Abstract
Polyunsaturated fatty acids (PUFAs) exhibit critical functions in biological systems and their importance during animal oocyte maturation has been increasingly recognized. However, the detailed mechanism of lipid transportation for oocyte development remains largely unknown. In this study, the transportation of yolk lipoprotein (lipid carrier) and the rate of lipid delivery into oocytes in live C. elegans were examined for the first time by using coherent anti-Stokes Raman scattering (CARS) microscopy. The accumulation of secreted yolk lipoprotein in the pseudocoelom of live C. elegans can be detected by CARS microscopy at both protein (~1665 cm−1) and lipid (~2845 cm−1) Raman bands. In addition, an image analysis protocol was established to quantitatively measure the levels of secreted yolk lipoprotein aberrantly accumulated in PUFA-deficient fat mutants (fat-1, fat-2, fat-3, fat-4) and PUFA-supplemented fat-2 worms (the PUFA add-back experiments). Our results revealed that the omega-6 PUFAs, not omega-3 PUFAs, play a critical role in modulating lipid/yolk level in the oocytes and regulating reproductive efficiency of C. elegans. This work demonstrates the value of using CARS microscopy as a molecular-selective label-free imaging technique for the study of PUFA regulation and oocyte development in C. elegans.
The successful development of oocytes and embryos is one of the most important events for species survival, in which unsaturated fatty acids act as fuel molecules, the building blocks of membranes, and signaling molecules in various pathways1. The biosynthesis of unsaturated fatty acids including polyunsaturated fatty acids (PUFAs) follows a conserved mechanism from bacteria to human2. Therefore, one can uncover the regulation of PUFAs by using different animal models. Similar results from mice and C. elegans suggest that sufficient oocyte PUFAs are necessary for fertilization because these PUFAs are precursors of the prostaglandins that control sperm-oocyte interactions3,4. Since C. elegans has a well-defined anatomy, short life cycle, and genetic similarity to the human genome, it has been widely used for studies of the relationship between lipid/PUFAs and the signaling pathways of reproductive growth5, development6, and reproduction7,8. Moreover, because of its optically transparent body, C. elegans is considered an excellent animal model for optical studies, such as high-speed super-resolution microscopy9, optogenetic manipulation10, and non-linear optical techniques11.
Yolk lipoprotein is the major carrier that transfers lipids/fatty acids from the intestine to oocytes in C. elegans12. It is believed that a continuum exists between nascent lipid droplets (LDs) and immature yolk particles during the formation of yolk lipoprotein, which is analogous to the production of lipoprotein in mammals13. However, the underlying mechanism of the incorporation of yolk lipoproteins/LDs remains unknown. The protein motif of yolk lipoprotein, vitellogenins, is homologous to human apoB14,15. The endocytosis of yolk lipoprotein in oocytes of C. elegans is mediated by a specific receptor, RME-2 (encoded by rme-2 gene), in a similar fashion as human apoB16. Lipid metabolism in oocytes is critical for the growth of oocytes, fertilization, and the development of early embryos1. Failure of proper lipid delivery leads to abnormal oocytes, a low egg production rate, and low viability of embryos16,17.
C. elegans can synthesize de novo a wide range of PUFAs by converting the oleic acid (18:1,n9) by a series of desaturases and elongases18. Figure S1a shows the biosynthesis of PUFAs and the genes involved in the desaturation: fat-1, fat-2, fat-3, and fat-4. Deficiency in PUFAs, due to the dysfunction of the desaturases, can cause defects in lipid regulation and reproduction. For example, the fat-2 mutant (lack of all PUFAs) decreases in size, number, content of LDs dramatically19, and has a significantly low brood size (~19%) and hatching rate (29%) compared to wild-type worms17. PUFAs have been characterized as the precursor of signaling factors that control the recruitment of sperm3,20,21 and are essential materials for synthesizing the lipid-rich layer of eggshells during oocyte maturation22. These findings suggested that PUFAs are important for oocyte development and reproduction. Although the process of oocyte development has been studied23,24, the detailed mechanism of how PUFAs modulate oocyte development remains unclear.
Since adult hermaphrodites turnover their entire gonad every 6.5 hours25, the cellular events, including oocyte growth, maturation, and ovulation, are highly coordinated to ensure successful fertilization23,24. The presence of lipids is necessary for the development of oocytes and embryos because it is a highly energy-consuming process26,27. Although some signaling factors such as maturation-promoting factor28 and major sperm protein29 have been characterized to regulate the development of oocytes, there is no in vivo evidence for delineating how PUFAs regulate oocyte development. A number of methods have been developed and applied to analyze lipid content in oocytes, including dye staining30, isotopic labeling31, gas chromatography (GC) and matrix assisted laser desorption/ ionization mass spectrometry (MALDI-MS)32. However, these methods need fixation, labeling, dissection, or purification. On the other hand, coherent Raman techniques have several advantages including non-invasiveness, high molecular specificity, and high image quality, which makes them powerful tools for characterization and quantification of the molecular dynamics in cells, tissues, and animal models33,34,35,36. Coherent Raman techniques, such as coherent anti-Stokes Raman scattering (CARS), have been applied to lipid studies in C. elegans since 200737; however, no studies on oocyte development have been reported.
CARS microscopy provides a means of imaging the reproductive system in C. elegans including germ line, oocytes, spermatheca, and embryos (Fig. S1b). In the present study, the fat mutants (fat-1~fat-4) were introduced as exemplars of animals, where yolk uptake is defective, to investigate which kind of PUFA (omega-3 or omega-6) is more critical to the transportation of yolk lipoprotein into oocytes. We showed that CARS microscopy is capable of detecting the aberrant accumulation of secreted yolk lipoprotein in the pseudocoelomic cavity without any labeling by using protein (~1665 cm−1) and lipid (~2845 cm−1) Raman bands. In addition, we established an image analysis protocol to identify and quantitatively measure the levels of secreted yolk lipoprotein accumulation in fat mutants and PUFA-supplemented fat-2 worms (the PUFA add-back experiments). Finally, we examined the lipid content in single oocytes, the development of the oocyte (including oocyte size, ovulation rate, and egg number), and quantitatively estimated the rate of lipid delivery into oocytes in C. elegans. Our data suggest that omega-6 PUFAs are a key regulator for lipid delivery and oocyte development in C. elegans.
