Abstract
Aim
It is not well known if advancing age influences normal rest or exercise pulmonary artery pressures. The purpose of the study was to evaluate the association of increasing age with measurements of pulmonary artery systolic pressure at rest and with exercise.
Subjects and methods
A total of 467 adults without cardiopulmonary disease and normal exercise capacity (age range: 18–85 years) underwent symptom-limited treadmill exercise testing with Doppler measurement of rest and exercise pulmonary artery systolic pressure.
Results
There was a progressive increase in rest and exercise pulmonary artery pressures with increasing age. Pulmonary artery systolic pressures at rest and with exercise were 25±5mmHg and 33±9mmHg, respectively, in those <40 years, and 30±5mmHg and 41±12mmHg, respectively, in those ≥70 years. While elevated left-sided cardiac filling pressures were excluded by protocol design, markers of arterial stiffness associated with the age-dependent effects on pulmonary pressures.
Conclusion
These data demonstrate that in echocardiographically normal adults, pulmonary artery systolic pressure increases with advancing age. This increase is seen at rest and with exercise. These increases in pulmonary pressure occur in association with decreasing transpulmonary flow and increases in systemic pulse pressure, suggesting that age-associated blood vessel stiffening may contribute to these differences in pulmonary artery systolic pressure.
Keywords: pulmonary artery systolic pressure, stress echocardiography
Introduction
Pulmonary artery (PA) systolic pressure may increase with exercise in the setting of hypoxia, chronic lung disease, heart failure, and in some patients with connective tissue disease or other conditions that are associated with pulmonary vascular disease (1, 2, 3, 4, 5, 6, 7). The clinical importance of pulmonary hypertension that develops with exercise, however, remains enigmatic in part because a clear delineation between normal and abnormal responses to exertion has not been established for individuals (7, 8). Prior studies have indicated that PA pressures at rest and with exercise are higher in highly trained athletes compared with healthy controls (in large part related to greater cardiac output-mediated transpulmonary flow) (9). Further data suggest that PA pressures are higher in subjects over the age of 50 years compared with younger individuals (10, 11, 12). However, these studies did not address or exclude the possible role of left ventricular (LV) filling pressures and the potential impact of diastolic dysfunction on pulmonary pressures. Due to the lack of data in this regard, at the Fourth World Symposium on pulmonary arterial hypertension (Dana Point, February 2008), a consensus opinion determined that the definition of exercise-induced pulmonary hypertension should be deferred (7). The normal range of PA systolic pressure changes with exercise and any association with age (particularly in patients over the age of 50) remains ill-defined.
The purpose of the present study was to evaluate the association of increasing age with measurements of right ventricular and PA hemodynamics at rest and with exercise in a cohort of adult subjects without cardiopulmonary disease and with at least satisfactory exercise capacity.
Methods
Study setting and population
This retrospective study was performed in the Mayo Clinic Cardiovascular Ultrasound Imaging and Hemodynamics Laboratory in Rochester, MN, with the approval of the Institutional Review Board. Study subjects (age range: 18–85 years) were selected from those patients who underwent clinically indicated exercise echocardiography over a 1-year period (n=4540). During this period, all patients also underwent routine Doppler assessment of LV diastolic function and pulmonary pressures at rest and with exercise as part of a prospective protocol (13). Clinical variables, brachial artery blood pressure, and body mass index (weight in kilograms divided by height in meters squared) were recorded at the time of the symptom-limited treadmill exercise stress echocardiogram according to the Bruce protocol. Pulse pressure (the difference between brachial systolic and diastolic blood pressures) was used as an index of systemic arterial stiffness (14). Systemic arterial stiffening results in the earlier return of reflected waves and thus increased systolic, decreased diastolic blood pressures, and increased pulse pressure (15). Medication use and pertinent medical history were abstracted from the medical record at the time of the echocardiogram and entered into a prospectively maintained database by specially trained nurses (16).
Exclusion criteria
In addition to requiring measurable Doppler signals for the assessment of PA systolic pressure and LV diastolic function at rest and post exercise, to be included subjects were required to have no evidence of (i) significant valvular heart disease (defined by more than mild stenosis and/or regurgitation of any cardiac valve or previous valve repair or replacement); (ii) coronary artery disease (history of myocardial infarction, coronary revascularization, or rest or stress wall motion abnormalities on echocardiography); (iii) LV systolic dysfunction (rest or peak exercise LV ejection fraction <50%); (iv) an elevation in LV filling pressures (E/e′ ratio at rest >15 or E/e′ immediately after exercise >13 or left atrial volume index ≥32cc/m2) (22, 23, 24); (v) atrial fibrillation/flutter; (vi) pulmonary airway or parenchymal disease (by history or available pulmonary function testing); (vii) obstructive sleep apnea (by history, overnight oximetry, and/or polysomnography); (viii) history of venous thrombo-embolic disease; (ix) history of connective tissue disease; (x) family or personal history of pulmonary arterial hypertension; (xi) congenital heart disease; (xii) human immunodeficiency viral (HIV) infection; (xiii) chronic liver disease (by history or evidence of synthetic dysfunction on serum testing); or (xiv) failure to achieve a satisfactory exercise capacity (i.e. with failure defined as <80% predicted functional aerobic capacity). A total of 469 subjects who met these criteria were included in the study group.
Doppler echocardiography
All echocardiograms were performed by registered diagnostic cardiac sonographers using standardized instruments and protocols and interpreted by an American Society of Echocardiography level 3 trained stress echocardiologist. In addition to standard two-dimensional, and color Doppler images, continuous-wave Doppler examination of tricuspid flow, pulsed-wave Doppler examination of mitral inflow, and tissue Doppler imaging of the medial mitral annulus were performed in each subject at rest and immediately following the regional wall motion assessment following a symptom-limited, Bruce protocol treadmill exercise test. The average time to record the peak TR velocity was 125 s, mitral annulus tissue Doppler 166 s, and the mitral inflow 180 s following the cessation of exercise.
