1 (10) | 25 (35) | NS |
| Hypertension (n%) | 15 (60) | 21 (58) | 4 (40) | 40 (56) | NS |
| Dyslipidaemia (n%) | 11 (44) | 18 (50) | 3 (30) | 32 (45) | NS |
| Smoking (n%) | 9 (36) | 16 (42) | 1 (10) | 26 (37) | NS |
| Chronic kidney disease (n%) | 4 (16) | 5 (14) | 2 (20) | 11 (15) | NS |
| Heart rate (bpm) | 68±8* | 65±8 | 62±4 | 66±8 | 0.05 |
| ARB and/or ACE inhibitors (n%) | 13 (52)* | 13 (36) | 1 (10) | 27 (38) | 0.05 |
| Calcium channel blockers (n%) | 7 (28) | 10 (28) | 2 (20) | 19 (27) | NS |
| Diuretics (n%) | 5 (20) | 5 (14) | 1 (10) | 11 (15) | NS |
| β-blockers (n%) | 10 (40) | 16 (44) | 3 (30) | 29 (41) | NS |
NS, non-significant.
*P<0.05 vs control group.
GLPSS and angiographic results
There was a significant inverse correlation between GLPSS and SS values (r2=0.3869, P<0.001). This correlation was weaker in the low-SS group (r2=0.1332, P<0.05), whereas it was lost in the high-SS group (r2=0.0002, P=NS) as shown in Fig. 2.
Figure 2.
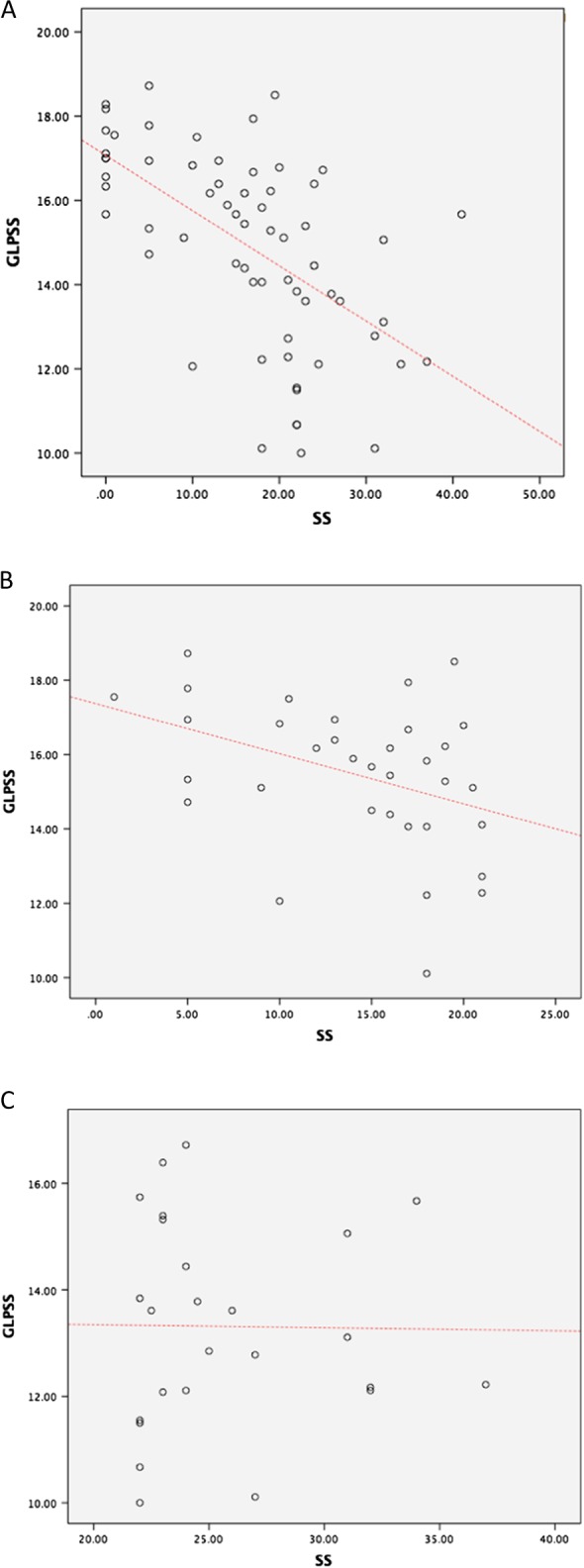
(A) Correlation between GLPSS and SS values. (B) Correlation between GLPSS and SS values for the low-SS group. (C) Correlation between GLPSS and SS values for the high-SS group.