Results
Detection of secreted yolk lipoprotein accumulated in the pseudocoelom of C. elegans by CARS imaging
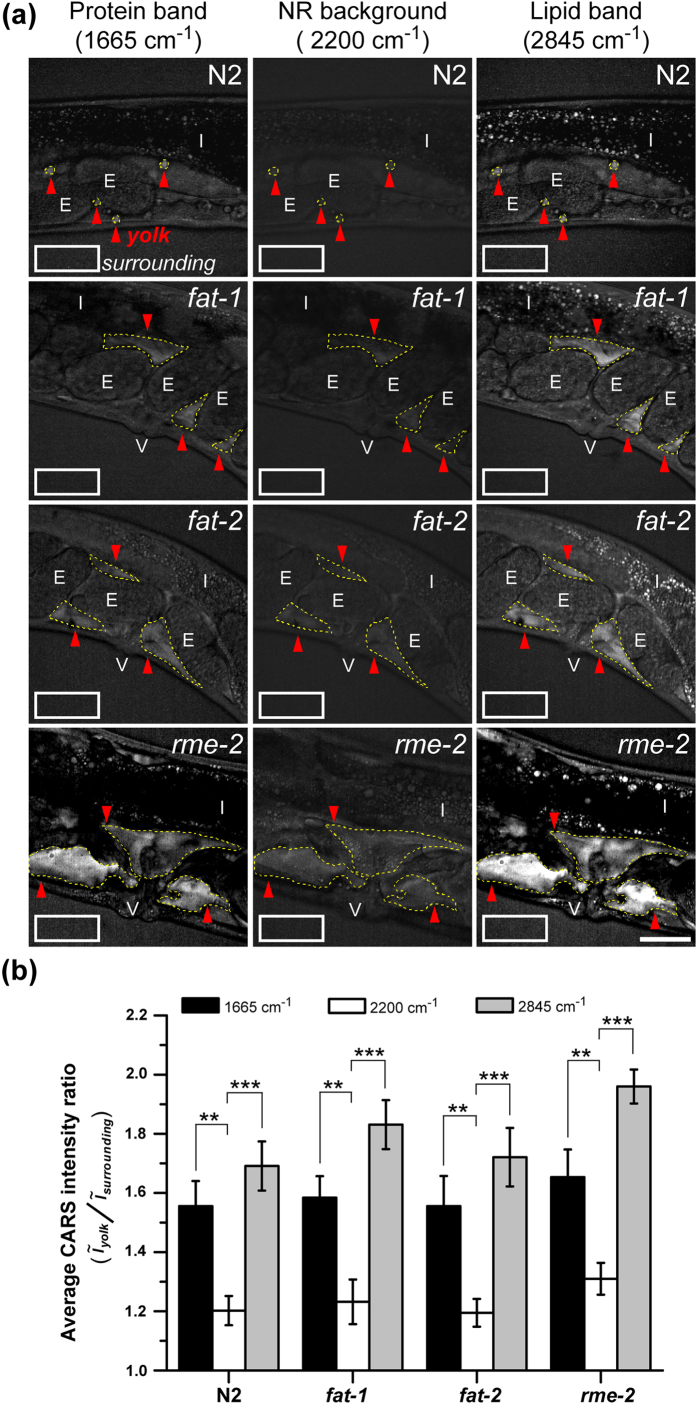
In addition to the use of the lipid Raman band at ~2845 cm−1 (CH2 stretching) for monitoring the accumulation of the secreted yolk lipoprotein in the pseudocoelomic cavity of uterus19, the protein Raman band at ~1665 cm−1 (amide I/C=C stretching)38 provides an additional marker for CARS imaging to identify the yolk lipoprotein accumulation. We first examined the accumulation of secreted yolk lipoprotein in one-day-adult (1D-Ad) rme-2, fat-1, and fat-2 mutants by Raman micro-spectroscopy. The Raman data confirm that 1665 cm−1 is a reliable protein Raman band (Fig. S2)38. We then used these two Raman bands at ~1665 cm−1 (protein band) and ~2845 cm−1 (lipid band) plus the non-resonant background at ~2200 cm−1 to image the aberrant accumulation of secreted yolk lipoprotein in these mutants and wild-type N2 worms (Fig. 1a). The CARS images show high co-localization between both Raman bands at ~1665 cm−1 and ~2845 cm−1 (Fig. S3). The average CARS intensity ratio between secreted yolk lipoprotein accumulations and surrounding buffer suggests that both the protein band ratio and lipid band ratio are significantly higher than the ratio at non-resonant background in all four C. elegans strains (Fig. 1b). These indicate that both CARS signals of protein and lipid bands provide a sufficient S/N ratio for in vivo imaging of secreted yolk lipoprotein accumulated in C. elegans.
Figure 1. The accumulation of secreted yolk lipoprotein in the pseudocoelom of N2, fat-1, fat-2, and rme-2 mutants.
(a) CARS Images of N2, fat-1, fat-2, and rme-2 mutants containing yolk lipoprotein accumulation measured at three different vibrational frequencies. Red arrows and white rectangles represent the regions of yolk lipoprotein accumulation and surrounding buffer, respectively. E stands for embryo, I stands for intestine, and V stands for vulva. Scale bar = 30 μm. (b) The average CARS intensity ratio between yolk lipoprotein accumulation (red arrows in (a)) and surrounding buffer (white rectangles in (a)) at three vibrational frequencies. Black bar for ~1665 cm−1 (protein band), white bar for ~2200 cm−1 (non-resonant background), and gray bar for ~2845 cm−1 (lipid band) (n = 10~15). All worms are 1D-Ad worms. Error bars represent the standard error of the mean (SEM). (P-value: *p < 0.05, **p < 0.01, ***p < 0.001).
We further used this method to measure the distribution of the protein/lipid ratio for lipid-enriched particles in the N2 intestine (data not shown). However, we were not able to precisely distinguish yolk particles from LDs in the intestine. This is because there are various yolk/LD intermediates during yolk biosynthesis and there are no specific protein or lipid markers that can definitively distinguish intestinal yolk and LDs at present13. Because CARS imaging has a higher S/N ratio of secreted yolk lipoprotein accumulation at lipid band (~2845 cm−1) than at protein band (~1665 cm−1), the CARS imaging of secreted yolk lipoprotein at ~2845 cm−1 was used for data collection and analysis for the following experiments.
Analysis of secreted yolk lipoprotein accumulation in the pseudocoelom of worms
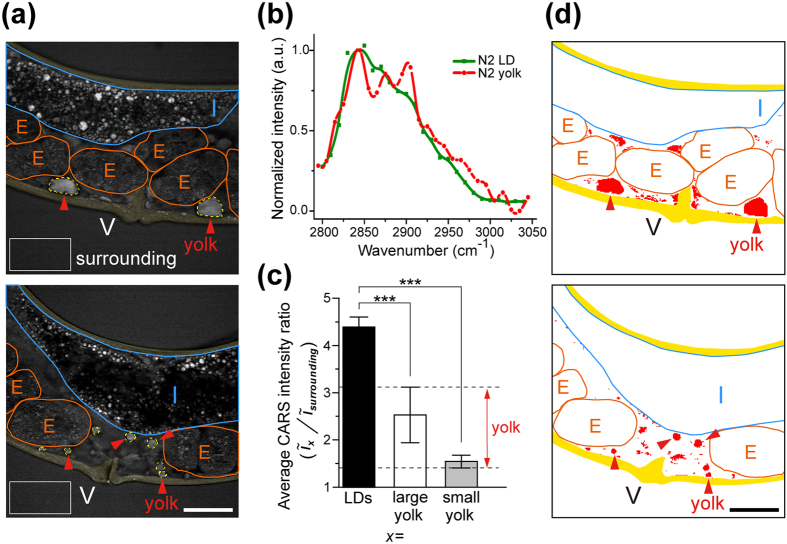
Here we examined the CARS signals at ~2845 cm−1 of the secreted yolk lipoprotein accumulations in the pseudocoelom (Fig. 2a) and the LDs in the skin-like hypodermal cells (Fig. S4). We found that both of them show strong lipid signal in CARS imaging (Fig. 2a and Fig. S4) and in normalized CARS spectrum (Fig. 2b). In contrast to LDs, the secreted yolk lipoprotein accumulation in pseudocoelom is larger in size with polygonal shapes and has weaker average CARS intensity. The average CARS intensity ratio between secreted yolk lipoprotein accumulation and the surrounding buffer (2.53 ± 0.59 and 1.69 ± 0.22 for large and small accumulations, respectively) is much weaker than the ratio between the LDs and surrounding buffer (4.39 ± 0.21) (Fig. 2c) in 1D-Ad wild-type worms. After excluding the internal organs/tissues such as intestine, oocytes/embryos, and body wall, we set a threshold at an intensity ratio within 1.47–3.12 to convert the CARS image into a binary image for extracting the component of secreted yolk lipoprotein accumulation in the pseudocoelomic cavity (as indicated by red arrows in Fig. 2a,d). We further introduced the signal of VIT-2-GFP reporter as a reference to test the detection limit and found that this thresholding method is capable of detecting the accumulation of secreted yolk lipoprotein with size ≥10 μm2 (Fig. S5). Accordingly, the size histogram of all the accumulations of secreted yolk lipoprotein in the pseudocoelomic cavity of uterus in 1D-Ad wild-type worms was plotted (Fig. S6a). After the number of occurrence was normalized by the number of worms (n = 57) (Fig. S6b) and multiplied by each binned area size, the area contribution curve of the wide-type worm can be obtained (Fig. S6c) (also see Methods for the details). The results show that the most dominant size of secreted yolk lipoprotein accumulation is 20~60 μm2 and the major contribution to the total area (~72%) of secreted yolk lipoprotein accumulation is <80 μm2 for N2 worms. This method was also applied to examine the size distribution of secreted yolk lipoproteins in the pseudocoelomic cavity of fat mutants.
