Abstract
Relapse after allogeneic hematopoietic stem cell transplantation (alloHSCT) remains one of the leading causes of mortality in patients with leukemia. Treatment options in this population remain limited, with concern for both increased toxicity and further relapse. We treated 18 patients with acute leukemia for marrow +/- extramedullary relapse after a prior alloHSCT, with a myeloablative cytoreductive regimen including clofarabine, melphalan, and thiotepa, followed by a second or third transplant (HSCT2/3) from the same or different donor. All patients were in remission at the time of HSCT2/3. All evaluable patients engrafted. The most common toxicity was reversible transaminitis associated with clofarabine. Two patients died from transplant-related causes. Seven patients relapsed post-HSCT2/3 and died of disease. Nine of 18 patients are alive and disease-free, with a three-year 49% probability of overall survival. Patients whose remission duration after initial alloHSCT was >6 months achieved superior outcomes (3-year OS 74%, 95% CI: 53-100%), compared with those relapsing within 6 months (0%), (p<0.001). This new cytoreductive regimen has yielded promising results with acceptable toxicity for second transplants in patients with high-risk ALL and AML who relapsed after a prior transplant, using various graft and donor options,. This approach merits further evaluation in collaborative group studies.
Introduction
Allogeneic hematopoietic stem cell transplantation (alloHSCT) plays an important role in the treatment of patients with high-risk hematologic malignancies. Nevertheless, relapse remains a challenging cause of treatment failure after alloHSCT, and is one of the leading causes of mortality for patients transplanted for acute leukemia. Treatment options for patients relapsing post-alloHSCT remain limited, and outcomes after attempts at salvage are often poor due to both increased toxicity and high rates of relapse. We report our experience with a new myeloablative cytoreductive regimen comprising clofarabine, melphalan, and thiotepa (Clo/Mel/Thio) used at Memorial Sloan Kettering Cancer Center for transplantation of hematologic malignancies, which included patients undergoing a second or third HSCT.
Patients and Methods
In 2006 we developed a phase 1/2 protocol (NCT00423514) incorporating escalating doses of clofarabine into a cytoreductive regimen for alloHSCT for patients with hematologic malignancies. Additional agents included melphalan and thiotepa. Grafts allowed on this protocol included unmodified bone marrow (BM), peripheral blood stem cells (PBSC), or umbilical cord blood (UCB). The maximum tolerated clofarabine dose reached was 20 mg/m2 for patients ≥18 years, while younger patients were able to tolerate 30 mg/m2. Subsequently, we added this cytoreductive regimen to our comprehensive T-cell depleted protocol (NCT01119066). We demonstrated reliable engraftment in both of these settings, as well as acceptable rates of toxicity and disease control across all enrolled patients.
We identified 47 patients with hematologic malignancies who relapsed after an initial allogeneic HSCT and received a subsequent transplant between November 2005 and December 2012. Nineteen patients received cytoreduction with Clo/Mel/Thio, including 18 patients with acute leukemia (and one patient with CML who was excluded from this analysis), while 28 patients received other regimens. Other regimens included reduced intensity conditioning (n=7), TBI-based myeloablative conditioning (n=13), and busulfan-based myeloablative conditioning (n=7). Informed consent was obtained with the approval of the MSK Institutional Review and Privacy Board.
Patient and first transplant characteristics
Eighteen patients were identified who received a second (n=16) or third transplant (n=2) after cytoreduction with Clo/Mel/Thio for acute leukemia. Patient and transplant characteristics are summarized in Table 1 and patient-specific data are delineated in Table 2. The median age of the cohort was 19.5 years. Patients had ALL or AML in second or third complete remission (CR2 or CR3). Seven patients had extramedullary disease. Fourteen of 18 patients had previously undergone myeloablative total body irradiation (TBI)-based cytoreduction, while the remaining four patients underwent myeloablative non-TBI based conditioning regimens.