A one-way between-groups analysis of variance with post hoc analysis with Bonferroni’s correction was conducted to explore the correlation of traditional echocardiographic parameters and GLPSS with SS values. Participants were divided into three groups according to their SS (no CAD: SS=0; low SS: <22; high SS: ≥22). There was a significant relationship between GLPSS and SS values. The rest of the parameters did not correlate with SS. The results are shown in Table 2.
Table 2.
Echocardiographic parameters.
| Parameters | High SS (n=25) | Low SS (n=36) | No CAD (n=10) | P-value |
|---|---|---|---|---|
| LV end-diastolic dimension (mm) | 42±6 | 44±4 | 45±3 | NS |
| LV end-systolic dimension (mm) | 29±5 | 30±4 | 30±4 | NS |
| LV ejection fraction (%) | 59±6 | 61±6 | 62±9 | NS |
| LV fractional shortening (%) | 31±6 | 32±6 | 33±9 | NS |
| GLPSS (%) | −13.31±1.91a,b | −15.61±1.90a | −17.39±1.15 | 0.05a,b |
NS, Non-significant.
aP<0.05 when compared with normal.
bP<0.05 when compared with low SS; High SS: SS≥22; low SS: SS<22.
Receiver operating characteristic analysis for the diagnosis of severe coronary artery disease
The results of ROC analysis for GLPSS, basal, mid and apical LPSS are shown in Fig. 3. The optimal cut-off of GLPSS, which discriminated patients with high SS from all participants, was −13.95% (sensitivity=71% and specificity=90%, AUC=0.846, 95%CI=0.75−0.95, P<0.001) and was the overall best parameter predicting high SS.
Figure 3.
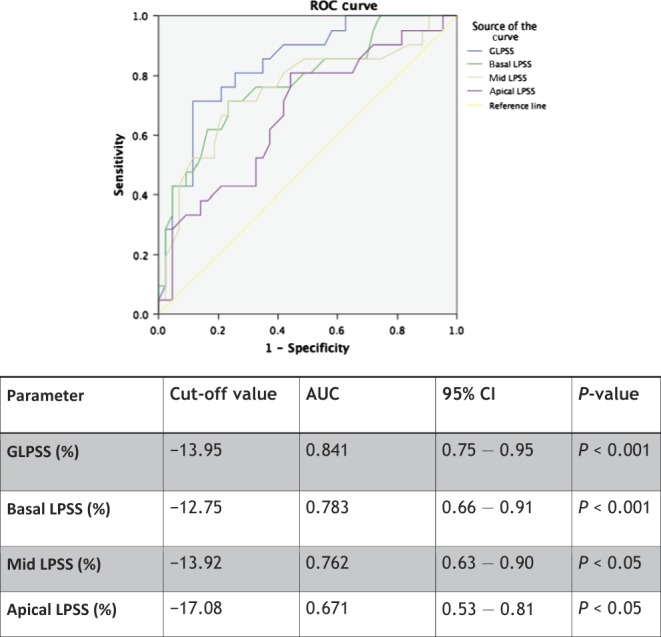
Predictive myocardial strain characteristics for the detection of high-SS (SS≥22) population.
Discussion
In this study, we found that resting GLPSS best predicts a high SS in patients with suspected CAD. There was a reverse linear correlation between GLPSS and the angiographically derived SS, which is widely used as a scoring system for the quantification of the complexity of CAD. Therefore, a good relationship between anatomy and function was found.
