Abstract
Because of an extra copy of the Aβ precursor protein gene on chromosome 21, Down syndrome (DS) individuals develop high levels of Aβ peptides and Alzheimer disease–like brain amyloidosis early in life. Here we show that the γ-secretase activating protein (GSAP), a key enzyme in amyloidogenesis, is increased in DS brains and specifically regulated at the transcriptional level by GATA1 transcription factor. The discovery of this novel pathway has translational implications for DS, because pharmacological inhibition of GSAP is an attractive and viable Aβ-lowering therapeutic strategy for this disorder.
Down syndrome (DS) results from the trisomy of chromosome 21 in humans and is the most common cause of genetically defined intellectual disability.1 Among the various triplicate genes in DS is the one encoding for Aβ precursor protein (APP), a transmembrane glycoprotein known for its association with the pathogenesis of Alzheimer disease (AD).2–4 APP overdosage is believed to be responsible for the high incidence of early onset AD-like amyloidosis observed in DS patients.
APP is cleaved by the sequential proteolytic activity of the β-secretase and the γ-secretase complex, which ultimately is responsible for the formation of the Aβ peptides.4 Because activation of the γ-secretase complex is the required final step for the formation of Aβ, in recent years there has been a tremendous effort to develop drugs that block this complex activity as a disease-modifying therapeutic approach to lowering Aβ levels.5 The γ-secretase activating protein (GSAP) is a newly recognized protein derived from a larger precursor molecule via a caspase-3–dependent proteolytic cleavage, which by directly interacting with key components of the γ-secretase complex acts as a rate-limiting step in Aβ formation.6,7 Recent evidence suggests that GSAP is increased in postmortem brain tissues of AD patients, and its pharmacological or genetic inhibition results in an amelioration of the AD-like amyloidotic phenotype in transgenic mouse models of the disease.7–9 However, no data are available on this protein in DS. Considering the similarity between AD and DS regarding the high levels and deposition of Aβ peptides, we hypothesized that this pathway could also be altered in brain tissues of DS patients.
Materials and Methods
Human Subjects
Protocol for the study received prior approval by the appropriate institutional review board. Human postmortem brain frontal cortex tissues were obtained from patients with a clinical and genetic diagnosis of DS (2 females and 3 males) and normal control subjects (4 female and 1 male). Average ages in the DS and control groups (55.8 ± 1.7 vs 64.6 ± 1.9 years) were not significantly different. The postmortem interval was not statistically different between the 2 groups (control, 5 ± 0.7 hours; DS, 10.3 ± 3.2 hours; p = 0.14). Postmortem diagnostic evaluation was performed in accordance with standard histopathological criteria, as previously described.10 All DS subjects had severe AD changes, with grading of A3, B3, C3 using the standard AD neuropathology system.11
Cell Culture and Treatments