Table 1. Patient and transplant characteristics.
| Overall No. (%) | |
|---|---|
| Age (median, range), in years | 19.5 (4.5-43.7) |
| Age category | |
| <18 years | 7 (55.6) |
| ≥18 years | 11 (44.4) |
| Sex | |
| Male / Female | 12 (66.7) / 6 (33.3) |
| Disease | |
| ALL | 12 (66.7) |
| AML | 6 (33.3) |
| Patient status | |
| CR 2 | 6 (33.3) |
| CR 3 | 12 (66.7) |
| HSCT | |
| 2nd | 16 (88.9) |
| 3rd | 2 (11.1) |
| Graft Source | |
| BM | 7 (38.9) |
| PBSC | 10 (55.6) |
| Double UCB | 1 (5.6) |
| Graft manipulation | |
| TCD | 5 (27.8) |
| Unmodified | 13 (72.2) |
| Donor Category | |
| Related | 8 (44.4) |
| Unrelated | 10 (55.6) |
| Match category | |
| Matched | 11 (61.1) |
| Mismatched | 6 (33.3) |
| Mismatched DUCB | 1 (5.6) |
| Donor relation to initial donor | |
| Different | 5 (27.8) |
| Same | 13 (72.2) |
| Remission duration after previous HSCT | |
| ≤6 months | 6 (33.3) |
| >6 months | 12 (66.7) |
Table 2. Specific patient and transplant characteristics and outcomes.
| Patient # |
Age (y) at SCT |
Gender | Diagnosis/ Stage |
Extramedullary Disease |
Prior transplant regimens |
Remission duration after initial HSCT (months) |
Time between previous and current HSCT (months) |
Donor match and relation |
Donor relationship to previous donor |
Graft source |
Graft manipulation |
Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 39 | F | ALL, pre-B/ CR3 | none | TBI/Etop Bu/Flu | 36 | 60.2 | 8/10, related | same | PBSC | none | alive |
| 2 | 8 | M | ALL, pre-B/ CR3 | none | TBI/Thio/Cy | 7 | 10.3 | 10/10, related | same | BM | none | deceased-relapse |
| 3 | 23 | M | ALL, pre-B/ CR3 | none | TBI/Thio/Cy | 4 | 8.4 | 10/10, related | same | BM | none | deceased -relapse |
| 4 | 5.4 | F | ALL, pre-B/ CR3 | none | TBI/Thio/Cy | 6 | 11.2 | 8/10, unrelated | same | BM | none | deceased -relapse |
| 5 | 4.5 | M | ALL, pre-B/ CR3 | CNS | TBI/Thio/Cy | 8 | 11.7 | 10/10, related | same | BM | none | alive |
| 6 | 5.2 | M | ALL, pre-B/ CR2 | CNS | TBI/Cy | 24 | 29.2 | 10/10, unrelated | different | BM | none | alive |
| 7 | 8 | M | ALL, pre-B/ CR2 | CNS | TBI/Thio/Cy | 12 | 15.6 | 10/10, related | same | PBSC | none | alive |
| 8 | 28.8 | F | ALL, pre-B/ CR2 | none | TBI/Thio/Flu | 4 | 8.5 | 10/10, related | same | PBSC | none | deceased -relapse |
| 9 | 9 | F | ALL, pre-B/ CR3 | none | TBI/Cy | 11 | 15.9 | 5/6 & 4/6, unrelated | different | cord | none | alive |
| 10 | 25.9 | M | ALL, pre-B/ CR3 | CNS | TBI/Thio/Flu | 31 | 36.6 | 10/10, unrelated | same | PBSC | CD34+ Isolex/E- | alive |
| 11 | 17.6 | M | ALL, pre-B/ CR3 | Testis, nodal, CNS | TBI/Cy | 72 | 73.9 | 10/10, related | same | BM | none | deceased -relapse |
| 12 | 26.7 | M | ALL, T-cell/ CR2 | 1° bone lesion | TBI/Cy | 24 | 31.2 | 10/10, related | same | PBSC | none | alive |
| 13 | 18.5 | M | AML / CR3 | none | Bu/Flu | 3.3 | 13.8 | 9/10, unrelated | different | PBSC | CD34+ CliniMACS | deceased -relapse |
| 14 | 40.8 | F | AML / CR2 | none | TBI/Thio/Cy | 11 | 14.9 | 10/10, unrelated | same | PBSC | none | deceased -relapse |
| 15 | 18.8 | M | AML / CR2 | none | Bu/Mel/Flu | 8 | 13.3 | 9/10, unrelated | different | PBSC | CD34+ CliniMACS | alive |
| 16 | 38.7 | M | AML / CR3 | none | Bu/Mel/Flu | 45.5 | 49.4 | 8/10, unrelated | same | PBSC | CD34+ CliniMACS | alive |
| 17 | 20.2 | F | AML / CR3 | none | Bu/Cy Mel/Flu | 5 | 8.5 | 9/10, unrelated | different | PBSC | CD34+ CliniMACS | deceased -infection |
| 18 | 43.7 | M | AML / CR3 | CNS | TBI/Thio/Cy | 4 | 9 | 10/10, unrelated | same | BM | none | deceased -TRM |
Second transplant regimen and characteristics
