In this issue of the American Journal of Hypertension, Naito et al. 1 report novel evidence that heterozygous mice deficient in the transferrin-1 receptor (TfR1) show protection against the development of hypoxia-induced pulmonary hypertension and vascular remodeling associated with this disease process. The depletion of TfR1 by siRNA also attenuated the proliferation of human pulmonary artery smooth muscle cells promoted by culturing with platelet-derived growth factor-BB. These observations provide new insight for identifying how an apparent iron deficiency and/or a disruption in aspects of iron metabolism participate in the development and expression of pulmonary hypertension seen in various forms of the human disease and animal disease models.2–6
This group has previously reported evidence for increased TfR1 in the monocrotaline rat model of pulmonary hypertension.6 It has also been observed that circulating soluble transferrin receptor levels are increased in patients with idiopathic pulmonary arterial hypertension.2 While most cells are thought to regulate iron uptake though TfR1 expression,7,8 very little is known about how this system and iron metabolism are functioning in the various cell types that contribute to pulmonary hypertension. Increased TfR1 expression is associated with an iron deficiency in cells, and antibodies for TfR1 have been used for targeting cancer cells.9 There are distinct similarities between cancer cells and changes in pulmonary arterial smooth muscle cells associated with pulmonary arterial hypertension.10 Data in the study by Naito et al. 1 demonstrate that a deficiency in TfR1 attenuates the growth of pulmonary arterial smooth muscle cells in vitro and pulmonary arterial remodeling in the in vivo chronic hypoxia mouse model studied. Thus, the TfR1 receptor and/or its influence on cellular iron availability could have an important role in controlling processes contributing to pulmonary arterial smooth muscle remodeling in various forms of pulmonary hypertension.
Several processes important in pulmonary hypertension development could potentially originate from an iron deficiency. The stabilization or increased expression of hypoxia-inducible factor (HIF) is an important aspect of pulmonary hypertension development which could be promoted by an iron deficiency or alterations in cellular iron metabolism.3 There is evidence for enhanced heme-deficient soluble guanylate cyclase (sGC) activity based on the therapeutic action of a sGC activator,11 which preferentially binds the heme site of this enzyme when the heme of sGC is oxidized and/or no longer bound to sGC. There is also evidence in pulmonary hypertension for a depletion of subunit 4 of the mitochondrial electron transport chain protein cytochrome oxidase,12 which is a very sensitive cellular indicator protein for depletion when there is a heme deficiency.13 Our lab has also detected evidence for the pulmonary hypertension mediator endothelin-1 causing an accumulation of protoporphyrin IX in a manner, which could reflect a deficiency in the availability of iron for the biosynthesis of heme by the mitochondrial ferrochelatase enzyme.14 The accumulation of protoporphyrin IX is a property of cancer cells which is used for diagnostic detection of these cells.15 Interestingly, the detection of increased zinc protoporphyrin IX in blood of humans with idiopathic pulmonary hypertension suggests that there is an iron deficiency or impairment of iron utilization for heme biosynthesis in hematopoietic cells.16 This is because ferrochelatase uses zinc when an adequate amount of mitochondrial iron is not available for heme biosynthesis. Our studies on pulmonary arteries isolated from the chronic hypoxia model for pulmonary hypertension detected increased ferrochelatase activity.14 While this could result from a HIF-promoted increase in expression of this enzyme, the iron-sulfur cluster dependence of the stability and activity of ferrochelatase17 suggests that a deficiency of iron availability for these clusters was not detected. Thus, some of the processes discussed above and others dependent on the availability of iron could contribute to pulmonary hypertension development.
The mechanism through which TfR1 is influencing pulmonary hypertension is not known. Since TfR1 normally functions through increasing cellular iron uptake, its primary action in enabling pulmonary arterial smooth muscle growth might not originate from creating an actual iron deficiency in these cells. While there is evidence that a deficiency in iron bound to iron regulatory proteins functioning to promote the expression of TfR1 and other proteins contributing to intracellular iron transport,7,8 essentially all aspects of the actual consequences on TfR1 on cellular iron metabolism and processes regulated by iron or the TfR1 relevant to systems influencing pulmonary hypertension development remain to be defined. For example, modulation of a mishandling of iron by TfR1 expression could be a more important factor than modulation of an actual cellular iron deficiency. Therapies delivering iron or creating an iron deficiency clearly influence the progression of pulmonary hypertension.4–6 However, it is not known if pulmonary hypertension creates an iron deficiency or if an iron deficiency associated with conditions leading to pulmonary hypertension enhances the development of this disease. Diseases that promote the development of pulmonary hypertension such as heart failure and chronic obstructive pulmonary disease show a high incidence of iron deficiency, but, the role of this deficiency in pulmonary hypertension development is not well defined.18,19 Humans documented to have idiopathic pulmonary arterial hypertension associated with a deficiency in iron appeared to benefit when treated with supplemental intravenous iron.5 In this study, the effects on skeletal muscle oxygen utilization appear to be most obvious beneficial effect in exercise testing examined.5 The results of the study by Naito et al. 1 suggest that 1 potentially beneficial effect of iron supplementation in pulmonary hypertension associated with an iron deficiency could be suppressing the increased expression of TfR1 and pathophysiological processes stimulated by its increased expression through relationships that are shown in Figure 1.