Determination of ventricular function
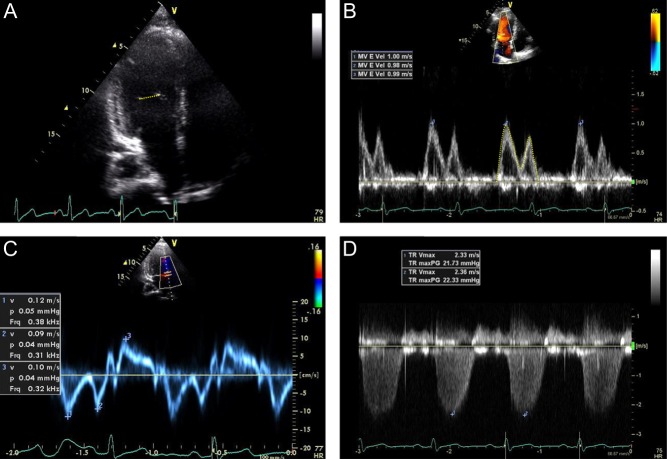
Left ventricular ejection fraction was assessed by a combination of the modified Quinones method (17) and visual assessment. Stroke volume at rest and with exercise was calculated from the LV inflow as the product of mitral valve area (derived from the diameter at the level of the mitral valve tips from the apical four chamber view) and the LV inflow TVI (time velocity integral) as measured by pulsed-wave Doppler of the mitral inflow at the level of the mitral valve tips from the apical four chamber view (18) (Fig. 1). Cardiac output at rest and with exercise was calculated as the product of stroke volume and heart rate and indexed to body surface area.
Figure 1.
Representative 2D and Doppler examples. Stroke volume was calculated from the LV inflow as the product of mitral valve area derived from the diameter at the level of the mitral valve tips from the apical four chamber view (A) and the LV inflow time velocity integral as measured by pulsed-wave Doppler of the mitral inflow at the level of the mitral valve tips from the apical four chamber view (B). The ratio of early mitral inflow peak velocity (B) to that of the early peak tissue velocity of the medial mitral annulus (C) was used as an estimate of LV filling pressure. The PA systolic pressure was calculated in standard fashion from the peak tricuspid regurgitant velocity (D).
Determination of PA pressures
Right ventricular systolic pressure and therefore PA systolic pressure was estimated by Doppler echocardiography by calculating the systolic right ventricular to right atrial pressure gradient using the modified Bernoulli equation (4 times the square of the peak tricuspid regurgitant velocity) and adding the right atrial pressure, assumed to be 5mmHg. The peak tricuspid regurgitant velocity was acquired from the view where the Doppler angle was most parallel to flow (incidence angle typically <20°) with the averaging of three consecutive values obtained in held expiration. None of the subjects had right ventricular outflow tract obstruction or pulmonic stenosis. Echo Doppler measurement of PA systolic pressure obtained in this manner has been shown to correlate well with invasive catheter-based measurements over a wide range of values (correlation coefficients ranging between 0.89 and 0.97) at our institution (19, 20) and others (3, 21).
Determination of LV diastolic pressures and LA volume
The ratio of early transmitral flow velocity (E) to early peak tissue velocity (e′) by spectral tissue Doppler of the medial mitral annulus was used as a Doppler-derived estimate of LV filling pressure (22, 23). Left atrial volume index was calculated by the area–length method from the apical four- and three chamber views, using the average of the two lengths and indexed to body surface area.
Statistical analysis
Statistical analyses were performed using JMP 8.0 (SAS Institute Inc, Cary, NC, USA). Continuous variables presented as mean±s.d. and 95% confidence intervals and tested between groups using analysis of variance, as appropriate. Categorical variables are presented as number and percentage, and comparisons were done using Pearson chi-square analysis. Variables that were significant in univariate analysis were selected in a stepwise forward-selection manner, with entry and retention set at a significance of 0.05. Upper normal values of right ventricular systolic pressure at rest and post exercise were determined from the upper 5% limits derived from semiparametric logistic regression of normative data factoring in age. For all analyses, a P<0.05 was considered to be statistically significant. The correlation coefficient in a subgroup (n=50) blinded assessment of interobserver variability was 0.92 for TR velocity, 0.85 for E/e′, and 0.77 for CO.
Results
PA pressure at rest
The study group of 469 subjects had a mean age±s.d. of 57±12 years and 292 (62%) were female. Baseline demographics of the cohort are given in Table 1. Overall, the tricuspid regurgitant peak velocity averaged 2.4±0.3m/s at rest. This corresponded to a PA systolic pressure of 28±5mmHg.
Table 1.
Baseline demographics (n=467).
| Age (years) | 57±12 |
| Sex (female) | 292 (62%) |
| Body mass index (kg/m2) | 27±5 |
| Systolic blood pressure (mmHg) | 124±19 |
| Diastolic blood pressure (mmHg) | 76±10 |
| Heart rate (beats per min) | 75±13 |
| Smoking history | |
| Current smoker | 57 (12%) |
| Ex-smoker | 132 (28%) |
| Systemic hypertension | 180 (38%) |
| Family history of coronary artery disease | 183 (39%) |
| Diabetes mellitus | 3 (0.6%) |
| Medication use | |
| ACE inhibitor/angiotensin receptor blocker | 70 (15%) |
| Beta blocker | 78 (17%) |
| Calcium channel blocker | 24 (5%) |
| Digoxin | 0 (0%) |
| Spironolactone | 1 (0%) |
| Indication for stress echocardiogram | |
| Atypical chest pain | 236 (50%) |
| Risk factors for coronary artery disease | 134 (29%) |
| Abnormal resting ECG | 46 (10%) |
| Risk stratification before noncardiac surgery | 38 (8%) |
| Abnormal coronary calcium scan | 15 (3%) |
With increasing age, the resting PA systolic pressure increased (r=0.28; P<0.0001; Fig. 2 and Table 2) in men (r=0.20; P<0.001) but to a greater degree in women (r=0.32; P<0.0001). After adjusting for age, no sex-associated difference in PA systolic pressure was found (P=0.73). Cardiac output at rest tended to decline with age (r=0.12; P<0.01; Table 2), suggesting that the association of increasing PA systolic pressure with advancing age was not mediated by increased transpulmonary flow. PA systolic pressure at rest did increase significantly with increasing systemic pulse pressure (r=0.33; P<0.0001), Fig. 3.