Patients received a novel chemotherapy-only based cytoreduction regimen, consisting of clofarabine, melphalan, and thiotepa. Clofarabine was administered at doses of 20 (n=16) or 30 (n=2) mg/m2/dose for five days on day -9 to day -5. Thiotepa was administered at a total dose of 10 mg/m2 as a single dose on day -4. Melphalan was administered at a dose of 70 mg/m2/dose on days -3 and -2. There were 1-3 day delays in cytoreduction in five patients due to transaminitis attributed to clofarabine. Filgrastim was administered from day +7 until engraftment for all patients. Patients received standard antimicrobial prophylaxis and transfusion support as per institutional guidelines.
Graft sources included bone marrow (n=7), peripheral blood (n=10), or double umbilical cord bloods (n=1). All bone marrow grafts were unmodified, and peripheral blood stem cell (PBSC) grafts were either unmodified (n=5) or T-cell depleted (TCD) (n=5). TCD was performed via CD34+ selection using the CliniMACS CD34 Reagent System (1) (Miltenyi Biotec, Bergisch Gladbach, Germany) (n=4), or with the ISOLEX 300i Magnetic Cell Separator (2) (Baxter Health care Corporation, Dearfield, IL, USA) followed by sheep RBC rosette depletion (E-rosetting) (3) (n=1). Patients who received unmodified grafts received tacrolimus plus either methotrexate or mycophenolate mophetil (MMF) for graft versus host disease (GVHD) prophylaxis. Patients who received TCD grafts received antithymocyte globulin (ATG) for rejection prophylaxis with no further agents for GVHD prophylaxis. Thirteen patients received grafts from the same donors as their prior transplants. Five patients received grafts from new donors, including matched (n=1) or mismatched (n=3) unrelated donors, and one patient received an unrelated double umbilical cord blood (DUCB) graft. Pre-transplant HLA typing was available at the allele level for HLA A, B, C, DRB1, DQB1 loci for bone marrow and peripheral blood grafts, but at only HLA A, B, DRB1 loci for the umbilical cord blood graft.
Outcome definitions
Neutrophil engraftment was defined as the first of 3 consecutive days of an absolute neutrophil count > 0.5 × 109/L. Platelet engraftment was defined as a platelet count >20 × 109/L without transfusion support. All patients surviving to engraftment were evaluated for acute GVHD. Patients surviving for at least 100 days were evaluated for chronic GVHD. GVHD was diagnosed and scored based on Center for International Blood and Marrow Transplant Research (CIBMTR) criteria for acute GVHD (4) and NIH consensus criteria for chronic GVHD (5). Regimen-related toxicities included all non-hematologic toxicities occurring early post-transplant (up to 100 days). These were graded according to standard NCI common toxicity criteria (CTCAE v.4.03).
Relapse was defined as evidence of hematologic, cytogenetic, and/or molecular recurrence of primary disease at any site, in host cells. Disease status and survival information was based on patient status at last follow up.
Statistical methods
Overall survival (OS) time was calculated from date of 2nd or 3rd transplant to date of death from any cause. Disease-free survival (DFS) time was calculated from date of 2nd or 3rd transplant to date of relapse or death from disease. The Kaplan-Meier method was used to estimate OS and DFS. The cumulative incidence of relapse was estimated in a competing risks framework, with deaths without relapse treated as a competing event. Differences in OS, DFS, and relapse between groups were tested using the permutation log-rank test. A p-value <0.05 was considered statistically significant. All statistical analyses were performed using R software version 3.1.1 (R Core Development Team, Vienna, Austria).