The N2A (neuro-2 A neuroblastoma) neuronal cells stably expressing human APP carrying the K670 N, M671 L Swedish mutation (APPswe) were grown, as previously described.12,13 For transfection, cells were grown to 70% confluence and transfected with 1 μg of empty vector (pMT2) or mouse GATA1 pMT2 plasmid (Addgene, Cambridge, MA) by using Lipofectamine 2000 Transfection Reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. After 24 hours of transfection, supernatants were collected, and cell pellets were harvested in lytic buffer for biochemical analyses or cells were collected for RNA extraction for reverse transcription polymerase chain reaction (RT-PCR) analyses. For siRNA knockdown studies, GATA1 siRNA (sc-35452) and negative control siRNAs (control siRNA-A, sc-37007) were all obtained from Santa Cruz Biotechnology (Dallas, TX). N2A-APPswe cells were reverse transfected with siRNA (100nM) using Lipofectamine 2000 according to the manufacturer’s instruction and as previously described.12,13
Quantitative Analysis of Aβ Peptides
Levels of Aβ1–40 and Aβ1–42 in supernatants were assayed by a sensitive sandwich enzyme-linked immunosorbent assay kit (Wako Chemicals, Richmond, VA), as previously described.12,13
Immunoblot Analysis
Proteins were extracted, sonicated, and centrifuged at 13,000rpm for 45 minutes at 4 °C, and supernatants were used for immunoblot analysis, as previously described.12–14 Actin was always used as an internal loading control. Primary antibodies used were as follows: anti-GSAP full length (1:200) and GSAP-16kDa (1:150; Thermo Scientific, Waltham, MA), anti-TMP21 (1:200), anti-CD147(1:200), anti-APP N-terminal raised against amino acids 66 to 81 for total APP (1:200; Milli-pore, Billerica, MA), anti–ADAM-10 (1:200; Millipore), anti-PS1 (1:200; Cell Signaling Technology, Danvers, MA), anti–BACE-1 (1:200; IBL America, Minneapolis, MN), anti-nicastrin (1:200; Cell Signaling Technology), anti–Pen-2 (1:100; Invitrogen), anti–APH-1 (1:150; Millipore), anti-sAPPα (1:200; Cell Signaling Technology), anti-sAPPβ (1:200; Cell Signaling Technology), anti–carboxyl-terminal fragments (CTFs; 1:200; Santa Cruz Biotechnology), anti–insulin-degrading enzyme (IDE; 1:200; Santa Cruz Biotechnology), anti-neprilysin (1:200; Santa Cruz Biotechnology), anti–apoli-poprotein E (apoE; 1:200), anti-GATA1 (1:200; Santa Cruz Biotechnology), anti-GATA2 (1:200; Santa Cruz Biotechnology), anti-JunD (1:200; Santa Cruz Biotechnology), anti–Ets-2 (1: 200; Santa Cruz Biotechnology), anti–caspase-3 (1:200; Santa Cruz Biotechnology), and anti–β-actin (1:200; Santa Cruz Biotechnology). IRDye infrared secondary antibodies were from LI-COR Bioscience (Lincoln, NE).
Immunohistochemical Analysis
Immunostaining was performed as reported previously by our group.12–14 Briefly, sections were blocked in 2% fetal bovine serum before incubation with primary anti-GSAP antibody overnight at 4 °C (1:100). Next, sections were incubated with biotinylated antimouse immunoglobulin G (Vector Laboratories, Burlingame, CA) and then developed by using the avidin–biotin complex method with 3,3′-diaminobenzidine as a chromogen. Light microscopic images were used to calculate the integrated optical density of GSAP using the software Image-Pro Plus for Windows version 5.0 (Media Cybernetics, Rockville, MD). The threshold optical density that discriminated staining from background was determined and kept constant for all quantifications.
Real-Time Quantitative RT-PCR Amplification
RNA was extracted and purified using the RNeasy Mini Kit (Qiagen, Valencia, CA), as previously described.12–14 Briefly, mouse GSAP gene was amplified by using the corresponding primers (SA Biosciences, Valencia, CA), and β-actin was always used as an internal control gene.
Data Analysis
One-way analysis of variance followed by Bonferroni multiple comparison tests and nonparametric t tests were performed using Prism 5.0 (GraphPad Software, San Diego, CA). All data are presented as mean ± standard error of the mean. Results are presented as percentage change from the mean value of the appropriate control group. Significance was set at p < 0.05.