Figure 2. Analysis of yolk lipoprotein accumulation in the pseudocoelomic cavity of one-day-adult (1D-Ad) wild-type (N2) worms.
(a) The CARS image of 1D-Ad N2 worms containing large (upper image) and small (lower image) yolk lipoprotein accumulation (red arrows) in their pseudocoelomic cavities. The white rectangles are selected for calculating the average CARS intensity of surrounding buffer. Yellow region stands for the skin-like hypodermal cells, E stands for embryo, I stands for intestine, and V stands for vulva. Scale bar = 30 μm. (b) The CARS spectra of yolk lipoprotein accumulation obtained from pseudocoelomic cavity and the lipid droplet (LD) obtained from the skin-like hypodermal cell in 1D-Ad N2 worms. (c) The average CARS intensity ratios between LDs/surrounding buffer and between (large and small) yolk lipoprotein accumulation/surrounding buffer. A threshold of 1.47–3.12 (in red color) was applied to the CARS image to enhance the boundary of yolk lipoprotein accumulation. Error bars represent the standard deviation. (P-value: *p < 0.05, **p < 0.01, ***p < 0.001) (d) After excluding the internal organs/tissues (such as intestine, oocyte/embryo, and body wall) and applying the threshold, the pixels with intensity ratio between 1.47–3.12 are converted into red color, and pixels with intensity ratio <1.47 and >3.12 are converted into white color. The boundary of yolk lipoprotein accumulation can be easily distinguished (indicated by red arrows).
PUFAs are required for the transportation of yolk lipoprotein into oocytes
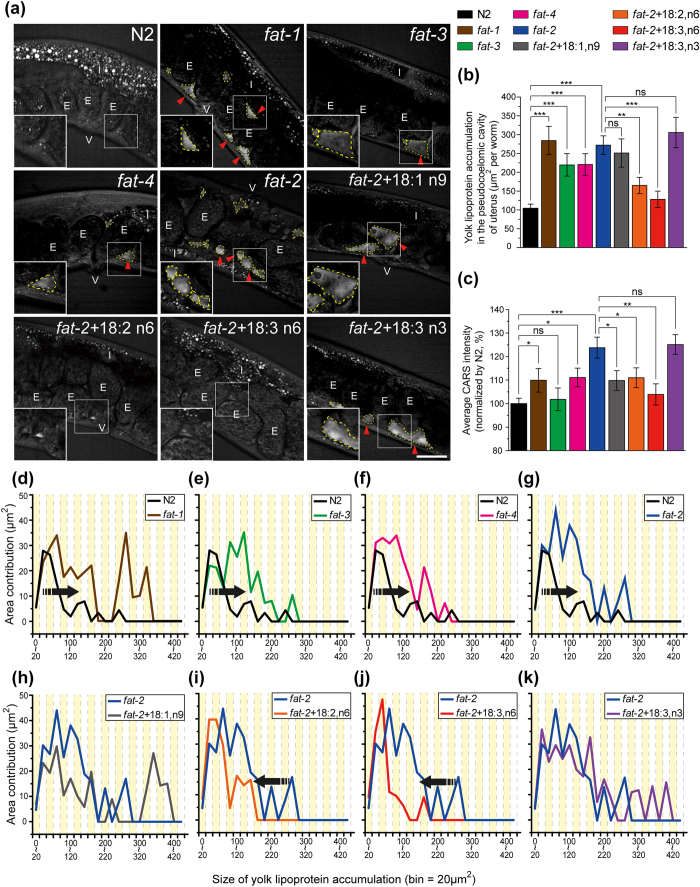
We next measured the accumulation level of secreted yolk lipoprotein in different mutants (fat-1, fat-2, fat-3, and fat-4) and examined the effects of various PUFA supplementations (18:1,n9, 18:2,n6, 18:3,n6, and 18:3,n3 fatty acids) to fat-2 mutants (Fig. 3a,b). These fat mutants were chosen because they exhibit different levels of defects in PUFA biosynthesis. Consequently, their phenotypes could act as markers for investigating the role of PUFAs in the transportation of yolk lipoprotein in C. elegans. The supplementation of various PUFAs to fat-2 worms was an add-back method to study the differences between complete deficiency in PUFAs (fat-2) and either partial (18:3,n6 and 18:3,n3) or total (18:2,n6) restoration of PUFA production in the intestine of fat-2 worms. The supplementation of MUFA (18:1,n9) was applied as a negative control. All the fat-1 to fat-4 mutants show generally enhanced CARS intensities (Fig. 3c) and dramatically increased levels of secreted yolk lipoprotein accumulation (Fig. 3b). The major sizes of accumulations in these mutants are all larger than N2 worms (Fig. 3d–g). The supplementation of omega-6 PUFAs in fat-2 worms exhibits appreciably reduced accumulation level (Fig. 3b), average CARS intensity (Fig. 3c), and the size of secreted yolk lipoprotein accumulation (Fig. 3i,j). However, we did not observe a significant difference after the dietary restoration of MUFA (Fig. 3b,c,h) and omega-3 PUFAs (Fig. 3b,c,k). These data indicate that PUFA is a key factor for the transportation of yolk lipoprotein. To further determine which type of PUFA (omega-6 or omega-3) is more critical for the oocyte development, it is necessary to examine the lipid content in oocytes.
Figure 3. Analysis of yolk lipoprotein accumulation in PUFA-deficient mutants.
(a) The CARS images of N2, PUFA-deficient mutants (fat-1, fat-2, fat-3, and fat-4), and fat-2 worms supplemented with PUFAs. Red arrows indicate the regions of yolk lipoprotein accumulation. E stands for embryo, I stands for intestine, and V stands for vulva. Scale bar = 30 μm. (b) The total area and (c) the average CARS intensity of yolk lipoprotein accumulations in the pseudocoelomic cavity of uterus in different worms. (n = 57 for N2, and n = 19~32 for mutants and PUFA-supplemented fat-2 worms) All worms are 1D-Ad worms. Error bars represent the SEM. (d–k) The area contribution curves of yolk lipoprotein accumulations in various strains. (P-value: *p < 0.05, **p < 0.01, ***p < 0.001, ns: not significant).