Results
Hematopoietic recovery
All patients engrafted neutrophils at a median of 12 days (range 9-25). Two patients died before documented platelet engraftment, while the remaining patients engrafted platelets at a median of 24 days (range 14-98). All surviving patients had normal blood counts, and all surviving patients with available chimerism data post-transplant (n=8) maintained 100% donor status at last follow-up.
Regimen related toxicity and GVHD
Grade 3-4 toxicities are summarized in Table 3, and included mucositis, hypoxia, and renal dysfunction; toxicities resolved in all patients except in two patients who suffered early transplant-related mortality (TRM). Overall toxicity with this regimen was comparable to that of other myeloablative transplant regimens. One exception was grade 3-4 transaminitis associated with clofarabine, which occurred transiently in 14/18 patients. TRM was due to veno-occlusive disease/sinusoidal obstructive syndrome (VOD/SOS) and multi-system organ failure in one patient and EBV post-transplant lymphoproliferative disease (PTLD) in another. These two patients both experienced grade 4-5 hypoxia/respiratory failure and grade 4-5 renal failure.
Table 3. Toxicity (grade III-IV) and HSCT outcome.
| Toxicity or outcome | Overall No. (%) |
|---|---|
| Mucositis | 4 (22) |
| Transaminitis | 14 (78) |
| Renal toxicity | 3 (17) |
| Hypoxia | 2 (11) |
| Respiratory failure | 2 (11) |
| Veno-occlusive disease (VOD) | 1 (6) |
|
| |
| Acute GVHD, grade II-IV | 3 (17) |
| Chronic GVHD | 3 (17) |
|
| |
| Transplant-related mortality | 2 (11) |
|
| |
| Relapse | 7 (39) |
Acute grade II-III GVHD was diagnosed in 3 patients (two with grade II and one with grade III), all recipients of unmodified grafts. No patient had grade IV GVHD. One patient, who received an unmodified PBSC graft from a 7/8 related donor, developed limited chronic GVHD. Two patients developed extensive chronic GVHD; one patient following an unmodified PBSC graft from a matched sibling, and the other patient following an unmodified BM graft from a partially matched (6/8) unrelated donor.
Outcome
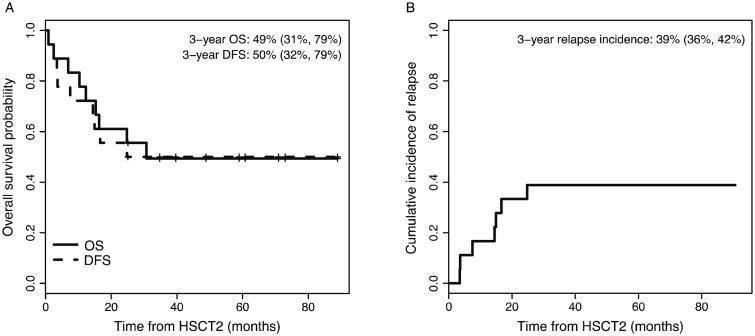
The three-year overall survival for the entire cohort was 49% (95% CI: 31%, 79%) with a median follow-up of 59 months (range: 25.1-88.9) (Figure 1). Seven patients relapsed post-HSCT, six patients following unmodified grafts and one patient following a CliniMACS CD34+-selected PBSC graft, and all patients who relapsed after this HSCT ultimately succumbed to their disease.
Figure 1.
Overall and disease-free survival curves (A), and cumulative incidence of relapse (B) across all patients.