Figure 2.
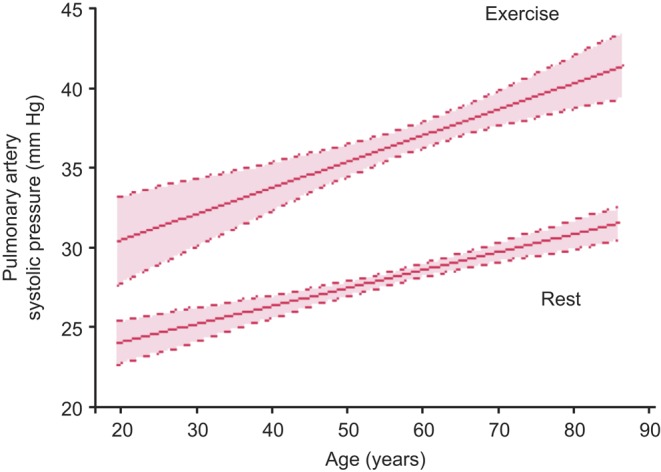
Age-dependent changes in pulmonary artery systolic pressure with exercise. There is a progressive rise in pulmonary artery systolic pressure at rest and with exercise with advancing age. Curves were fitted to the data using linear regression analysis. Dashed lines indicate the upper and lower 95% confidence limits for the mean values.
Table 2.
Resting Doppler-derived hemodynamics according to age.
| Age (years) | <40 (n=38) | 40–49 (n=93) | 50–59 (n=127) | 60–69 (n=127) | >70 (n=84) |
|---|---|---|---|---|---|
| Before exercise | |||||
| Tricuspid regurgitant peak velocity (m/s*) | 2.3±0.2 | 2.4±0.2 | 2.4±0.3 | 2.4±0.2 | 2.5±0.3 |
| (2.2, 2.3) | (2.3, 2.4) | (2.3, 2.4) | (2.4, 2.5) | (2.5, 2.6) | |
| Pulmonary artery systolic pressure (mmHg*, **) | 25±5 | 28±4 | 28±5 | 29±5 | 30±5 |
| (24–26) | (27–28) | (27–29) | (28–30) | (29–31) | |
| 33 | 35 | 37 | 39 | 41 | |
| Cardiac output (L/min) | 7.9±3.1 | 7.7±2.4 | 7.2±2.5 | 7.1±2.4 | 6.8±2.0 |
| (6.7, 9.0) | (7.1, 8.2) | (6.8, 7.7) | (6.6, 7.6) | (6.4, 7.3) | |
| PASP/Cardiac output (mmHg/L min−1) | 3.8±1.7 | 4.0±1.5 | 4.2±1.4 | 4.5±1.8 | 4.8±1.3 |
| (3.2, 4.5) | (3.6, 4.4) | (3.9, 4.5) | (4.2, 5.2) | (4.5, 5.2) |
Data presented as mean±s.d. and (upper and lower 95% confidence intervals)
Upper normal defined as the 5% limit derived from semiparametric logistic regression of normative data factoring in age.
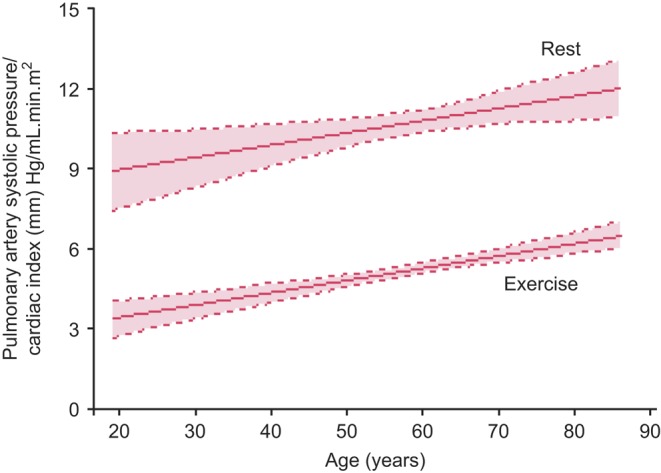
Figure 3.
Age-dependent changes in pulmonary artery systolic pressure adjusted for cardiac index with exercise. Progressive rise in pulmonary artery systolic pressure at rest and with exercise persists with advancing age after adjusting for transpulmonary flow (cardiac index). Curves were fitted to the data using linear regression analysis. Dashed lines indicate the upper and lower 95% confidence limits for the mean values.