Quantitative analysis of lipid content in −1 to −4 oocytes during oocyte development
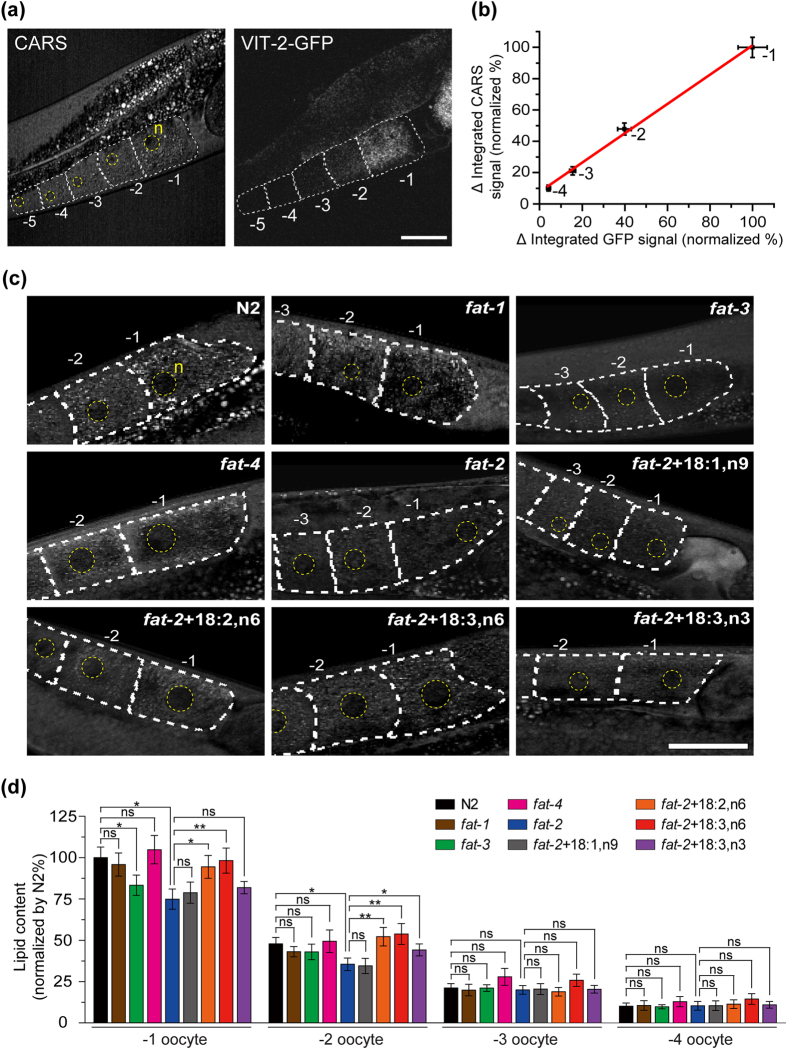
We simultaneously obtained both CARS and two-photon excitation fluorescence images of the oocytes in the wild-type worms with vit-2p::vit-2::gfp transgene expression (Fig. 4a) and, then analyzed the integrated CARS and GFP fluorescence signals (see Methods for the definition of integrated CARS/GFP signal) in these images to investigate the lipid delivery into oocytes by yolk lipoprotein during oocyte development in C. elegans. The GFP fluorescence signal shows the distribution of the GFP-tagged yolk lipoprotein (VIT-2-GFP), whereas the CARS signal shows the lipid content in oocytes. We noticed that the −1 oocyte has the highest GFP fluorescence signal and decreasing signal in the following oocytes (−2, −3, −4 and −5), indicating that VIT-2-GFP yolk lipoprotein complexes transport into the proximal oocytes that are nearly mature (−1 and −2 oocytes), but not to the immature oocytes such as the −5 oocyte. This is because the GFP signal in the −5 oocyte was almost non-detectable and could be ignored (Fig. 4a). To confirm the linear correlation between lipid carrier (revealed by GFP signal) and lipid stored (revealed by CARS signal) in each oocyte, Fig. 4b shows the plot of Δ integrated CARS signal versus Δ integrated GFP signal for each oocyte (−1, −2, −3 and −4). The Δ integrated CARS signal was defined as the difference in integrated CARS signal of each oocyte to that of the −5 oocyte (as an offset), and so was the Δ integrated GFP signal. The strong linear correlation (R2 > 0.99) between them indicates that CARS signal can reveal the amount of lipid delivered into oocytes by yolk lipoproteins.
Figure 4. Quantitative analysis of lipid content in oocytes.
(a) CARS and two-photon excitation fluorescence images of the oocytes of a wild-type worm with vit-2p::vit-2::gfp transgene expression. n: nucleus. (b) The correlation between Δ integrated GFP signal and Δ integrated CARS signal of each oocyte. The red line represents the linear regression (R2 > 0.99). Each data point represents the average ± SEM (n = 17). (c) The CARS images of the oocytes and (d) The normalized amount of lipid stored in the oocytes of N2, fat-1, fat-3, fat-4, fat-2, and fat-2 worms supplemented with 18:1,n9, 18:2,n6, 18:3,n6, and 18:3,n3 (n = 9~11). All are 1D-Ad worms. Error bars represent the SEM. Scale bar = 30 μm. (P-value: *p < 0.05, **p < 0.01, ***p < 0.001. ns: not significant).
We further examined the oocytes in fat-1 to fat-4 mutants, and PUFA-supplemented fat-2 mutant (Fig. 4c). We found that N2, fat-1, and fat-4 worms show a similar level of lipid content in their oocytes, but fat-2 and fat-3 mutants showed a discernible decrease of lipid content in the −2 or −1 oocytes (Fig. 4d). This implies that the delivery of lipid into oocytes is not affected by the deficiency of omega-3 PUFAs in fat-1 mutants. The fat-3 mutants, which lose most of their PUFAs due to the dysfunction of delta-6 desaturase, show less lipid content in its −1 oocytes. The loss of both omega-3 and omega-6 PUFAs in fat-2 compromised the delivery of lipid into oocytes. The average lipid content in both −1 and −2 oocytes of fat-2 mutant is ~25% less than N2 worms. Supplementation with omega-6 PUFAs to fat-2 mutant could recover the lipid content in the −1 and −2 oocytes to a level close to that of N2 worms, while supplementation with MUFA (18:1,n9) or omega-3 PUFA (18:3,n3) did not significantly improve the level of lipid storage in oocytes in fat-2 mutants (p = 0.666 for 18:1,n9 and p = 0.329 for 18:3,n3) (Fig. 4d). These data suggest that omega-6 PUFAs are necessary for the lipid delivery into oocytes in C. elegans, which is critical for oocyte development and embryonic development.
Omega-6 PUFAs modulate oocyte development in C. elegans
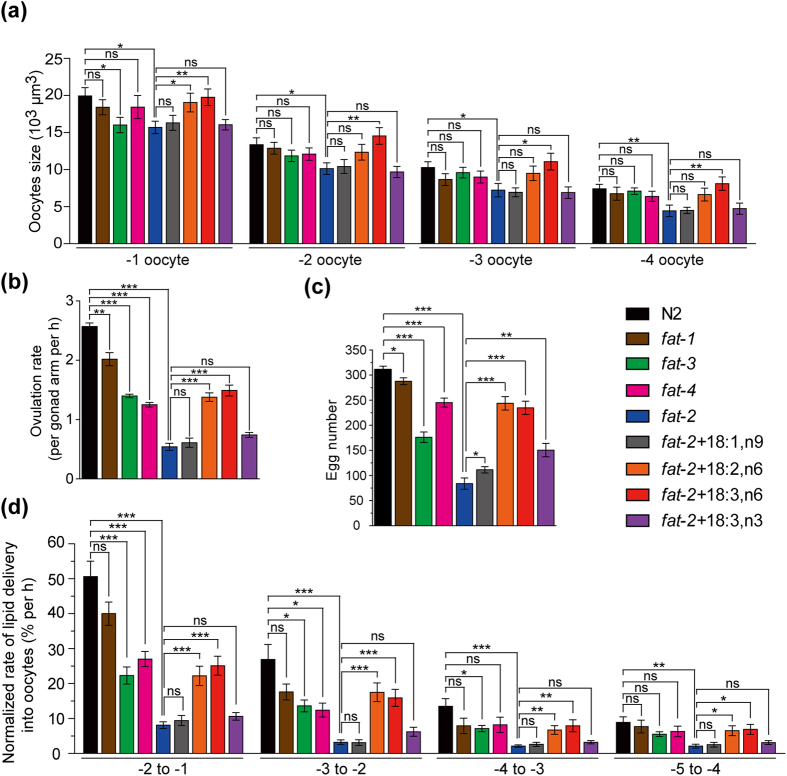
In oocyte development, the size of nearly mature oocytes (the oocytes in the proximal gonad arm) will increase dramatically, which is one of the most important landmark events24. We analyzed the cellular volume of the −1 to −4 oocytes in N2, fat-1, fat-3, fat-4, as well as fat-2 with or without PUFA-supplementation by CARS images (Fig. 5a). The estimated size of the −1 oocyte in N2 worms is ~20000 ± 1100 μm3 (Fig. 5a), which is consistent with the volume of fully mature oocytes (21700 ± 4000 μm3)39. The fat-1 and fat-4 oocytes show similar volume, but fat-2 and fat-3 oocytes are ~20% smaller compared to the average size of the −1 oocyte in N2 (Fig. 5a). In the PUFA-supplement experiments, we found that omega-6 PUFAs, but not omega-3 PUFAs, recover the oocyte volume of fat-2 to a level close to N2 (Fig. 5a). The correlation analysis between the oocyte size and lipid uptake suggests that these two parameters are highly correlated (Pearson’s product-moment coefficient r = 0.89) (Fig. S7). It is known that the lipid provision in eggs is important for the development of embryo26,27. Our data show that fat-2 mutants have a ~25% lower amount of lipid stored (Fig. 4d) and smaller oocyte size (Fig. 5a) in the −1 oocyte compared to the control, implying that more than a 25% loss of lipid in the oocyte that can cause significantly lethal embryonic defects in C. elegans.