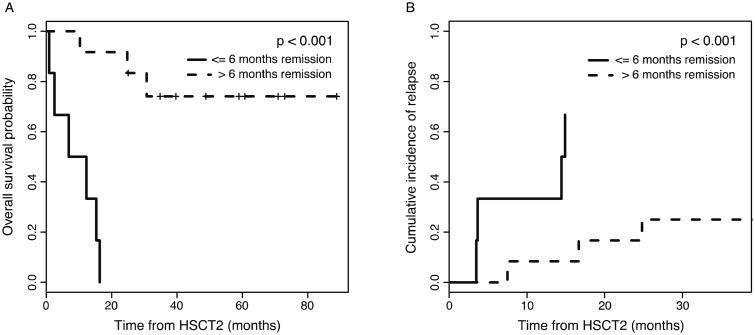
Outcome measures were analyzed in the context of prognostic and risk factors. There was no significant difference in OS or incidence of relapse for patients with the following characteristics: (1) age < or ≥ 21 years, (2) grafts from same or different donor, (3) myeloid vs. lymphoid disease, (4) second or third complete remission at present HSCT, (5) TCD vs. unmodified grafts. The only significant risk factor was remission duration following prior transplant (Figure 2). Patients who relapsed more than 6-months post-transplant (n=12) had a superior outcome, with a 3-year OS of 74% (95% CI: 53%-100%), as compared to patients who relapsed within 6-months (n=6) following their prior HSCT (no long-term survivors) (p<0.001). The cause of mortality in the latter population was relapse (n=4) or transplant-related (n=2). The cumulative incidence of relapse was also significantly lower in the late-relapse group (p<0.001; Figure 2). It is important to note that among patients with extramedullary disease at relapse following their prior HSCT, who received Clo/Mel/Thio conditioning for their second allo-HSCT, only one died of relapse in the form of nodal disease, one died of transplant-related mortality, and five are long-term survivors.
Figure 2.
Overall survival (A) and cumulative incidence of relapse (B) stratified by remission duration of ≤6 months or >6 months, demonstrating significantly improved survival and lower incidence of relapse for patients with remission durations greater than 6 months following their initial allo-HSCT (p<0.001).
We compared results of this Clo/Mel/Thio regimen to that of patients who underwent second transplants in remission using other regimens at our institution during the same time period. Of 28 second or third transplants performed using cytoreductive regimens other than Clo/Mel/Thio, 22 were performed for acute leukemia, and 13 of those patients were in complete remission at HSCT. The majority of these patients (12/13) had received non-TBI containing cytoreduction, primarily busulfan based (n=10), for their first transplant, whereas 14/18 patients in our cohort had received TBI containing cytoreductive regimens for their first transplant. Only 2/13 (15%) of these patients are long-term survivors. Causes of death of this patient cohort included relapse (n=7; 54%) or TRM (n=4; 31%).
Discussion
Allogeneic HSCT plays an important role in the treatment of high-risk leukemia. Unfortunately, relapse after alloHSCT is still one of the most frequent causes of death in these patients. These patients have a dismal prognosis, with long-term survival rates of <10% in most historical series (6-9). Long-term survivors after a post-transplant relapse are almost exclusively recipients of a secondary alloHSCT, and this has therefore become the recommended therapeutic approach for this patient population (10). Relapses are common among patients who undergo a secondary alloHSCT, occurring in more than 50% of patients. TRM is also high, exceeding 40%, and long-term DFS is below 30% even in more modern series (11-16). Approaches using reduced-intensity or non-myeloablative conditioning regimens have been unable to improve either relapse or non-relapse mortality, and ultimately have rates of long-term overall survival comparable to those of earlier studies (18-29%) (17-19). We report a novel cytoreductive regimen with activity in this very high-risk group of patients, with a 3-year disease-free survival of 50% (95% CI 32%-79%), as well as tolerable toxicity with a low transplant-related mortality rate of 11%.
The challenge in designing second myeloablative transplant regimens for patients who have relapsed after HSCT is the need to balance potent anti-leukemic effects with acceptable toxicity, especially in patients who have received prior TBI. Clofarabine was approved in 2004 for the treatment of relapsed or refractory ALL, and has been further studied as an effective regimen in both MDS and AML (20-23). In 2006, we developed a phase 1-2 protocol incorporating clofarabine at escalating doses into a cytoreductive regimen for hematologic malignancies in order to avoid the use of TBI. Melphalan and thiotepa were chosen as additional myeloablative (Mel/Thio), anti-leukemic (Mel/Thio), and immunosuppressive (Thio) agents with non-overlapping toxicities. We postulated that the potent anti-leukemic effects of clofarabine would obviate the need for TBI in these high-risk patients, as well as provide additional immune suppression to allow consistent engraftment, regardless of graft source or manipulation. Indeed, in our series, all patients engrafted with donor cells, and all survivors had full donor chimerism at last follow-up. In addition, this phase 1-2 trial and other recent studies (24) have shown that clofarabine dosing was well tolerated at 30 mg/m2/dose x5 in combination with other agents in pediatric patients.