Results
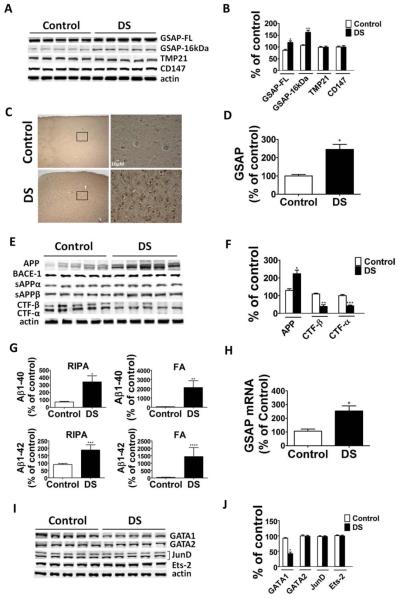
Compared with the age-matched control group, brain samples of DS patients had significant increases in both GSAP-16kDa fragment levels and its precursor protein, full-length GSAP (GSAP-FL; Fig 1). By contrast, no significant differences between the 2 groups were detected for 2 other distinct γ-secretase modulatory proteins: TMP21 and CD147.15,16 In accordance with the Western blot results, immunohistochemical staining showed increased GSAP immunoreactivity in the frontal cortex of DS patients when compared with controls. As predicted, DS brains had higher total APP levels and lower carboxyl-terminal fragment (CTF)-β and CTF-α, but no changes were observed in the steady state levels of BACE-1, ADAM-10, or the 4 components of the γ-secretase complex between the 2 groups.
FIGURE 1.
γ-Secretase activating protein (GSAP) is upregulated in Down syndrome brains. (A) Representative Western blot analysis of full-length GSAP (GSAP-FL), GSAP-16kDa, TMP21, and CD147 in frontal cortex homogenates of Down syndrome patients (DS) and age-matched control samples. (B) Densitometric analyses of the immunoreactivities to the antibodies shown in A (n = 5 control, n = 5 DS; *p = 0.004, **p = 0.0002; unpaired t test). (C) Representative brain sections from DS brains and controls samples immunostained for GSAP (original magnification, × 4 and ×40; scale bar = 10 μM). (D) Quantitative analysis of the immunoreactivity for GSAP shown in the previous panel (n = 3 control, n = 3 DS; *p = 0.0001; unpaired t test). (E) Representative Western blot analysis of Aβ precursor protein (APP), BACE-1, sAPPα, sAPPβ, carboxyl-terminal fragment (CTF)-β, and CTFα in frontal cortex homogenates from DS brain samples and controls. (F) Densitometric analyses of the immunoreactivities to the antibodies shown in F (n = 5 control, n = 5 DS; *p = 0.003, **p = 0.0002, ***p < 0.0001; unpaired t test). (G) Radioimmunoprecipitation assay (RIPA)-soluble and formic acid (FA)-extractable Aβ1–40 and Aβ1–42 levels in frontal cortex of DS brain samples and controls were measured by sandwich enzyme-linked immunosorbent assay (n = 5 control, n = 5 DS; *p = 0.01, **p = 0.02, ***p = 0.02, ****p = 0.05; unpaired t test). (H) Relative mRNA levels for GSAP in frontal cortex homogenates from DS brain samples and controls, as determined by real-time quantitative reverse transcription polymerase chain reaction amplification (n = 5 control, and n = 5 DS; *p = 0.007; unpaired t test). (I) Representative Western blot analysis of GATA1, GATA2, JunD, and Ets-2 in frontal cortex homogenates from DS brain samples and controls. (J) Densitometric analyses of the immunoreactivities to the antibodies shown in I (n = 5 control, and n = 5 DS; *p < 0.0001; unpaired t test). Values represent mean ± standard error of the mean. Results are presented as percentage change from the mean value of the control group. [Color figure can be viewed in the online issue, which is available at www.annalsofneurology.org.]
These changes were associated with a significant increase in the amount of both radioimmunoprecipitation assay–soluble and formic acid–soluble Aβ1–40 and Aβ1–42 peptides in the brains from DS patients (see Fig 1G). No significant correlation was found between the levels of GSAP-FL and the amount of Aβ1–40 and Aβ1–42 in DS samples (r2 = 0.37, p = 0.28; r2 = 0.23, p = 0.43). Finally, we found no differences between DS and controls when the steady state levels of 2 main Aβ-degrading enzymes, neprilysin and IDE, as well as apolipoprotein E (apoE), an Aβ chaperone, were assayed (data not shown).