Figure 5. Analyses of oocyte growth, ovulation rate, and egg number of N2 worms and PUFA-deficient mutants.
(a) The cellular size of the oocytes (n = 9~11), (b) ovulation rate (n = 20~32), and (c) egg number (n = 10~13) of N2, fat-1, fat-3, fat-4, fat-2, and fat-2 worms supplemented with 18:1,n9, 18:2,n6, 18:3,n6, and 18:3,n3. (d) The normalized rate of lipid delivery into oocytes in N2, fat-1, fat-3, fat-4, fat-2, and fat-2 worms supplemented with 18:1,n9, 18:2,n6, 18:3,n6, and 18:3,n3. The data are normalized by wild-type (N2). All worms are 1D-Ad worms. Error bars represent SEM. (P-value: *p < 0.05, **p < 0.01, ***p < 0.001. ns: not significant).
We next analyzed the ovulation rate and egg number in N2, fat-1, fat-3, fat-4, and fat-2 with or without the addition of PUFAs. Compared to N2 worms (~2.5 oocyte per gonad arm per h), we found a slight decrease in fat-1 worms (~2 oocyte per gonad arm per h), an appreciable decrease in fat-3 worms (~1.4 oocyte per gonad arm per h) and fat-4 worms (~1.25 oocyte per gonad arm per h), and a significant decrease in fat-2 worms (~0.5 oocyte per gonad arm per h) in their ovulation rates (Fig. 5b). Addition of omega-6 PUFAs to fat-2 worms can partially recover the defect of oocyte ovulation (~1.5 oocyte per gonad arm per h; Fig. 5b), but addition of 18:1,n9 and 18:3,n3 show no significant increase in the ovulation rate of fat-2 worms. The results of egg number have a similar trend. The egg number (Fig. 5c) has a slight decrease in fat-1 worms, an appreciable decrease in fat-3 and fat-4 worms, and a significant decrease in fat-2 worms with respect to N2 worms. Addition of omega-6 PUFAs to fat-2 has a pronounced effect on the recovery of the number of eggs (75% of wild-type).
Oocyte development in C. elegans is a continuous process and the reproduction line of oocytes is highly time-dependent. The average transition time from “−n” position to “−(n − 1)” position can be determined as the average time to ovulate a mature oocyte, which is the inverse the ovulation rate. We further calculated the increase of lipid content between −n and −(n − 1) from Fig. 4d. We quantified the rate of lipid delivery into single oocytes as [(the increase of lipid content between adjacent oocytes)/(the average time to ovulate a oocyte)] in wild-type worms, fat-1, fat-3, fat-4, and fat-2 mutants, as well as PUFA-supplemented fat-2 worms during oocyte development in live C. elegans (Fig. 5d). Compared to the transition of −2 to −1 oocyte in wild-type N2 worms, the lipid delivery rate is slightly lower in fat-1 (~80%), significantly lower in fat-3 (~40%) and fat-4 (~50%), and dramatically lower in fat-2 (~15%). For the fat-2 worms with PUFA-supplementation, we found that omega-6 PUFAs, but not omega-3 PUFA and MUFA, restitute the defect in the lipid delivery rate in fat-2 worms.
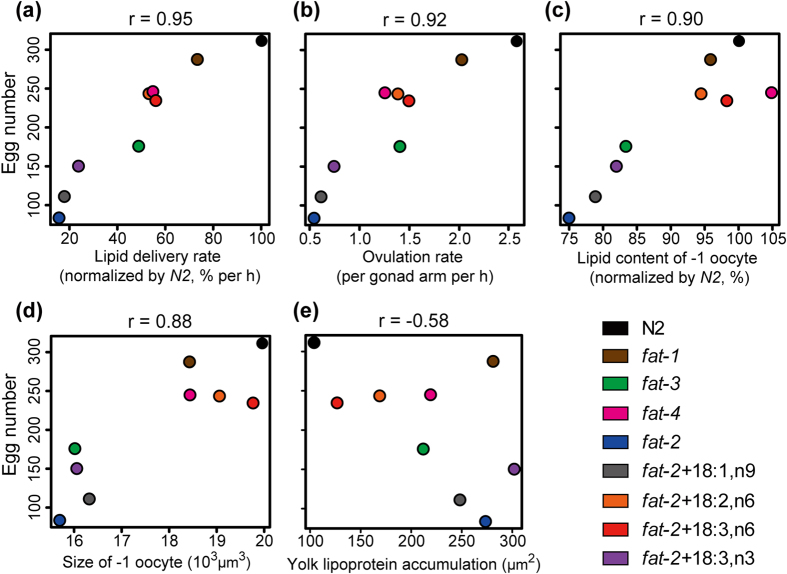
We further analyzed the correlations between the final output results (egg number) and various quantitative measurements by using R40. We found that the egg number has highly positive correlations with lipid delivery rate, ovulation rate, lipid content, and oocyte size (r = 0.95, 0.92, 0.90, and 0.88, respectively; Fig. 6a–d), and a moderately negative correlation with the yolk lipoprotein accumulation (r = −0.58; Fig. 6e). The abnormal accumulation of yolk lipoprotein in fat-1 originates from the over-production of lipid/protein complexes (please see Discussion). If we exclude the data point of fat-1 in Fig. 6e, then the correlation becomes a strongly negative correlation (r = −0.84), while other correlations are not significantly affected (r = 0.94 for lipid delivery rate; r = 0.91 for ovulation rate; r = 0.92 for lipid content; r = 0.91 for oocyte size). These results suggest that the yolk transportation controlled by omega-6 PUFAs could be one of the important factors that influence the development of oocyte in C. elegans.
Figure 6. The correlation between various quantitative results and egg number.
The scatter plots of (a) lipid delivery rate, (b) ovulation rate, (c) lipid content, (d) oocyte size, and (e) total area of yolk lipoprotein accumulation versus egg number, together with the values of the Pearson’s product-moment correlation coefficient (r). The analyses were conducted using R40.
Discussion
The C. elegans is a good exemplar to verify which kind of PUFA is more critical for the transportation of yolk lipoprotein by 1) designing an input condition (fat mutants and the PUFA add-back experiments); 2) quantitatively measuring the parameters involving the development of oocytes (aberrant yolk accumulation, lipid content, lipid delivery rate, oocyte size, and ovulation rate); and 3) eventually collecting the final output results (the reproductive activity, i.e. the egg number). We showed that CARS is particularly useful in detecting the aberrant accumulation of yolk lipoprotein, revealing the oocyte lipid content, and estimating the relative rate of lipid delivery into oocytes during oocyte development in live C. elegans, demonstrating an alternative label-free method to study the relationship between lipoprotein regulation and oocyte development.
One of the most important issues regarding the study of yolk lipoprotein is to understand the underlying mechanism of how lipids are packaged into the lipid carrier for export. We have demonstrated that the accumulation of secreted yolk lipoprotein in pseudocoelom of live C. elegans can be quantitatively measured by CARS microscopy at both protein (~1665 cm−1) and lipid (~2845 cm−1) Raman bands. However, the measurement of both protein and lipid ratios of intestinal lipid-enriched particles (including mature/immature yolk and lipid storage particles) is not sufficient to identify yolk in the intestine. Yolk lipoproteins are made in the intestine, secreted, and taken up by the developing oocytes. It is believed that lipid molecules translocate between LDs and yolk lipoproteins in the intestine of C. elegans13. So far, there is no direct evidence to elucidate the interactions between them in the intestine and the underlying mechanism of yolk formation remains unknown. Thus, specific markers or new methods are needed for characterizing those lipid-enriched particles in the intestine.