Despite very poor outcomes for patients relapsing after alloHSCT, subgroups have emerged for whom second alloHSCT appears to be more beneficial. Specifically, patients who were initially transplanted in CR and those with longer remissions (>6 months) following first alloHSCT consistently had better outcomes than those re-transplanted with disease or following an early relapse (≤6 months) (11-14, 16, 25-27). Data regarding survival or relapse differences in other subgroups (e.g., age, GVHD, new donor selection) have been inconsistent across studies or shown to be insignificant in larger series (11, 13, 16, 17, 28-30). As CR status at the time of second transplant has been consistently crucial to long-term disease-free survival in this patient population (14, 15, 28), it is important to note that all patients in this series were in remission at the time of transplant. Thirteen of 18 patients were disease-free by cytogenetic or molecular assessment, and the remainder were in CR as assessed by morphology alone. As in previous series, remission duration after initial transplant was significantly correlated with relapse; patients who relapsed >6 months after their prior transplant had relapse rates of only 8% (1/12) at 1 year and 25% (3/12) at 3 years, while those who relapsed within 6 months of their initial alloHSCTs either died of TRM (2/6) or eventually relapsed and died of their disease (4/6). Our results demonstrate the efficacy of this regimen with respect to leukemia control, as well as its safety, with non-relapse mortality of only 11% in a heavily pre-treated patient population.
The graft versus leukemia (GvL) effect has often been implicated in successful outcomes following a second HSCT, and is often attributed to the T-cell component of the graft. Donor lymphocyte infusions (DLI) and transplant regimens with haploidentical grafts have been used for post-transplant relapse, hoping to leverage this effect (9, 31). In our cohort we did not find any difference in outcomes between our T-cell replete and T-cell depleted grafts, demonstrating the robustness of this regimen for TCD alloHSCTs. This is consistent with prior reports showing similar OS and RFS in patients with AML or ALL who received TCD grafts and unmodified grafts (32-34).
We report a novel chemotherapy-only cytoreductive regimen for patients who have relapsed acute leukemia after prior alloHSCT, with promising outcomes for this very poor-risk group. Transplant-related mortality was low in this series of high-risk patients, and rates of disease-free survival similar to patients with late-stage hematologic malignancies (35, 36). Other advantages of this regimen include its apparent efficacy for both myeloid and lymphoid malignancies, its tolerability by both children and adults, and its ability to support consistent engraftment regardless of graft source or manipulation. This salvage treatment offers an excellent choice for cytoreduction for second alloHSCT in relapsed patients and merits further study and evaluation in collaborative group studies.
Acknowledgments
This work was supported in part by Genzyme-Sanofi and by NIH/NCI Cancer Center Support Grants P30 CA008748 and P01 CA023766.
Footnotes
The authors declare no other conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Peters C, Matthes-Martin S, Fritsch G, et al. Transplantation of highly purified peripheral blood CD34+ cells from HLA-mismatched parental donors in 14 children: evaluation of early monitoring of engraftment. Leukemia. 1999;13:2070–2078. doi: 10.1038/sj.leu.2401577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collins NH, Carabasi MH, Bleau S, et al. New technology for the depletion of T cells from soybean lectin agglutinated, HLA-matched bone marrow grafts for leukemia: initial laboratory and clinical results. Prog Clin Biol Res. 1992;377:427–439. [PubMed] [Google Scholar]