Figure 1.
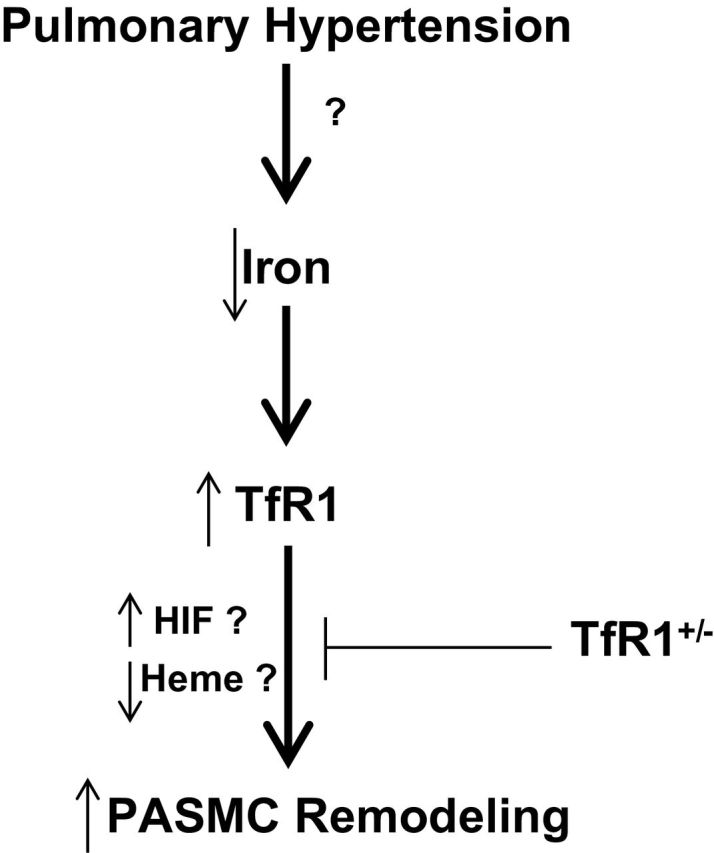
Model hypothesizing how an iron deficiency associated with pulmonary hypertension could promote pulmonary vascular remodeling through increasing the expression of transferrin Receptor 1 (TfR1). While increased hypoxia-inducible factors (HIF) and impaired heme biosynthesis are iron (Fe) regulated processes occurring in pulmonary hypertension, the direct influence of TfR1 expression on these processes, or iron metabolism in pulmonary arterial smooth muscle cells under conditions relevant to pulmonary hypertension have not yet been established.
DISCLOSURE
M.S.W. is an Inventor on a patent held by New York Medical College for targeting protoporphyrin IX for smooth muscle relaxation. There are no other disclosures or conflicts of interest for the authors.
ACKNOWLEDGMENT
The authors’ laboratories are supported by NIH grant R01HL115124.
REFERENCES
- 1. Naito Y, Hosokawa M, Sawada H, Oboshi M, Hirotani S, Iwasaku T, Okuhara Y, Morisawa D, Eguchi A, Nishimura K, Soyama Y, Fujii K, Mano T, Ishihara M, Tsujino T, Masuyama T. Transferrin receptor 1 in chronic hypoxia-induced pulmonary vascular remodeling. Am J Hypertens, 2016; 29:713–726. [DOI] [PubMed] [Google Scholar]
- 2. Rhodes CJ, Howard LS, Busbridge M, Ashby D, Kondili E, Gibbs JS, Wharton J, Wilkins MR. Iron deficiency and raised hepcidin in idiopathic pulmonary arterial hypertension: clinical prevalence, outcomes, and mechanistic insights. J Am Coll Cardiol 2011; 58:300–309. [DOI] [PubMed] [Google Scholar]
- 3. Robinson JC, Graham BB, Rouault TC, Tuder RM. The crossroads of iron with hypoxia and cellular metabolism. Implications in the pathobiology of pulmonary hypertension. Am J Respir Cell Mol Biol 2014; 51:721–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cotroneo E, Ashek A, Wang L, Wharton J, Dubois O, Bozorgi S, Busbridge M, Alavian KN, Wilkins MR, Zhao L. Iron homeostasis and pulmonary hypertension: iron deficiency leads to pulmonary vascular remodeling in the rat. Circ Res 2015; 116:1680–1690. [DOI] [PubMed] [Google Scholar]
- 5. Ruiter G, Manders E, Happé CM, Schalij I, Groepenhoff H, Howard LS, Wilkins MR, Bogaard HJ, Westerhof N, van der Laarse WJ, de Man FS, Vonk-Noordegraaf A. Intravenous iron therapy in patients with idiopathic pulmonary arterial hypertension and iron deficiency. Pulm Circ 2015; 5:466–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Naito Y, Hosokawa M, Hao H, Sawada H, Hirotani S, Iwasaku T, Okuhara Y, Eguchi A, Hirota S, Ohyanagi M, Tsujino T, Masuyama T. Impact of dietary iron restriction on the development of monocrotaline-induced pulmonary vascular remodeling and right ventricular failure in rats. Biochem Biophys Res Commun 2013; 436:145–151. [DOI] [PubMed] [Google Scholar]
- 7. Wang J, Pantopoulos K. Regulation of cellular iron metabolism. Biochem J 2011; 434:365–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mühlenhoff U, Hoffmann B, Richter N, Rietzschel N, Spantgar F, Stehling O, Uzarska MA, Lill R. The role of mitochondria and the CIA machinery in the maturation of cytosolic and nuclear iron–sulfur proteins. Eur J Cell Biol. 2015; 94: 280–291. [DOI] [PubMed] [Google Scholar]