PA pressure with exercise
Exercise duration declined with increasing age (r=0.47; P<0.0001) as did peak heart rate (r=0.59; P<0.0001) and exercise cardiac output (r=0.28; P<0.0001) (Table 3). Immediately after symptom-limited treadmill exercise (mean exercise duration 8.9±2.5min; 122±27% of predicted functional aerobic capacity), tricuspid regurgitant peak velocity increased to 2.8±0.4m/s, which corresponded to an estimated PA systolic pressure of 37.0±9.5mmHg with an average difference from rest to exercise of 8.7±8.1mmHg (Table 3). Smoking status was not associated with PA systolic pressure with exercise (P=0.3). Exercise PA systolic pressure was similar (mean and 95% C.I.) between those with left atrial volume indices of ≤22cc/m2 (37 (35, 38)), 23–28cc/m2 (37 (35, 38)), and 29–32cc/m2 (38 (36, 39)); P=0.65. There was no association between the exercise PA systolic pressure or the change in PA systolic pressure with exercise and the workload achieved, expressed as a percent of predicted functional aerobic capacity (r=0.04; P=0.4). The change in PA systolic pressure with exercise was similar between men and women (P=0.3). The use of an antihypertensive was associated with a mildly higher resting average PA systolic pressure (30±5mmHg vs 28±5mmHg at rest) and post exercise (39±8mmHg vs 36±8mmHg) (Supplementary Tables 1 and 2, see section on supplementary data given at the end of this article). PA systolic pressures with exercise increased with advancing age (Fig. 2 and Table 2) driven mostly by differences in resting tricuspid regurgitant velocity/pressure (Table 4). With increasing age, exercise duration, peak exercise heart rates, and cardiac outputs all declined. The ratio of PA systolic pressure to cardiac output/index ratios declined with exercise although remained positively associated with advancing age (Fig. 3). After adjusting for age and body mass index or resting tricuspid regurgitant velocity, systemic pulse pressure remained positively associated with exercise PA systolic pressure (Table 4, Figure 4). Smoking status was not associated with a difference in PA systolic pressure before (28±5 vs 28±5, P=0.4) or after 38±10 vs 37±9, P=0.25) exercise.
Table 3.
Clinical and echocardiographic exercise characteristics according to age.
| Age (years) | <40 (n=38) | 40–49 (n=93) | 50–59 (n=127) | 60–69 (n=127) | >70 (n=84) |
|---|---|---|---|---|---|
| Exercise duration (min*) | 10.8±2 | 10.2±2.4 | 9.3±2.3 | 8.3±2.3 | 7.0±1.9 |
| (10.1, 11.5) | (9.7, 10.7) | (8.9, 9.7) | (7.9, 8.7) | (6.6, 7.4) | |
| % of predicted functional aerobic capacity* | 101±11 | 115±23 | 125±26 | 124±26 | 131±34 |
| (97, 105) | (111, 120) | (121, 130) | (120, 129) | (124, 139) | |
| Peak systolic blood pressure (mmHg*) | 155±23 | 167±26 | 163±21 | 170±23 | 166±26 |
| (147, 163) | (160, 171) | (159, 167) | (166, 174) | (160, 172) | |
| Systemic pulse pressure (mmHg) | 39±19 | 44±21 | 40±19 | 37±22 | 33±21 |
| (33, 45) | (39, 48) | (37, 43) | (33, 40) | (28, 37) | |
| Peak heart rate (beats per min*) | 175±19 | 167±17 | 157±15 | 147±16 | 136±17 |
| (169, 181) | (164, 171) | (154, 160) | (144, 149) | (132, 140) | |
| Tricuspid regurgitant peak velocity (m/s*) | 2.6±0.3 | 2.8±0.4 | 2.8±0.4 | 2.8±0.4 | 3.0±0.5 |
| (2.5, 2.7) | (2.7, 2.8) | (2.7, 2.8) | (2.7, 2.9) | (2.9, 3.1) | |
| Pulmonary artery systolic pressure (mmHg*, **) | 33±7 | 36±8 | 36±9 | 37±10 | 41±12 |
| (31–35) | (34–37) | (34–38) | (35–39) | (38–43) | |
| 50 | 53 | 54 | 54 | 56 | |
| Delta pulmonary artery systolic pressure from baseline (mmHg*) | 7±7 | 8±8 | 9±8 | 9±8 | 10±9 |
| (5,10) | (7, 10) | (7, 10) | (7, 10) | (8, 12) | |
| Cardiac output (L/min*) | 16.6±5.8 | 17.4±4.8 | 14.7±4.9 | 14.6±4.6 | 12.8±3.7 |
| (14.4, 18.7) | (16.2, 18.6) | (16.2, 18.6) | (13.7, 15.5) | (11.9, 13.7) | |
| PASP/cardiac output (mmHg/L min−1) | 2.3±1.3 | 2.2±0.7 | 2.7±1.1 | 2.8±1.2 | 3.3±1.1 |
| (1.8, 2.7) | (2.0, 2.4) | (2.5, 2.9) | (2.6, 3.0) | (3.1, 3.6) | |
| Delta PASP/cardiac output from baseline (mmHg/L min−1) | 0.99±1.6 | 1.03±1.1 | 1.56±2.1 | 1.33±1.5 | 1.83±2.2 |
| (0.4, 1.6) | (0.76, 1.31) | (1.2, 2.0) | (1.0, 1.6) | (1.3, 2.4) |
Data presented as mean±s.d. and (upper and lower 95% confidence intervals)
Upper normal defined as the 5% limit derived from semi-parametric logistic regression of normative data factoring in age.
Table 4.