Because we observed that DS brain samples had a significant increase in the steady state levels of GSAP protein, we wanted to see whether this occurred also at the mRNA level. Quantitative real-time RT-PCR showed that compared with controls, DS brains had a significant increase in GSAP mRNA levels, suggesting a transcriptional regulation of this protein (see Fig 1H). Next, by using an online software program (ie, PROMO) to predict transcription factor binding sites on GSAP gene sequence, we found that GATA1, GATA2, Ets-2, and Jun D transcriptional factors were all potential candidates. Western blot analyses showed that compared with the age-matched control group, brain homogenates from DS patients had significantly lower levels of GATA1. By contrast, no significant differences were observed between the 2 groups for GATA2, Ets-2, and Jun D steady state levels (see Fig 1I, J).
No significant correlation was observed between the levels of GATA1 and the amount of Aβ peptides in DS samples (r2 = 0.14, p = 0.53; r2 = 0.66, p = 0.1).
Because previous studies showed that the active fragment of GSAP, GSAP-16kDa, derives from its precursor protein GSAP-FL via a caspase-3–dependent cleavage,6 next we investigated whether this pathway was altered. Western blot analyses showed no significant differences between the 2 groups for both procaspase-3 and caspase-3 (data not shown).
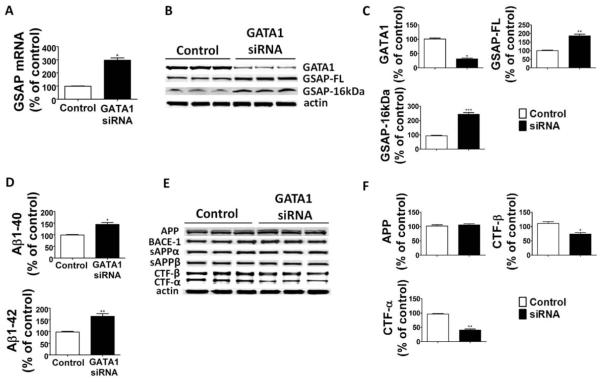
To establish a biological link between transcriptional regulation of GSAP mRNA levels and GATA1, next we used GATA1 siRNA to knock down this transcription factor in neuronal cells. As shown in Figure 2A, cells treated with this siRNA showed a significant decrease in GATA1 levels, which was associated with an increase in GSAP mRNA. The same treatment resulted also in a significant elevation of GSAP-FL and GSAP-16kDa steady state levels and Aβ peptides in the supernatants (see Fig 2). Further, compared with controls, cells treated with GATA1 siRNA had a significant decrease in both CTF-β and CTF-α, but no changes in the steady state levels of APP, sAPP, BACE-1, ADAM-10, or the 4 components of the γ-secretase complex.
FIGURE 2.
GATA1 gene knockdown increases γ-secretase activating protein (GSAP) levels and Aβ formation. N2A-APPswe cells were transfected with GATA1 siRNA or scrambled siRNA-A (control), and supernatants and cell lysates were collected for analysis. (A) Relative mRNA levels for GSAP in cells treated with GATA1 siRNA or control scrambled siRNA (n = 3 control, and n = 3 GATA1 siRNA; *p =: 0.0001; unpaired t test). (B) Representative Western blot analysis of GATA1, full-length GSAP (GSAP-FL), and GSAP-16kDa in cells treated with GATA1 siRNA or control scrambled siRNA. (C) Densitometric analyses of the immunoreactivities to the antibodies shown in B (n = 3 for control, n = 3 for GATA1 siRNA; *p = 0.0001, **p = 0.001, ***p = 0.0003; unpaired t test). (D) Aβ1–40 and Aβ1–42 levels in conditioned medium from cells treated with GATA1 siRNA or control scrambled siRNA were measured by sandwich enzyme-linked immunosorbent assay (n = 3 control, n = 3 GATA1 siRNA; *p = 0.002, **p = 0.001; unpaired t test). (E) Representative Western blot analysis of Aβ precursor protein (APP), BACE-1, sAPPα, sAPPβ, carboxyl-terminal fragment (CTF)-β, and CTF-α, in cells treated with GATA1 siRNA or control scrambled siRNA. (F) Densitometric analyses of the immunoreactivities to the antibodies shown in E (n = 3 control, n = 3 GATA1 siRNA; *p = 0.009, **p = 0.0002; unpaired t test). Values represent mean ± standard error of the mean. Results are presented as percentage change from the mean value of the control group.