- 9. Daniels TR, Delgado T, Rodriguez JA, Helguera G, Penichet ML. The transferrin receptor part I: biology and targeting with cytotoxic antibodies for the treatment of cancer. Clin Immunol 2006; 121:144–158. [DOI] [PubMed] [Google Scholar]
- 10. Archer SL, Gomberg-Maitland M, Maitland ML, Rich S, Garcia JG, Weir EK. Mitochondrial metabolism, redox signaling, and fusion: a mitochondria-ROS-HIF-1alpha-Kv1.5 O2-sensing pathway at the intersection of pulmonary hypertension and cancer. Am J Physiol Heart Circ Physiol 2008; 294:H570–H578. [DOI] [PubMed] [Google Scholar]
- 11. Chester M, Seedorf G, Tourneux P, Gien J, Tseng N, Grover T, Wright J, Stasch JP, Abman SH. Cinaciguat, a soluble guanylate cyclase activator, augments cGMP after oxidative stress and causes pulmonary vasodilation in neonatal pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 2011; 301:L755–L764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bonnet S, Michelakis ED, Porter CJ, Andrade-Navarro MA, Thébaud B, Bonnet S, Haromy A, Harry G, Moudgil R, McMurtry MS, Weir EK, Archer SL. An abnormal mitochondrial-hypoxia inducible factor-1alpha-Kv channel pathway disrupts oxygen sensing and triggers pulmonary arterial hypertension in fawn hooded rats: similarities to human pulmonary arterial hypertension. Circulation 2006; 113:2630–2641. [DOI] [PubMed] [Google Scholar]
- 13. Atamna H, Liu J, Ames BN. Heme deficiency selectively interrupts assembly of mitochondrial complex IV in human fibroblasts: revelance to aging. J Biol Chem 2001; 276:48410–48416. [DOI] [PubMed] [Google Scholar]
- 14. Alhawaj R, Patel D, Kelly MR, Sun D, Wolin MS. Heme biosynthesis modulation via δ-aminolevulinic acid administration attenuates chronic hypoxia-induced pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 2015; 308:L719–L728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ohgari Y, Nakayasu Y, Kitajima S, Sawamoto M, Mori H, Shimokawa O, Matsui H, Taketani S. Mechanisms involved in delta-aminolevulinic acid (ALA)-induced photosensitivity of tumor cells: relation of ferrochelatase and uptake of ALA to the accumulation of protoporphyrin. Biochem Pharmacol 2005; 71:42–49. [DOI] [PubMed] [Google Scholar]
- 16. Decker I, Ghosh S, Comhair SA, Farha S, Tang WH, Park M, Wang S, Lichtin AE, Erzurum SC. High levels of zinc-protoporphyrin identify iron metabolic abnormalities in pulmonary arterial hypertension. Clin Transl Sci 2011; 4:253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Crooks DR, Ghosh MC, Haller RG, Tong WH, Rouault TA. Posttranslational stability of the heme biosynthetic enzyme ferrochelatase is dependent on iron availability and intact iron-sulfur cluster assembly machinery. Blood 2010; 115:860–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nickol AH, Frise MC, Cheng HY, McGahey A, McFadyen BM, Harris-Wright T, Bart NK, Curtis MK, Khandwala S, O’Neill DP, Pollard KA, Hardinge FM, Rahman NM, Armitage AE, Dorrington KL, Drakesmith H, Ratcliffe PJ, Robbins PA. A cross-sectional study of the prevalence and associations of iron deficiency in a cohort of patients with chronic obstructive pulmonary disease. BMJ Open 2015; 5:e007911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. von Haehling S, Jankowska EA, van Veldhuisen DJ, Ponikowski P, Anker SD. Iron deficiency and cardiovascular disease. Nat Rev Cardiol 2015; 12:659–669. [DOI] [PubMed] [Google Scholar]