Association of clinical and echocardiographic variables with exercise pulmonary artery systolic pressure.
| Univariate | Multivariate model 1 | Multivariate model 2 | ||||
|---|---|---|---|---|---|---|
| β coefficient (s.e.m.) | P value | β coefficient (s.e.m.) | P value | β coefficient (s.e.m.) | P value | |
| Age per 10 year | 1.6 (0.3) | <0.0001 | 1.03 (0.37) | 0.006 | ||
| Female sex | −0.51 (0.8) | 0.54 | ||||
| Body mass index (kg/m2) | 0.16 (0.09) | 0.08 | 0.23 (0.09) | 0.01 | ||
| TR peak velocity at rest (m/s) | 17.4 (1.4) | <0.0001 | 18.1 (1.5) | <0.0001 | ||
| Pulse pressure at rest (per 10mmHg) | 1.71 (0.3) | <0.0001 | 0.68 (0.3) | 0.01 | 1.38 (0.3) | <0.0001 |
| Systolic BP at rest (per 10mmHg) | 1.15 (0.2) | <0.0001 | ||||
| History of hypertension | 3.93 (0.9) | <0.0001 | ||||
| Current or prior smoker | 1.13 (0.9) | 0.2 | ||||
| Duration of exercise (min) | −0.70 (0.17) | <0.0001 | ||||
| Cardiac index with exercise (mL/min/m2) | −0.007 (0.2) | 0.97 | ||||
| Systolic BP post exercise (per 10mmHg) | 0.02 (0.008) | 0.04 | 0.4 (0.2) | <0.02 | ||
| Pulse pressure post exercise (per 10mmHg) | 0.02 (0.009) | 0.04 | 0.4 (0.2) | 0.05 | ||
Multivariate analysis with (model 1) and without (model 2) the inclusion of tricuspid regurgitant peak velocity at rest.
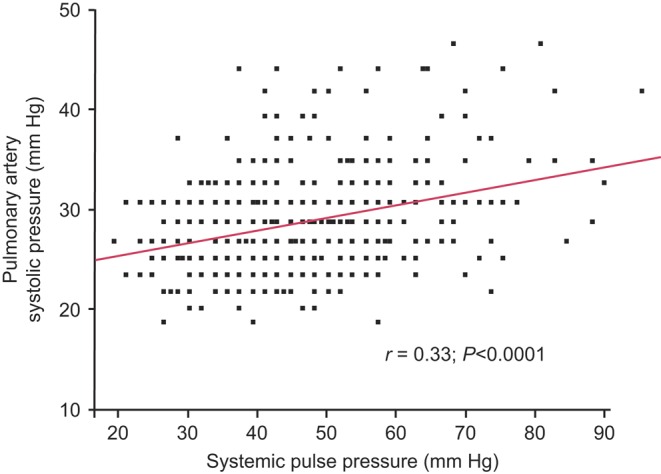
Figure 4.
Association between resting pulmonary artery systolic pressure and systemic pulse pressure. There is a rise in pulmonary artery systolic pressure at rest as systemic pulse pressure rises.
Association of age with transpulmonary resistance
The ratio of PA systolic pressure to cardiac output as a measure of transpulmonary resistance was 4.3mmHg/Lmin−1 and was similar between sexes and across body mass indices. However, there was an age-related increase (Table 2), lowest in subjects 18–40 years (3.8±1.7) and highest in those over the age of 70 years (4.8±1.3; P<0.0001). Overall, the PA systolic pressure to cardiac output ratio decreased in all subjects with exercise, but remained higher in older subjects (Table 3).
Diastolic function and PA pressure
Of the study group, 156 (33%) subjects had evidence of mild diastolic dysfunction (delayed relaxation pattern) at rest but no evidence of elevated LV filling pressures. By protocol design (excluding patients with left atrial enlargement or elevated E/e′ at rest or with exercise), no subjects had elevated LV filling pressures (i.e. moderate or severe diastolic dysfunction). Patients with mild diastolic dysfunction were older than those with normal LV diastolic function (62±11 vs 54±12 years, P<0.0001). Compared with those with normal diastolic function, subjects with mild LV diastolic dysfunction had similar PA systolic pressures at rest (rest: 28.2±5.5mmHg vs 28.6±4.5mmHg, P=0.4) and with exercise (36.9±9.6mmHg vs 37.4±9.4mmHg, P=0.6).
Discussion
These data demonstrate that in echocardiographically normal subjects, PA systolic pressure increases with advancing age. This increase is seen at rest and with exercise. These increases occur in association with decreasing transpulmonary flow and increases in systemic pulse pressure, suggesting that age-associated blood vessel stiffening may contribute to these differences in PA systolic pressure.
Factors that determine PA pressure include intrinsic properties of the pulmonary vascular bed, transpulmonary flow (cardiac output), and left atrial pressure. By design, all subjects in this study had normal or low left atrial/LV filling pressures at rest as assessed by 2D and Doppler echocardiography. LV filling pressures with exercise were also assessed and subjects were also excluded if they had evidence of an increase in filling pressures with exercise. Moreover, subjects with mild diastolic dysfunction at rest were similar to those who had normal diastolic function with regard to resting and exercise PA systolic pressure. Elevated LV filling pressures are a potentially common cause of pulmonary hypertension in the elderly including a rise in PA pressures on exercise Doppler echocardiography (25, 26). Mahjoub and coworkers (25) and Ha and coworkers (26) have also demonstrated higher PA pressures post exercise in elderly subjects. However, unlike in those studies, here in this cohort, abnormalities of diastolic function on echo do not appear to underlie the age-related differences in PA systolic pressure seen. The differences being that in our study, abnormalities of diastolic function were excluded by study design. A modest decrease in cardiac output with advancing age was seen at rest and with exercise. Therefore, the age-dependent increase seen in PA systolic pressure does not appear to be due to increased transpulmonary flow, thus implicating intrinsic changes in the pulmonary arterial circulation (27).