Left ventricular wall motion at rest can be normal, even in patients with severe CAD. Therefore, it would be useful if another resting parameter could help in the discrimination of patients with severe CAD from those with less severe or no CAD. Studies (9, 10) have shown that longitudinal strain correlates well with the presence and severity of CAD, but none has investigated whether a correlation exists with SS. We found that a GLPSS cut-off value of −13.95% on the vendor non-specific software used in this study predicts the detection of a high SS among patients with suspected CAD with good sensitivity and specificity. Reduced GLPSS, therefore, increases the pretest probability for the presence of severe CAD and may enable earlier recognition of patients who are more likely to have complex CAD on angiogram and for whom coronary artery bypass surgery might be the most appropriate therapeutic option.
Although SS was designed to characterise coronary anatomy based on nine anatomic criteria such as lesion location and complexity, Tanaka and coworkers showed that it correlates well with myocardial ischaemia as assessed by stress SPECT (12). The correlation we observed between GLPSS and the SS might reflect the underlying relationship between SS and possible microcirculatory damage. The weak but significant correlation between SS and GLPSS that we observed in all coronary artery disease patients as well as in the low-SS group at rest was also found by Tanaka and coworkers during stress SPECT (12). In addition, they found that stress SPECT did not correlate well with the high SS values, which is again consistent with our observation of poor correlation between GLPSS and SS values for the high-SS group. This can probably be attributed to the increasing complexity of the lesions for higher SS. Calculation of SS takes into account not only the number of coronary lesions but also their anatomical characteristics such as tortuosity and calcification. A higher SS, therefore, does not necessarily reflect an increase in the extent of myocardial ischaemia, which may explain the loss of correlation in the high-SS group.
Our study confirms the results of previous studies that investigated the role of speckle-tracking echocardiography for diagnosing CAD in patients with normal resting echocardiogram (6, 7, 9, 10). Strain echocardiography is a simple, inexpensive and risk-free diagnostic tool, which should become a routine part of the evaluation of patients suspected of having CAD.
Limitations
There are a number of limitations in this study. Our control group consists of a small number of patients. The number of patients admitted to our institution, a tertiary university hospital, might cause a possible selection bias. Some of the baseline characteristics of the population that are known to affect GLPSS were not equally distributed among the participants (age, heart rate, hypertension and some of the medications) and may have contributed to reduced GLPSS values. Cardiac MRI was not performed to look for myocardial fibrosis, which could explain reduced strain values in some of those patients. Finally, we did not use intravascular ultrasound or functional studies (functional flow reserve) for a more accurate measurement of myocardial ischaemia.
The results of this study may also not be readily applicable in clinical practice. We conducted this study due to the need for a simple, non-invasive method to identify patients with coronary artery disease. We hypothesised that GLPSS correlates with coronary artery disease severity. The aim of this study is to investigate this hypothesis-generating idea, which can improve the selection of patients who are referred for coronary angiography. Our results show that GLPSS can help in the identification of patients with severe coronary artery disease in the form of high SYNTAX score. It definitely needs further evaluation before being recommended for clinical use.
Conclusions
The results of our study suggest that GLPSS aids in the detection of patients with increased SYNTAX score on coronary angiogram. We showed that there is an inverse correlation between resting GLPSS and SS as assessed by coronary angiography. In patients with the highest SS, however, the correlation with GLPSS was less significant. GLPSS is a useful tool for the initial evaluation of patients with suspected CAD.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Acknowledgements
We deeply thank the Foundation for Education and European Culture in Greece for the help and financial support, which made this research possible.