- 3.Reisner Y, Kapoor N, Kirkpatrick D, et al. Transplantation for severe combined immunodeficiency with HLA-A,B,D,DR incompatible parental marrow cells fractionated by soybean agglutinin and sheep red blood cells. Blood. 1983;61:341–348. [PubMed] [Google Scholar]
- 4.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 5.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11:945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 6.Frassoni F, Barrett AJ, Granena A, et al. Relapse after allogeneic bone marrow transplantation for acute leukaemia: a survey by the E.B.M.T. of 117 cases. Br J Haematol. 1988;70:317–320. doi: 10.1111/j.1365-2141.1988.tb02488.x. [DOI] [PubMed] [Google Scholar]
- 7.Mehta J, Powles R, Treleaven J, et al. Outcome of acute leukemia relapsing after bone marrow transplantation: utility of second transplants and adoptive immunotherapy. Bone Marrow Transplant. 1997;19:709–719. doi: 10.1038/sj.bmt.1700720. [DOI] [PubMed] [Google Scholar]
- 8.Mortimer J, Blinder MA, Schulman S, et al. Relapse of acute leukemia after marrow transplantation: natural history and results of subsequent therapy. J Clin Oncol. 1989;7:50–57. doi: 10.1200/JCO.1989.7.1.50. [DOI] [PubMed] [Google Scholar]
- 9.Bejanyan N, Weisdorf DJ, Logan BR, et al. Survival of patients with acute myeloid leukemia relapsing after allogeneic hematopoietic cell transplantation: a center for international blood and marrow transplant research study. Biol Blood Marrow Transplant. 2015;21:454–459. doi: 10.1016/j.bbmt.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thakar MS, Forman SJ. ASH evidence-based guidelines: is there a role for second allogeneic transplant after relapse? Hematology Am Soc Hematol Educ Program. 2009:414–418. doi: 10.1182/asheducation-2009.1.414. [DOI] [PubMed] [Google Scholar]
- 11.Bosi A, Laszlo D, Labopin M, et al. Second allogeneic bone marrow transplantation in acute leukemia: results of a survey by the European Cooperative Group for Blood and Marrow Transplantation. J Clin Oncol. 2001;19:3675–3684. doi: 10.1200/JCO.2001.19.16.3675. [DOI] [PubMed] [Google Scholar]
- 12.Kishi K, Takahashi S, Gondo H, et al. Second allogeneic bone marrow transplantation for post-transplant leukemia relapse: results of a survey of 66 cases in 24 Japanese institutes. Bone Marrow Transplant. 1997;19:461–466. doi: 10.1038/sj.bmt.1700680. [DOI] [PubMed] [Google Scholar]
- 13.Michallet M, Tanguy ML, Socie G, et al. Second allogeneic haematopoietic stem cell transplantation in relapsed acute and chronic leukaemias for patients who underwent a first allogeneic bone marrow transplantation: a survey of the Societe Francaise de Greffe de moelle (SFGM) Br J Haematol. 2000;108:400–407. doi: 10.1046/j.1365-2141.2000.01851.x. [DOI] [PubMed] [Google Scholar]
- 14.Mrsic M, Horowitz MM, Atkinson K, et al. Second HLA-identical sibling transplants for leukemia recurrence. Bone Marrow Transplant. 1992;9:269–275. [PubMed] [Google Scholar]
- 15.Kato M, Horikoshi Y, Okamoto Y, et al. Second allogeneic hematopoietic SCT for relapsed ALL in children. Bone Marrow Transplant. 2012;47:1307–1311. doi: 10.1038/bmt.2012.29. [DOI] [PubMed] [Google Scholar]
- 16.Ruutu T, de Wreede LC, van Biezen A, et al. Second allogeneic transplantation for relapse of malignant disease: retrospective analysis of outcome and predictive factors by the EBMT. Bone Marrow Transplant. 2015;50:1542–1550. doi: 10.1038/bmt.2015.186. [DOI] [PubMed] [Google Scholar]
- 17.Christopoulos P, Schmoor C, Waterhouse M, et al. Reduced-intensity conditioning with fludarabine and thiotepa for second allogeneic transplantation of relapsed patients with AML. Bone Marrow Transplant. 2013;48:901–907. doi: 10.1038/bmt.2012.267. [DOI] [PubMed] [Google Scholar]
- 18.Devine SM, Sanborn R, Jessop E, et al. Fludarabine and melphalan-based conditioning for patients with advanced hematological malignancies relapsing after a previous hematopoietic stem cell transplant. Bone Marrow Transplant. 2001;28:557–562. doi: 10.1038/sj.bmt.1703198. [DOI] [PubMed] [Google Scholar]