Our findings are supported by previous small cardiac catheterization cohort series that have demonstrated a trend toward increasing PA pressures both at rest and with exercise with age (28, 29, 30). However, due to the study designs, these studies did not have the distribution of age ranges from young adult to elderly to adequately assess the association of varying age and pulmonary pressure. Resting PA pressures have also been found to correlate with age in a patient cohorts evaluated by echocardiography. Resting PA systolic pressures have been associated with echo findings such as increased wall thickness and E/e′, suggesting that at least part of this association may have been related to LV diastolic dysfunction and/or other cardiovascular diseases (not excluded or directly evaluated in these series) (31, 32, 33). A recent community-based echocardiography study also demonstrated a correlation between increasing age and increasing resting PA pressure (10). In that study, which included a comprehensive assessment of LV diastolic function, these age-related increases in PA pressures correlated with an age-dependent elevation in LV filling pressures (10). Furthermore, these findings also correlated with systemic vascular stiffening (10). Our study demonstrates that in the presence of normal LV filling pressures, the association of age and increasing PA pressures both at rest and following exercise persists, implicating intrinsic pulmonary arterial changes with age; these findings are supported by a correlation with increasing systemic vascular stiffening.
Strengths and limitations
The strengths of the current study include the large cohort of subjects over a wide age distribution and the comprehensive uniformly performed echocardiographic assessment of rest and stress wall motion assessment and Doppler evaluation of LV diastolic function and pulmonary pressures. Although Doppler measurement of PA pressure correlates well with simultaneous invasive catheter measures, direct measurements remain the gold standard and furthermore allow for measurement rather than estimates of mean pulmonary pressure and pulmonary vascular resistance as well as measures of PA pulse pressure and pulmonary capacitance/stiffening (8, 34) An invasive study would also have facilitated more direct and simultaneous measurement of cardiac output. However, this study could not have been performed invasively. While the ratio of PA systolic pressure to cardiac index is a noninvasive correlate of pulmonary vascular resistance, other Doppler measures of pulmonary artery hemodynamics such as pulmonary vascular resistance (e.g. by the method of Abbas) or capacitance would have been of interest in this cohort (35, 36). Furthermore, there is an association of changes in correlates of systemic vascular stiffness with echo-derived measures of pulmonary vascular stiffness beyond simply PA pressure elevation. Here, we used an unorthodox site for cardiac output measurement which allowed simultaneous measurement of cardiac output and diastolic function, thereby improving acquisition time though this is less accurate than cardiac output measurements from the LV outflow tract and potentially overestimated (18). However, the role of cardiac output measurements in this study was to exclude higher cardiac outputs in older rather than younger patients and the accuracy of the data is likely sufficient for this aim. Subjects were carefully selected to be considered as ‘overtly healthy and disease free’; however, despite the rigorous clinical and echocardiographic based exclusion criteria, we cannot exclude that potential that the findings are confounded by undiagnosed subclinical disease. The method of exercise also deserves mention. The upright treadmill stress allows for a higher achieved workload than supine bike assessment but does not allow for Doppler assessment at peak exercise. The continuous-wave tricuspid regurgitant velocity signal was acquired early in the recovery period (approximately 2min) after symptom-limited exercise. The timeline of changes in pulmonary pressures in the immediate postexercise period is not well known, and hence, it is possible that pulmonary pressures and cardiac output will change during the brief time interval from peak exercise to their measurement in early recovery. The most likely finding would be an early decline in these values leading to an underestimated reflection of true peak exercise measures. However, this protocol is easy to perform and can readily be applied to standard treadmill stress echocardiography currently performed for the evaluation of possible myocardial ischemia, without negating the diagnostic accuracy of the wall motion assessment, potentially serving as an easy screen at no additional cost. These data provide reference values in clinically normal subjects. While the inclusion criteria are the following: an absence of left atrial enlargement, an elevated E/e′ at rest or with exercise should exclude an elevated left atrial pressure, we cannot account for modest variation in left atrial pressures among subjects. Finally, right atrial pressure in normal subjects in the supine position approximates 5mmHg; the uniform assignment of this value rather than individually estimated right atrial pressure based on 2D and Doppler data at rest could introduce a modest error, as right atrial pressure may increase or decrease modestly with exercise in normal subjects (37).
Conclusions
In this study, we delineate PA systolic pressures in adults overtly healthy and disease free at rest and with exercise over a wide range of ages. This work provides an insight into the ‘physiologic’ effects of the aging process on pulmonary hemodynamics and serves as a reference for clinical patients to assess the magnitude of superimposed disease-related effects. We present evidence of an age-associated increase in PA systolic pressure both at rest and with exercise, with data that suggest these age-related changes are independent of abnormalities in LV diastolic function, elevated of LV filling pressure or trans-pulmonary blood flow. Therefore, age-related increases in PA systolic pressure are likely related to intrinsic changes in the pulmonary arterial bed.
Supplementary data
This is linked to the online version of the paper at http://dx.doi.org/10.1530/ERP-16-0006.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This work did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector.