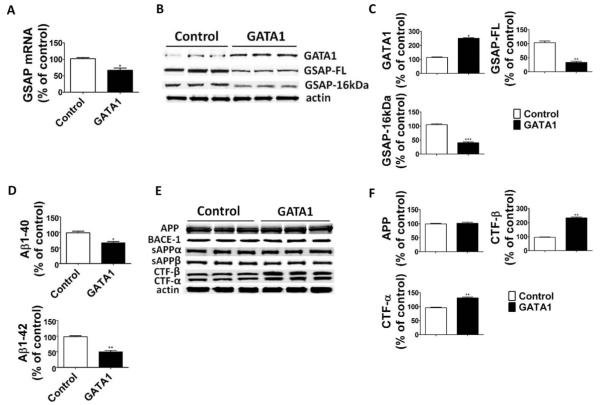
To further confirm these findings, next we transiently transfected neuronal cells with GATA1 cDNA plasmid, and evaluated the effect of GATA1 overexpression on GSAP gene transcription, its protein levels, and Aβ production. Western blot analyses showed that compared with the control, GATA1 cDNA transfected cells had a significant increase in the steady state levels of GATA1 that was accompanied by a decreased GSAP mRNA level (Fig 3). This effect was associated with significantly lower GSAP-FL and GSAP-16kDa steady state levels and Aβ peptides. Finally, compared with controls, cells overexpressing GATA1 had a significant increase in both CTF-β and CTF-α, but no changes in the steady state levels of APP, sAPP, BACE-1, ADAM-10, or the 4 components of the γ-secretase complex.
FIGURE 3.
GATA1 overexpression decreases γ-secretase activating protein (GSAP) levels and reduces Aβ formation. N2A-APPswe cells were transfected overnight with GATA1 cDNA or empty vector (control). Supernatant and cell lysates were collected for analysis. (A) Relative mRNA levels for GSAP in cells transfected with GATA1 cDNA or empty vector (n = 3 control, and n = 3 GATA1; *p = 0.01; unpaired t test). (B) Representative Western blot analysis of GATA1, full-length GSAP (GSAP-FL), and GSAP-16kDa in cells transfected with GATA1 cDNA or empty vector. (C) Densitometric analyses of the immunoreactivities to the antibodies shown in B (n = 3 control, n = 3 GATA1; *p < 0.0001, **p = 0.0006, ***p = 0.0001; unpaired t test). (D) Aβ1–40 and Aβ1–42 levels in conditioned medium from cells transfected with GATA1 cDNA or empty vector were measured by sandwich enzyme-linked immunosorbent assay (n = 3 control, n = 3 GATA1; *p = 0.003, **p = 0.0002; unpaired t test). (E) Representative Western blot analysis of Aβ precursor protein (APP), BACE-1, sAPPα, sAPPβ, carboxyl-terminal fragment (CTF)-β, and CTF-α in cells transfected with GATA1 cDNA or empty vector. (F) Densitometric analyses of the immunoreactivities to the antibodies shown in E (n = 3 control, n = 3 GATA1; **p = 0.0008; unpaired t test). Values represent mean ± standard error of the mean. Results are presented as percentage change from the mean value of the control group.
Discussion
In the current paper, we provide the first experimental evidence that compared with healthy controls DS brains have significantly higher levels of GSAP protein and mRNA. This increase is specific, because when we assayed for 2 other distinct γ-secretase modulator proteins we did not observe any significant differences between the 2 groups. In addition, we demonstrate that GSAP increase is specifically regulated at the transcriptional level by the GATA1 transcription factor, but not others factors whose binding sites are also recognized on the GSAP gene sequence.