References
- 1.Nesbitt GC, Mankad S, Oh JK. 2009. Strain imaging in echocardiography: methods and clinical applications. International Journal of Cardiovascular Imaging 25 (Supplement 1) 9–22. 10.1007/s10554-008-9414-1 [DOI] [PubMed] [Google Scholar]
- 2.Elhendy A, van Domburg RT, Bax JJ, Roelandt JR. 2000. Significance of resting wall motion abnormalities in 2-dimensional echocardiography in patients without previous myocardial infarction referred for pharmacologic stress testing. Journal of the American Society of Echocardiography 13 1–8. 10.1016/s0894-7317(00)90036-1 [DOI] [PubMed] [Google Scholar]
- 3.Jamal F, Sutherland GR, Weidemann F, D’hooge J, Bijnens B, Derumeaux G. 2002. Can changes in systolic longitudinal deformation quantify regional myocardial function after an acute infarction? An ultrasonic strain rate and strain study. Journal of the American Society of Echocardiography 15 723–730. 10.1067/mje.2002.118913 [DOI] [PubMed] [Google Scholar]
- 4.Williams RI, Payne N, Phillips T, D’hooge J, Fraser AG. 2005. Strain rate imaging after dynamic stress provides objective evidence of persistent regional myocardial dysfunction in ischaemic myocardium: regional stunning identified? Heart 91 152–160. 10.1136/hrt.2003.027490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liang HY, Cauduro S, Pellikka P, Wang J, Urheim S, Yang EH, Rihal C, Belohlavek M, heria B, Miller FA, et al. 2006 Usefulness of two-dimensional speckle strain for evaluation of left ventricular diastolic deformation in patients with coronary artery disease. American Journal of Cardiology 98 1581–1586. 10.1016/j.amjcard.2006.07.038 [DOI] [PubMed] [Google Scholar]
- 6.Nucifora G, Schuijf JD, Delgado V, Bertini M, Scholte AJ, Ng AC, van Werkhoven JM, Jukema JW, Holman ER, van der Wall EE, et al. , . 2010. Incremental value of subclinical left ventricular systolic dysfunction for the identification of patients with obstructive coronary artery disease. American Heart Journal 159 148–157. 10.1016/j.ahj.2009.10.030 [DOI] [PubMed] [Google Scholar]
- 7.Eek C, Grenne B, Brunvand H, Aakhus S, Endresen K, Smiseth OA, Edvardsen T, Skulstad H. 2010. Strain echocardiography predicts acute coronary occlusion in patients with non-ST-segment elevation acute coronary syndrome. European Journal of Echocardiography 11 501–508. 10.1093/ejechocard/jeq008 [DOI] [PubMed] [Google Scholar]
- 8.Gjesdal O, Helle-Valle T, Hopp E, Lunde K, Vartdal T, Aakhus S, Smith HJ, Ihlen H, Edvardsen T. 2008. Noninvasive separation of large, medium, and small myocardial infarcts in survivors of reperfused ST-elevation myocardial infarction: a comprehensive tissue Doppler and speckle-tracking echocardiography study. Circulation Cardiovascular Imaging 1 189–196. 10.1161/CIRCIMAGING.108.784900 [DOI] [PubMed] [Google Scholar]
- 9.Choi JO, Cho SW, Song YB, Cho SJ, Song BG, Lee SC, Park SW. 2009. Longitudinal 2D strain at rest predicts the presence of left main and three vessel coronary artery disease in patients without regional wall motion abnormality. European Journal of Echocardiography 10 695–701. 10.1093/ejechocard/jep041 [DOI] [PubMed] [Google Scholar]
- 10.Montgomery DE, Puthumana JJ, Fox JM, Ogunyankin KO. 2012. Global longitudinal strain aids the detection of non-obstructive coronary artery disease in the resting echocardiogram. European Heart Journal: Cardiovascular Imaging 13 579–87. 10.1093/ejechocard/jer282 [DOI] [PubMed] [Google Scholar]
- 11.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, et al. , . 2015. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. European Heart Journal: Cardiovascular Imaging 16 233–270. 10.1093/ehjci/jev014 [DOI] [PubMed] [Google Scholar]
- 12.Tanaka H, Chikamori T, Hida S, Igarashi Y, Shiba C, Usui Y, Hatano T, Yamashina A. 2013. Relationship of SYNTAX score to myocardial ischemia as assessed on myocardial perfusion imaging. Circulation Journal 77 2772–2777. 10.1253/circj.cj-13-0099 [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a