References
- 1.Maréchaux S, Pinçon C, Le Tourneau T, de Groote P, Huerre C, Asseman P, Van Belle E, Nevière R, Bauters C, Deklunder G, et al. 2008. Cardiac correlates of exercise induced pulmonary hypertension in patients with chronic heart failure due to left ventricular systolic dysfunction. Echocardiography 25 386–393. 10.1111/j.1540-8175.2007.00616.x [DOI] [PubMed] [Google Scholar]
- 2.Steen V, Chou M, Shanmugam V, Mathias M, Kuru T, Morrissey R. 2008. Exercise-induced pulmonary arterial hypertension in patients with systemic sclerosis. Chest 134 146–151. 10.1378/chest.07-2324 [DOI] [PubMed] [Google Scholar]
- 3.Himelman RB, Stulbarg M, Kircher B, Lee E, Kee L, Dean NC, Golden J, Wolfe CL, Schiller NB. 1989. Noninvasive evaluation of pulmonary artery pressure during exercise by saline-enhanced Doppler echocardiography in chronic pulmonary disease. Circulation 79 863–871. 10.1161/01.CIR.79.4.863 [DOI] [PubMed] [Google Scholar]
- 4.Grünig E, Janssen B, Mereles D, Barth U, Borst MM, Vogt IR, Fischer C, Olschewski H, Kuecherer HF, Kübler W. 2000. Abnormal pulmonary artery pressure response in asymptomatic carriers of primary pulmonary hypertension gene. Circulation 102 1145–1150. 10.1161/01.CIR.102.10.1145 [DOI] [PubMed] [Google Scholar]
- 5.Oelberg DA, Marcotte F, Kreisman H, Wolkove N, Langleben D, Small D. 1998. Evaluation of right ventricular systolic pressure during incremental exercise by Doppler echocardiography in adults with atrial septal defect. Chest 113 1459–1465. 10.1378/chest.113.6.1459 [DOI] [PubMed] [Google Scholar]
- 6.Grünig E, Weissmann S, Ehlken N, Fijalkowska A, Fischer C, Fourme T, Galié N, Ghofrani A, Harrison RE, Huez S, et al. 2009. Stress Doppler echocardiography in relatives of patients with idiopathic and familial pulmonary arterial hypertension: results of a multicenter European analysis of pulmonary artery pressure response to exercise and hypoxia. Circulation 119 1747–1757. [DOI] [PubMed] [Google Scholar]
- 7.Badesch DB, Champion HC, Sanchez MA, Hoeper MM, Loyd JE, Manes A, McGoon M, Naeije R, Olschewski H, Oudiz RJ, et al. 2009. Diagnosis and assessment of pulmonary arterial hypertension. Journal of the American College of Cardiology 54 S55–S66. [DOI] [PubMed] [Google Scholar]
- 8.McGoon MD, Kane GC. 2009. Pulmonary hypertension: diagnosis and management. Mayo Clinic Proceedings 84 191–207. 10.4065/84.2.191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bossone E, Rubenfire M, Bach DS, Ricciardi M, Armstrong WF. 1999. Range of tricuspid regurgitation velocity at rest and during exercise in normal adult men: implications for the diagnosis of pulmonary hypertension. Journal of the American College of Cardiology 33 1662–1666. 10.1016/S0735-1097(99)00055-8 [DOI] [PubMed] [Google Scholar]
- 10.Lam CS, Borlaug BA, Kane GC, Enders FT, Rodeheffer RJ, Redfield MM. 2009. Age-associated increases in pulmonary artery systolic pressure in the general population. Circulation 119 2663–2670. 10.1161/CIRCULATIONAHA.108.838698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kovacs G, Berghold A, Scheidl S, Olschewski H. 2009. Pulmonary arterial pressure during rest and exercise in healthy subjects: a systematic review. European Respiratory Journal 34 888–894. 10.1183/09031936.00145608 [DOI] [PubMed] [Google Scholar]
- 12.Ehrsam RE, Perruchoud A, Oberholzer M, Burkart F, Herzog H. 1983. Influence of age on pulmonary haemodynamics at rest and during supine exercise. Clinical Science 65 653–660. 10.1042/cs0650653 [DOI] [PubMed] [Google Scholar]
- 13.Grewal J, McCully RB, Kane GC, Lam C, Pellikka PA. 2009. Left ventricular function and exercise capacity. JAMA 301 286–294. 10.1001/jama.2008.1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mitchell GF, Parise H, Benjamin EJ, Larson MG, Keyes MJ, Vita JA, Vasan RS, Levy D. 2004. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: the Framingham Heart Study. Hypertension 43 1239–1245. 10.1161/01.HYP.0000128420.01881.aa [DOI] [PubMed] [Google Scholar]
- 15.Kelly R, Hayward C, Avolio A, O’Rourke M. 1989. Noninvasive determination of age-related changes in the human arterial pulse. Circulation 80 1652–1659. 10.1161/01.CIR.80.6.1652 [DOI] [PubMed] [Google Scholar]
- 16.Kane GC, Hepinstall MJ, Kidd GM, Kuehl CA, Murphy AT, Nelson JM, Schneider L, Stussy VL, Warmsbecker JA, Miller FA, Jr, et al. 2008. Safety of stress echocardiography supervised by registered nurses: results of a 2-year audit of 15,404 patients. Journal of the American Society of Echocardiography 21 337–341. 10.1016/j.echo.2007.08.028 [DOI] [PubMed] [Google Scholar]
- 17.Quinones MA, Waggoner AD, Reduto LA, Nelson JG, Young JB, Winters WL, Jr, Ribeiro LG, Miller RR. 1981. A new, simplified and accurate method for determining ejection fraction with two-dimensional echocardiography. Circulation 64 744–753. 10.1161/01.CIR.64.4.744 [DOI] [PubMed] [Google Scholar]
- 18.Dittmann H, Voelker W, Karsch KR, Seipel L. 1987. Influence of sampling site and flow area on cardiac output measurements by Doppler echocardiography. Journal of the American College of Cardiology 10 818–823. 10.1016/S0735-1097(87)80275-9 [DOI] [PubMed] [Google Scholar]
- 19.Chan KL, Currie PJ, Seward JB, Hagler DJ, Mair DD, Tajik AJ. 1987. Comparison of three Doppler ultrasound methods in the prediction of pulmonary artery pressure. Journal of the American College of Cardiology 9 549–554. 10.1016/S0735-1097(87)80047-5 [DOI] [PubMed] [Google Scholar]