Due to the small size of our sample, we were not able to find any statistically significant correlations between GSAP, GATA1, and Aβ peptide levels. Additionally, despite the observation that GSAP-FL as well as its cleaved product GSAP-16kDa were both increased, we did not observe any changes in caspase-3. This suggests that there is no correlation between GSAP transcriptional regulation and its cleavage process.
The discovery that GATA1 is implicated in regulating GSAP does not come as a surprise, because this transcription factor has been previously involved in DS. Thus, somatic mutations in the GATA1 gene resulting in lower levels of normal GATA1 and expression of a shorter and inactive form called GATA1-s have been reported in DS.17–19 Based on our results, we hypothesize that normally GATA1 acts as a transcription repressor for GSAP gene expression. Interestingly, a previous study suggested that GATA1 is also a transcription repressor for synapse-related genes.20
Because GSAP levels have already been shown to be altered in AD brains, our finding represents an extension to DS, which is also characterized by high levels of Aβ production and deposition, and also suggests that GSAP is probably modulated by its target. With the knowledge that activation of the γ-secretase complex is the required final step for Aβ peptide generation, in recent years its pharmacological blockade has been pursued as a potential therapeutic approach to effectively lowering Aβ peptides level in vivo.21 However, γ-secretase is known to process multiple substrates in addition to APP, most notably notch, and this has severely limited the clinical development of inhibitors that directly and irreversibly target this enzyme.22 Because GSAP interacts with this protease to facilitate Aβ formation without affecting notch, it represents a potential new relevant target for a safer anti-Aβ therapy.23 For these reasons, our study has important translational implications for DS therapy, as pharmacological inhibition of GSAP is an attractive and viable Ab-lowering strategy for this disorder.
Acknowledgment
This work was supported by grants from the Alzheimer Art Quilt Initiative (D.P.), Wanda Simone Endowment for Neuroscience (D.P.), and NIH (AG08051, T.W.).
Footnotes
Author Contributions
J.C. and D.P. designed experiments; J.C. performed major in vivo and in vitro biochemistry experiments, including quantitative analysis of Aβ peptides, immunoblot analysis, immunohistochemistry analysis, and real-time PCR; T.W. provided human postmortem brain samples of the DS patients and age-matched controls; J.C. and D.P. wrote the paper; J.C., T.W., and D.P. read and approved the final version of the manuscript.
Potential Conflicts of Interest
Nothing to report.
References
- 1.Rachidi M, Lopes C. Mental retardation and associated neurological dysfunctions in Down syndrome. A consequence of dysregulation in critical chromosome 21 genes and associated molecular pathways. Eur J Paediatr Neurol. 2008;12:168–182. doi: 10.1016/j.ejpn.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 2.Roberson ED, Mucke L. 100 years and counting: prospects for defeating Alzheimer’s disease. Science. 2006;314:781–784. doi: 10.1126/science.1132813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 4.O’Brien RJ, Wong PC. Amyloid precursor protein processing and Alzheimer’s disease. Annu Rev Neurosci. 2011;34:185–204. doi: 10.1146/annurev-neuro-061010-113613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolfe MS. Inhibition and modulation of γ-secretase for Alzheimer’s disease. Neurotherapeutics. 2008;5:391–398. doi: 10.1016/j.nurt.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chu J, Li JG, Joshi YB, et al. Gamma secretase-activating protein is a substrate for caspase-3: implications for Alzheimer’s disease. Biol Psychiatry. 2015;77:720–728. doi: 10.1016/j.biopsych.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He G, Luo W, Li P, et al. Gamma-secretase activating protein, a therapeutic target for Alzheimer’s disease. Nature. 2010;467:95–98. doi: 10.1038/nature09325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Satoh J, Tabunoki H, Ishida T, et al. Immunohistochemical characterization of γ-secretase activating protein expression in Alzheimer’s disease brain. Neuropathol App Neurobiol. 2012;38:132–141. doi: 10.1111/j.1365-2990.2011.01206.x. [DOI] [PubMed] [Google Scholar]