There are two major yolk lipoprotein complex forms in C. elegans, the B dimer and A complex41. The B dimer is composed of two yp170B polypeptides, which is encoded by the vit-2 gene41,42. The A complex is composed of yp170A, yp115, and yp88. The yp170A is encoded by vit-5, while yp115 and yp88 are the two smaller yolk lipoproteins cleaved from a precursor protein encoded by vit-641,42. The exogenous vit-2::gfp is widely used as a fluorescent marker to indicate the distribution of GFP-tagged B dimer yolk lipoprotein complex (yp170B). To quantify the amount of delivered lipid in oocytes, the contribution of other lipid delivery pathways should be considered. In this work, we compared the integrated GFP signal and integrated CARS signal collected from the oocytes of the wild-type worms with vit-2p::vit-2::gfp transgene expression, and discovered a strong linear correlation between them (R2 > 0.99). The interception of Fig. 4b is not zero at the axis of the Δ integrated CARS signal, which could be due to the contribution of the A complex, the endogenous B dimer, or an unknown lipid delivery pathway that cannot be detected by the fluorescent signal of GFP-tagged yp170B. Since the expression of GFP-tagged yolk lipoprotein may not accurately reflect the amount of total yolk lipoprotein, along with some possible perturbation from exogenous protein expression, the proposed label-free CARS imaging method is more reliable for quantifying and comparing the uptake of yolk lipoprotein/oocyte lipid content among different strains.
The normalized rate of lipid delivery in oocyte transitions in live C. elegans is established for the first time. We discovered a 50% lipid increase during the transition of the −2 to −1 oocyte in all of our experimental strains. This result is consistent with the observation of the GFP signal in the N2 worms expressing VIT-2-GFP, indicating that the major target of yolk lipoprotein complexes is the near mature oocytes (−2 and −1 oocytes). Both fat-3 and fat-4 mutants show decreased lipid delivery rates from −5 to −1 oocytes (49% and 54% of the N2 worms, respectively). The fat-2 mutant exhibits a dramatic decrease in the lipid delivery rate (only ~16% of the control). Although fat-1 mutant shows slightly lower oocyte lipid content and delivery rate than the control, the oocyte growth and reproduction of fat-1 mutant are not affected. The rescue experiments show that omega-6 PUFAs, but not omega-3 PUFAs, can increase the lipid delivery rate in fat-2 mutants. These results suggest that due to the lack of omega-6 PUFAs, the development of oocytes takes a much longer time (e.g. low ovulation rate) to acquire sufficient nutrients for embryonic development. The delivery of lipid into oocytes can be more efficient with sufficient omega-6 PUFAs.
The roles of omega-3 and -6 PUFAs in oocytes and embryo development are still in debate. It is not clear why the fat-1 mutant has non-detectable omega-3 PUFAs and increased levels of omega-6 PUFAs (about 13-fold higher arachidonic acid levels than wild-type worms) but shows no apparent defects in reproduction18,43, while fat-2 and fat-3 mutant shows reduced brood size and live progeny17,44. We noticed that fat-1 mutant shows a high level of secreted yolk lipoprotein accumulation, but the oocyte lipid content in the fat-1 mutant is similar to the level of wild-type N2 worms. It is found that the overexpression of PUFAs can negatively regulate DAF-16/FOXO to promote the protein expression level of yolk lipid protein (vitellogenins) in the intestine23. Based on our findings, we suggest that the over-production of omega-6 PUFAs in fat-1 mutant results in the generation of abundant yolk lipoprotein, which exceeds the uptake limit of oocytes. As a result, the fat-1 mutant shows abnormal yolk lipoprotein accumulation in pseudocoelom but no apparent defect in reproduction, suggesting that omega-3 PUFAs are not critical for the reproductive process. Edmonds et al. reported that supplementation with arachidonic acid (20:4,n6) reduces the accumulation of yolk lipoprotein in pseudocoelom of fat-2(wa17); vit-2p::vit-2::gfp transgenic worms21. Watts et al. found that biochemical complementation with the omega-6 PUFA (18:3,n6) restores the reproductive defect in the fat-3 mutant44. Together with our results from PUFA-supplementation experiments, all evidence strongly supports that omega-6 PUFAs, but not omega-3 PUFAs, are required for yolk lipoprotein transportation and oocyte development in C. elegans.
It is found that Omega-6 PUFAs such as linoleic (18:2,n6) and arachidonic (20:4,n6) acids are considerably enriched in the oocytes of cow, sheep and pigs1. In human oocytes, the most abundant fatty acids are saturated fatty acids (~79% of total fatty acids) followed by MUFA (~14%) and omega-6 PUFAs (~5%) from fertilization-failed human oocytes45. These data indicate that omega-6 PUFAs are associated with the development of oocytes in mammals. The results of in vitro incubation of bovine oocytes with omega-6 PUFA show that linoleic acid (18:2,n6) can stimulate lipid accumulation in maturing bovine oocytes in a dose dependent manner (in the 0~50 μM range)31. Microarray analysis reveals that the expression of the LDL receptor increases more than 10-fold during the maturation of human oocytes46,47. Since both yolk lipoprotein receptor (RME-2) and human LDL receptor are in the LDL receptor family16, it is possible that the regulatory effect of omega-6 PUFAs found in this study may similarly influence the endocytosis of lipoprotein in mammals.
In conclusion, we established an experimental method to quantitatively measure the level of aberrant yolk lipoprotein accumulation and lipid delivery into oocytes in living C. elegans without any labeling. We also demonstrated the first systematic examination of yolk lipoprotein transportation, lipid delivery, and oocyte development in wide-type, PUFA-deficient mutants, and various PUFA-supplemented fat-2 worms. Together with the results of egg number, we conclude that the omega-6 PUFAs is an important factor that modulates the delivery of lipid and further regulates the development of oocyte in C. elegans. This work remarks the value of using CARS as a molecular-selective label-free imaging technique for the study of yolk lipoprotein, PUFA regulation, and oocyte development in C. elegans, with implications for application to developmental biology and reproductive biology.
Methods
Worms and reagents
Wild-type (Bristol N2), DH1390 [rme-2(b1008)], BX24 [fat-1(wa9)], BX26 [fat-2(wa17)], BX30 [fat-3(wa22)], and BX17 [fat-4(wa14)] mutant C. elegans were obtained from the Caenorhabditis Genetics Center. All strains used in this study were cultured on nematode growth medium (NGM) plates seeded with E. coli OP50 and incubated at 20 °C. The late L4 larvae were picked onto plates and incubated at 20 °C to mature into young adults. After that, the worms were incubated another 24 h for them to grow into one-day-adult (1D-Ad) worms. Levamisole was purchased from Sigma (St. Louis, Missouri) and used to paralyze worms for microscopy.
Dietary supplementation of fatty acids in worms
Fatty-acid-containing NGM plates were prepared as previously described48. Briefly, fatty acids were added slowly to the cooled NGM media to a final concentration of ~0.2 mM. Plates were seeded with OP50 bacteria and kept in the dark overnight. OP50 bacteria accumulated PUFAs into their lipids at ranges from 1–5%48.
Raman micro-spectroscopy
Raman measurements of secreted yolk lipoprotein accumulation in C. elegans were performed by a Raman microscope (HR800, Horiba Scientific, Kyoto, Japan). A HeNe laser beam (632.8 nm) was focused on the sample through a 50× objective. The Raman scattering was collected by the same objective and delivered to an 80-cm spectrograph equipped with a liquid nitrogen-cooled CCD for spectral analysis. Raman spectra were collected in the region of 900–1,800 cm−1 with ∼60 s acquisition time.