- 20.Currie PJ, Seward JB, Chan KL, Fyfe DA, Hagler DJ, Mair DD, Reeder GS, Nishimura RA, Tajik AJ. 1985. Continuous wave Doppler determination of right ventricular pressure: a simultaneous Doppler-catheterization study in 127 patients. Journal of the American College of Cardiology 6 750–756. 10.1016/S0735-1097(85)80477-0 [DOI] [PubMed] [Google Scholar]
- 21.Yock PG, Popp RL. 1984. Noninvasive estimation of right ventricular systolic pressure by Doppler ultrasound in patients with tricuspid regurgitation. Circulation 70 657–662. 10.1161/01.CIR.70.4.657 [DOI] [PubMed] [Google Scholar]
- 22.Ommen SR, Nishimura RA, Appleton CP, Miller FA, Oh JK, Redfield MM, Tajik AJ. 2000. Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: a comparative simultaneous Doppler-catheterization study. Circulation 102 1788–1794. 10.1161/01.CIR.102.15.1788 [DOI] [PubMed] [Google Scholar]
- 23.Burgess MI, Jenkins C, Sharman JE, Marwick TH. 2006. Diastolic stress echocardiography: hemodynamic validation and clinical significance of estimation of ventricular filling pressure with exercise. Journal of the American College of Cardiology 47 1891–1900. 10.1016/j.jacc.2006.02.042 [DOI] [PubMed] [Google Scholar]
- 24.Tsang TS, Barnes ME, Gersh BJ, Bailey KR, Seward JB. 2002. Left atrial volume as a morphophysiologic expression of left ventricular diastolic dysfunction and relation to cardiovascular risk burden. American Journal of Cardiology 90 1284–1289. 10.1016/S0002-9149(02)02864-3 [DOI] [PubMed] [Google Scholar]
- 25.Majoub H, Levy F, Cassol M, Meimoun P, Peltier M, Rusinaru D, Tribouilloy C. 2009. Effects of age on pulmonary artert systolic pressure at rest and during exercise in normal adults. European Journal of Echocardiography 10 635–640. 10.1093/ejechocard/jep024 [DOI] [PubMed] [Google Scholar]
- 26.Ha JW, Choi D, Park S, Shim CY, Kim JM, Moon SH, Lee HJ, Choi EY, Chung N. 2009. Determinants of exercise-induced pulmonary hypertension in patients with normal left ventricular ejection fraction. Heart 95 490–494. 10.1136/hrt.2007.139295 [DOI] [PubMed] [Google Scholar]
- 27.Stanek V, Widimsky J, Hurych J. 1969. The effect of age on the pressure-flow relationship and on the capacity of the pulmonary vascular bed with special reference to the condition of high flow. Progress in Respiration Research 5 375–38. 10.1159/000391578 [DOI] [Google Scholar]
- 28.Emirgil C, Sobol BJ, Campodonico S, Herbert WH, Mechkati R. 1967. Pulmonary circulation in the aged. Journal of Applied Physiology 23 631–640. [DOI] [PubMed] [Google Scholar]
- 29.Granath A, Jonsson B, Strandell T. 1964. Circulation in healthy old men, studied by right heart catheterization at rest and during exercise in supine and sitting position. Acta Medica Scandinavica 176 425–446. 10.1111/j.0954-6820.1964.tb00949.x [DOI] [PubMed] [Google Scholar]
- 30.Davidson WR, Jr, Fee EC. 1990. Influence of aging on pulmonary hemodynamics in a population free of coronary artery disease. American Journal of Cardiology 65 1454–1458. 10.1016/0002-9149(90)91354-9 [DOI] [PubMed] [Google Scholar]
- 31.McQuillan BM, Picard MH, Leavitt M, Weyman AE. 2001. Clinical correlates and reference intervals for pulmonary artery systolic pressure among echocardiographically normal subjects. Circulation 104 2797–2802. 10.1161/hc4801.100076 [DOI] [PubMed] [Google Scholar]
- 32.Van de Veire NR, De Backer J, Ascoop AK, Middernacht B, Velghe A, Sutter JD. 2006. Echocardiographically estimated left ventricular end-diastolic and right ventricular systolic pressure in normotensive healthy individuals. International Journal of Cardiovascular Imaging 22 633–641. 10.1007/s10554-006-9082-y [DOI] [PubMed] [Google Scholar]
- 33.Dib JC, Abergel E, Rovani C, Raffoul H, Diebold B. 1997. The age of the patient should be taken into account when interpreting Doppler assessed pulmonary artery pressures. Journal of the American Society of Echocardiography 10 72–73. 10.1016/S0894-7317(97)80035-1 [DOI] [PubMed] [Google Scholar]
- 34.Mahapatra S, Nishimura RA, Sorajja P, Cha S, McGoon MD. 2006. Relationship of pulmonary arterial capacitance and mortality in idiopathic pulmonary arterial hypertension. Journal of the American College of Cardiology 47 799–803. 10.1016/j.jacc.2005.09.054 [DOI] [PubMed] [Google Scholar]
- 35.Abbas AE, Fortuin FD, Schiller NB, Appleton CP, Moreno CA, Lester SJ. 2003. A simple measure for noninvasive estimation of pulmonary vascular resistance. Journal of the American College of Cardiology 41 1021–1027. 10.1016/S0735-1097(02)02973-X [DOI] [PubMed] [Google Scholar]
- 36.Mahapatra S, Nishimura RA, Oh JK, McGoon MD. 2006. The prognostic value of pulmonary vascular capacitance determined by Doppler echocardiography in patients with pulmonary arterial hypertension Journal of the American Society of Echocardiography 19 1045–1050. 10.1016/j.echo.2006.03.008 [DOI] [PubMed] [Google Scholar]
- 37.Andersen MJ, Ersbøll M, Bro-Jeppesen J, Gustafsson F, Hassager C, Køber L, Borlaug BA, Boesgaard S, Kjærgaard J, Møller JE. 2012. Exercise hemodynamics in patients with and without diastolic dysfunction and preserved ejection fraction after myocardial infarction. Exercise hemodynamics in patients with and without diastolic dysfunction and preserved ejection fraction after myocardial infarction. Circulation. Heart Failure 5 444–451. 10.1161/CIRCHEARTFAILURE.112.967919 [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a