- 9.Chu J, Lauretti E, Craige CP, Praticò D. Pharmacological modulation of GSAP reduces amyloid-β levels and tau phosphorylation in a mouse model of Alzheimer’s disease with plaques and tangles. J Alzheimers Dis. 2014;41:729–737. doi: 10.3233/JAD-140105. [DOI] [PubMed] [Google Scholar]
- 10.Wegiel J, Kuchna I, Nowicki K, et al. Intraneuronal Abeta immuno-reactivity is not a predictor of brain amyloidosis-beta or neurofibrillary degeneration. Acta Neuropathol. 2007;113:389–402. doi: 10.1007/s00401-006-0191-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Montine TJ, Phelps CH, Beach TG, et al. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach. Acta Neuropathol. 2012;123:1–11. doi: 10.1007/s00401-011-0910-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chu J, Giannopoulos PF, Ceballos-Diaz C, et al. Adeno-associated virus-mediated brain delivery of 5-lipoxygenase modulates the AD-like phenotype of APP mice. Mol Neurodegen. 2012;7:1–10. doi: 10.1186/1750-1326-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chu J, Giannopoulos PF, Ceballos-Diaz C, et al. 5-Lipoxygenase gene transfer worsens memory, amyloid and tau brain pathologies in a mouse model of AD. Ann Neurol. 2012;72:442–454. doi: 10.1002/ana.23642. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Chu J, Li JG, Praticò D. Zileuton improves memory deficits, amyloid and tau pathology in a mouse model of Alzheimer’s disease with plaques and tangles. PLoS One. 2013;8:1–8. doi: 10.1371/journal.pone.0070991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen F, Hasegawa H, Schmitt-Ulms G, et al. TMP21 is a presenilin complex component that modulates gamma-secretase but not epsilon-secretase activity. Nature. 2006;440:1208–1212. doi: 10.1038/nature04667. [DOI] [PubMed] [Google Scholar]
- 16.Zhou S, Zhou H, Walian PJ, Jap BK. Regulation of gamma-secretase activity in Alzheimer’s disease. Biochemistry. 2007;46:2553–2563. doi: 10.1021/bi602509c. [DOI] [PubMed] [Google Scholar]
- 17.Wechsler J, Greene M, McDevitt MA, et al. Acquired mutations in GATA1 in the megakaryoblastic leukemia of Down syndrome. Nat Genet. 2002;32:148–152. doi: 10.1038/ng955. [DOI] [PubMed] [Google Scholar]
- 18.Mundschau G, Gurbuxani S, Gamis AS, et al. Mutagenesis of GATA1 is an initiating event in Down syndrome leukemogenesis. Blood. 2003;101:4298–4300. doi: 10.1182/blood-2002-12-3904. [DOI] [PubMed] [Google Scholar]
- 19.Hitzler JK, Cheung J, Li Y, et al. GATA1 mutations in transient leukemia and acute megakaryoblastic leukemia of Down syndrome. Blood. 2003;101:4301–4304. doi: 10.1182/blood-2003-01-0013. [DOI] [PubMed] [Google Scholar]
- 20.Kang HJ, Voleti B, Hajszan T, et al. Decreased expression of synapse-related genes and loss of synapses in major depressive disorder. Nat Med. 2012;18:1413–1417. doi: 10.1038/nm.2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crump CJ, Johnson DS, Li YM. Development and mechanism of γ-secretase modulators for Alzheimer’s disease. Biochemistry. 2013;52:3197–3216. doi: 10.1021/bi400377p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jurisch-Yaksi N, Sannerud R, Annaert W. A fast growing spectrum of biological functions of γ-secretase in development and disease. Biochim Biophys Acta. 2013;1828:2815–2827. doi: 10.1016/j.bbamem.2013.04.016. [DOI] [PubMed] [Google Scholar]
- 23.Gandy S. Lifelong management of amyloid beta metabolism to prevent Alzheimer’s disease. N Engl J Med. 2012;367:864–866. doi: 10.1056/NEJMe1207995. [DOI] [PMC free article] [PubMed] [Google Scholar]