Analysis of secreted yolk lipoprotein accumulation in the pseudocoelomic cavity of uterus
The following is the process for quantifying yolk lipoprotein accumulation in the pseudocoelomic cavity of uterus (here we used 1D-Ad N2 worms as an example): First, the measured sizes of yolk lipoprotein accumulations from all worms were pooled together to plot the size histogram. Figure S6a shows the size histogram of yolk lipoprotein accumulations from total 57 1D-Ad N2 worms. Second, the number of occurrence per binned area size (0~20 μm2, 20~40 μm2, 40~60 μm2, etc.) was divided by the number of worms (n = 57) to yield the distribution of the average frequency to find a corresponding size of accumulation in a worm (Fig. S6b). Finally, the area contribution histogram (Fig. S6c) was obtained by multiplying the frequency in Fig. S6b by each binned area size. The area contribution curve (red line in Fig. S6c) provides the contribution of each accumulation size in the pseudocoelomic cavity of uterus in a worm. The integration of the whole area contribution curve gives the total area of yolk lipoprotein accumulations in the pseudocoelomic cavity of uterus in a worm.
CARS/TPEF microscopy
Figure S8 describes the setup of the CARS microscope. The acquisition times were ∼5 s for a CARS image and ∼2 s for a two-photon excitation fluorescence (TPEF) image. CARS or TPE-F image was obtained within an appropriate field of view (∼141 × 141 μm2) allowing minimal non-uniformity of illumination. To preserve the largest dynamic range for further CARS image analysis, the data were recorded as 12-bit gray-level images. All images were analyzed by the ImageJ software49. The CARS spectrum was analyzed from the CARS images with vibrational frequencies in the range of 2,800–3,050 cm−1.
Quantitative analysis of CARS/TPEF signal in individual oocytes
The quantitative analysis of lipid content in oocytes was modified from our previous image analysis method50,51. CARS images of oocytes were taken at 2845 cm−1 and analyzed by image J software. Background intensity, which was mainly contributed from the non-resonant signal of non-lipid-rich cellular structures, was determined and subtracted from the average CARS intensity of the oocyte nucleus. The integrated CARS intensity was calculated by summing the square roots of the background-subtracted CARS intensities of the pixels that belongs to the oocyte (Fig. S9), which yields a numerical value for the lipid content in the single oocyte. For the integrated GFP intensity, the background intensity was defined as the average fluorescence intensity in the VIT-2::GFP negative region for which the definition was described by Yi et al.19. The integrated GFP intensity was then determined by the summation of the background-subtracted GFP intensities of the pixels that belongs to the oocyte.
Estimation of oocyte size
The single oocyte cell was assumed as a cylinder24, so its volume = π × length × (width/2)2. Area and length measurements of oocytes were analyzed with ImageJ software.
Egg number and oocyte ovulation assay
The egg number was used to quantify the number of eggs laid over the worm’s reproductive lifetime. The egg number of each worm was defined as the sum of laid eggs (including non-hatched and hatched eggs). The procedure of the oocyte ovulation assay was described by Brisbin et al.52. The number of oocytes/embryos in 1D-Ad worms was counted by the Nikon SMZ645 microscope. Then, the 1D-Ad worms were picked into a new plate and incubated for 5–8 h at 20 °C to lay eggs. The number of eggs laid on the plate as well as the number of eggs retained in worms were then counted. Ovulation rate, the average number of oocytes ovulated in 1 h per gonad arm, was determined by the following calculation: (the number of eggs laid on the plate + the number of oocytes/embryos retained in worms) − (the number of oocytes/embryos originally in the worms), and then averaged for the time period (5–8 h) as well as the number of gonad arms.
Additional Information
How to cite this article: Chen, W.-W. et al. Specific polyunsaturated fatty acids modulate lipid delivery and oocyte development in C. elegans revealed by molecular-selective label-free imaging. Sci. Rep. 6, 32021; doi: 10.1038/srep32021 (2016).
Supplementary Material
Acknowledgments
The authors thank Caenorhabditis Genetics Center for providing the mutant strains. The authors thank Prof. Arnold Stern (NYU School of Medicine) for his careful reading and Dr. Margaret Hsin-Jui Kuo (Academia Sinica) for her valuable discussion. This work is supported by the Ministry of Science and Technology of the Republic of China (MOST 103-2113-M-001-017-MY3, MOST 103-2321-B-182-013-MY3; MOST 103-2811-B-182-010; MOST 104-2811-B-182-021) and Minister of Education (EMRPD1D0651 and EMRPD1E1431).
Footnotes
Author Contributions W.-W.C. and Y.-H.Y. participated in the design and discussion of the study. W.-W.C. performed the experiments, analyzed the data, and wrote the manuscript. Y.-H.Y. prepared and characterized wild type and various mutants of C. elegans. C.-H.C. and W.-W.C. constructed the CARS/TPEF microscope. K.-C.H. and T.-H.M. assisted the preparation of C. elegans. Y.-C.L. assisted the study of CARS imaging experiments. S.J.L. and T.-C.C. led the project, interpreted the data, and wrote the manuscript.
References
- Dunning K. R., Russell D. L. & Robker R. L. Lipids and oocyte developmental competence: the role of fatty acids and beta-oxidation. Reproduction 148, R15–R27 (2014). [DOI] [PubMed] [Google Scholar]
- Aguilar P. S. & de Mendoza D. Control of fatty acid desaturation: a mechanism conserved from bacteria to humans. Mol Microbiol. 62, 1507–1514 (2006). [DOI] [PubMed] [Google Scholar]
- Kubagawa H. M. et al. Oocyte signals derived from polyunsaturated fatty acids control sperm recruitment in vivo. Nat. Cell. Biol. 8, 1143–1183 (2006). [DOI] [PubMed] [Google Scholar]
- Tamba S. et al. Timely interaction between prostaglandin and chemokine signaling is a prerequisite for successful fertilization. P. Natl. Acad. Sci. USA 105, 14539–14544 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branicky R., Desjardins D., Liu J. L. & Hekimi S. Lipid Transport and Signaling in Caenorhabditis elegans. Dev. Dynam. 239, 1365–1377 (2010). [DOI] [PubMed] [Google Scholar]
- Vrablik T. L. & Watts J. L. Emerging roles for specific fatty acids in developmental processes. Gene. Dev. 26, 631–637 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S. M., Cottee P. A. & Miller M. A. Sperm and Oocyte Communication Mechanisms Controlling C. elegans Fertility. Dev. Dynam. 239, 1265–1281 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrablik T. L. & Watts J. L. Polyunsaturated fatty acid derived signaling in reproduction and development: Insights from Caenorhabditis elegans and Drosophila melanogaster. Mol. Reprod. Dev. 80, 244–259 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter P. W. & Shroff H. Faster fluorescence microscopy: advances in high speed biological imaging. Curr. Opin. Chem. Biol. 20, 46–53 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packer A. M., Roska B. & Hausser M. Targeting neurons and photons for optogenetics. Nat. Neurosci. 16, 805–815 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumbusch A., Langbein W. & Borri P. Nonlinear vibrational microscopy applied to lipid biology. Prog. Lipid Res. 52, 615–632 (2013). [DOI] [PubMed] [Google Scholar]
- Kimble J. & Sharrock W. J. Tissue-Specific Synthesis of Yolk Proteins in Caenorhabditis-Elegans. Dev. Biol. 96, 189–196 (1983). [DOI] [PubMed] [Google Scholar]
- Lemieux G. A. & Ashrafi K. Neural Regulatory Pathways of Feeding and Fat in Caenorhabditis elegans. Annu. Rev. Genet. 49, 413–438 (2015). [DOI] [PubMed] [Google Scholar]
- Smolenaars M. M. W., Madsen O., Rodenburg K. W. & Van der Horst D. J. Molecular diversity and evolution of the large lipid transfer protein superfamily. J. Lipid Res. 48, 489–502 (2007). [DOI] [PubMed] [Google Scholar]
- Spieth J., Nettleton M., Zuckeraprison E., Lea K. & Blumenthal T. Vitellogenin Motifs Conserved in Nematodes and Vertebrates. J. Mol. Evol. 32, 429–438 (1991). [DOI] [PubMed] [Google Scholar]
- Grant B. & Hirsh D. Receptor-mediated endocytosis in the Caenorhabditis elegans oocyte. Mol. Biol. Cell 10, 4311–4326 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock T. J., Browse J. & Watts J. L. Fatty acid desaturation and the regulation of adiposity in Caenorhabditis elegans. Genetics 176, 865–875 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts J. L. & Browse J. Genetic dissection of polyunsaturated fatty acid synthesis in Caenorhabditis elegans. P. Natl. Acad. Sci. USA 99, 5854–5859 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi Y. H. et al. Lipid droplet pattern and nondroplet-like structure in two fat mutants of Caenorhabditis elegans revealed by coherent anti-Stokes Raman scattering microscopy. J. Biomed. Opt. 19, 011011 (2014). [DOI] [PubMed] [Google Scholar]
- Hoang H. D., Prasain J. K., Dorand D. & Miller M. A. A Heterogeneous Mixture of F-Series Prostaglandins Promotes Sperm Guidance in the Caenorhabditis elegans Reproductive Tract. Plos Genet. 9, e1003271 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmonds J. W. et al. Insulin/FOXO Signaling Regulates Ovarian Prostaglandins Critical for Reproduction. Dev. Cell 19, 858–871 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston W. L. & Dennis J. W. The eggshell in the C. elegans oocyte-to-embryo transition. Genesis 50, 333–349 (2012). [DOI] [PubMed] [Google Scholar]
- Kim S., Spike C. & Greenstein D. Control of oocyte growth and meiotic maturation in Caenorhabditis elegans. Adv. Exp. Med. Biol. 757, 277–320 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarter J., Bartlett B., Dang T. & Schedl T. On the control of oocyte meiotic maturation and ovulation in Caenorhabditis elegans. Dev. Biol. 205, 111–128 (1999). [DOI] [PubMed] [Google Scholar]
- Hirsh D., Oppenheim D. & Klass M. Development of the reproductive system of Caenorhabditis elegans. Dev. Biol. 49, 200–219 (1976). [DOI] [PubMed] [Google Scholar]
- Wathes D. C., Abayasekara D. R. E. & Aitken R. J. Polyunsaturated fatty acids in male and female reproduction. Biol. Reprod. 77, 190–201 (2007). [DOI] [PubMed] [Google Scholar]
- Sturmey R. G., Reis A., Leese H. J. & McEvoy T. G. Role of Fatty Acids in Energy Provision During Oocyte Maturation and Early Embryo Development. Reprod. Domest. Anim. 44, 50–58 (2009). [DOI] [PubMed] [Google Scholar]
- Burrows A. E. et al. The C. elegans Myt1 ortholog is required for the proper timing of oocyte maturation. Development 133, 697–709 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M. A. et al. A sperm cytoskeletal protein that signals oocyte meiotic maturation and ovulation. Science 291, 2144–2147 (2001). [DOI] [PubMed] [Google Scholar]
- Genicot G., Leroy J. L. M. R., Van Soom A. & Donnay I. The use of a fluorescent dye, Nile red, to evaluate the lipid content of single mammalian oocytes. Theriogenology 63, 1181–1194 (2005). [DOI] [PubMed] [Google Scholar]
- Carro M., Buschiazzo J., Rios G. L., Oresti G. M. & Alberio R. H. Linoleic acid stimulates neutral lipid accumulation in lipid droplets of maturing bovine oocytes. Theriogenology 79, 687–694 (2013). [DOI] [PubMed] [Google Scholar]
- Ferreira C. R. et al. Single embryo and oocyte lipid fingerprinting by mass spectrometry. J. Lipid Res. 51, 1218–1227 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camp C. H. & Cicerone, M. T. Chemically sensitive bioimaging with coherent Raman scattering. Nat. Photonics 9, 295–305 (2015). [Google Scholar]
- Pezacki J. P. et al. Chemical contrast for imaging living systems: molecular vibrations drive CARS microscopy. Nat. Chem. Biol. 7, 137–145 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le T. T., Yue S. & Cheng J. X. Shedding new light on lipid biology with coherent anti-Stokes Raman scattering microscopy. J. Lipid Res. 51, 3091–3102 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans C. L. & Xie X. S. Coherent Anti-Stokes Raman Scattering Microscopy: Chemical Imaging for Biology and Medicine. Annu. Rev. Anal. Chem. 1, 883–909 (2008). [DOI] [PubMed] [Google Scholar]
- Hellerer T. et al. Monitoring of lipid storage in Caenorhabditis elegans using coherent anti-Stokes Raman scattering (CARS) microscopy. P. Natl. Acad. Sci. USA 104, 14658–14663 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camp C. H. et al. High-speed coherent Raman fingerprint imaging of biological tissues. Nat. Photonics 8, 627–634 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolke U., Jezuit E. A. & Priess J. R. Actin-dependent cytoplasmic streaming in C-elegans oogenesis. Development 134, 2227–2236 (2007). [DOI] [PubMed] [Google Scholar]
- R: A language and environment for statistical computing. (R Foundation for Statistical Computing, Vienna, Austria, 2015). Available at: http://www.R-project.org/.
- Sharrock W. J., Sutherlin M. E., Leske K., Cheng T. K. & Kim T. Y. 2 Distinct Yolk Lipoprotein Complexes from Caenorhabditis-Elegans. J. Biol. Chem. 265, 14422–14431 (1990). [PubMed] [Google Scholar]
- Spieth J. & Blumenthal T. The Caenorhabditis-Elegans Vitellogenin Gene Family Includes a Gene Encoding a Distantly Related Protein. Mol. Cell. Biol. 5, 2495–2501 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn-Kirby A. H. et al. Specific polyunsaturated fatty acids drive TRPV-dependent sensory signaling in vivo. Cell 119, 889–900 (2004). [DOI] [PubMed] [Google Scholar]
- Watts J. L., Phillips E., Griffing K. R. & Browse J. Deficiencies in C20 polyunsaturated fatty acids cause behavioral and developmental defects in Caenorhabditis elegans fat-3 mutants. Genetics 163, 581–589 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matorras R. et al. Fatty acid composition of fertilization-failed human oocytes. Hum. Reprod. 13, 2227–2230 (1998). [DOI] [PubMed] [Google Scholar]
- van Montfoort A. P. A., Plosch T., Hoek A. & Tietge U. J. F. Impact of maternal cholesterol metabolism on ovarian follicle development and fertility. J. Reprod. Immunol. 104, 32–36 (2014). [DOI] [PubMed] [Google Scholar]
- Grondahl M. L. et al. The dormant and the fully competent oocyte: comparing the transcriptome of human oocytes from primordial follicles and in metaphase II. Mol. Hum. Reprod. 19, 600–617 (2013). [DOI] [PubMed] [Google Scholar]
- Deline M. L., Vrablik T. L. & Watts J. L. Dietary Supplementation of Polyunsaturated Fatty Acids in Caenorhabditis elegans. Jove-J. Vis. Exp., e50879 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abramoff M. D. M., Paulo J., Ram & Sunanda J. Image processing with Image J. Biophotonics international 11, 36–42 (2004). [Google Scholar]
- Chen W. W. et al. Automated quantitative analysis of lipid accumulation and hydrolysis in living macrophages with label-free imaging. Anal. Bioanal. Chem. 405, 8549–8559 (2013). [DOI] [PubMed] [Google Scholar]
- Chien C. H., Chen W. W., Wu J. T. & Chang T. C. Investigation of lipid homeostasis in living Drosophila by coherent anti-Stokes Raman scattering microscopy. J. Biomed. Opt. 17, 126001 (2012). [DOI] [PubMed] [Google Scholar]
- Brisbin S. et al. A Role for C. elegans Eph RTK Signaling in PTEN Regulation. Dev. Cell. 17, 459–469 (2009). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.