- 19.Grullich C, Bertz H, Spyridonidis A, Muller CI, Finke J. A fludarabine, thiotepa reduced toxicity conditioning regimen designed specifically for allogeneic second haematopoietic cell transplantation after failure of previous autologous or allogeneic transplantation. Bone Marrow Transplant. 2008;41:845–850. doi: 10.1038/sj.bmt.1705989. [DOI] [PubMed] [Google Scholar]
- 20.Cooper TM, Alonzo TA, Gerbing RB, et al. AAML0523: A report from the Children's Oncology Group on the efficacy of clofarabine in combination with cytarabine in pediatric patients with recurrent acute myeloid leukemia. Cancer. 2014;120:2482–2489. doi: 10.1002/cncr.28674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Locke F, Agarwal R, Kunnavakkam R, et al. A novel clofarabine bridge strategy facilitates allogeneic transplantation in patients with relapsed/refractory leukemia and high-risk myelodysplastic syndromes. Bone Marrow Transplant. 2013;48:1437–1443. doi: 10.1038/bmt.2013.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shukla N, Kobos R, Renaud T, Steinherz LJ, Steinherz PG. Phase II trial of clofarabine with topotecan, vinorelbine, and thiotepa in pediatric patients with relapsed or refractory acute leukemia. Pediatr Blood Cancer. 2014;61:431–435. doi: 10.1002/pbc.24789. [DOI] [PubMed] [Google Scholar]
- 23.Vigil CE, Tan W, Deeb G, et al. Phase II trial of clofarabine and daunorubicin as induction therapy for acute myeloid leukemia patients greater than or equal to 60 years of age. Leuk Res. 2013;37:1468–1471. doi: 10.1016/j.leukres.2013.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nelken B, Cave H, Leverger G, et al. A phase I study of clofarabine with multiagent chemotherapy in childhood high risk relapse of acute lymphoblastic leukemia (VANDEVOL study of the French SFCE acute leukemia committee) Pediatr Blood Cancer. 2015 doi: 10.1002/pbc.25751. [DOI] [PubMed] [Google Scholar]
- 25.Duncan CN, Majhail NS, Brazauskas R, et al. Long-term survival and late effects among one-year survivors of second allogeneic hematopoietic cell transplantation for relapsed acute leukemia and myelodysplastic syndromes. Biol Blood Marrow Transplant. 2015;21:151–158. doi: 10.1016/j.bbmt.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Konuma T, Kato S, Ooi J, et al. Second allogeneic transplantation using unrelated cord blood for relapsed hematological malignancies after allogeneic transplantation. Leuk Lymphoma. 2015:1–7. doi: 10.3109/10428194.2015.1045900. [DOI] [PubMed] [Google Scholar]
- 27.Naik S, Martinez C, Leung K, et al. Outcomes after Second Hematopoietic Stem Cell Transplantations in Pediatric Patients with Relapsed Hematological Malignancies. Biol Blood Marrow Transplant. 2015;21:1266–1272. doi: 10.1016/j.bbmt.2015.02.024. [DOI] [PubMed] [Google Scholar]
- 28.Christopeit M, Kuss O, Finke J, et al. Second allograft for hematologic relapse of acute leukemia after first allogeneic stem-cell transplantation from related and unrelated donors: the role of donor change. J Clin Oncol. 2013;31:3259–3271. doi: 10.1200/JCO.2012.44.7961. [DOI] [PubMed] [Google Scholar]
- 29.Eapen M, Giralt SA, Horowitz MM, et al. Second transplant for acute and chronic leukemia relapsing after first HLA-identical sibling transplant. Bone Marrow Transplant. 2004;34:721–727. doi: 10.1038/sj.bmt.1704645. [DOI] [PubMed] [Google Scholar]
- 30.Spyridonidis A, Labopin M, Schmid C, et al. Outcomes and prognostic factors of adults with acute lymphoblastic leukemia who relapse after allogeneic hematopoietic cell transplantation. An analysis on behalf of the Acute Leukemia Working Party of EBMT. Leukemia. 2012;26:1211–1217. doi: 10.1038/leu.2011.351. [DOI] [PubMed] [Google Scholar]
- 31.Tischer J, Engel N, Fritsch S, et al. Second haematopoietic SCT using HLA-haploidentical donors in patients with relapse of acute leukaemia after a first allogeneic transplantation. Bone Marrow Transplant. 2014;49:895–901. doi: 10.1038/bmt.2014.83. [DOI] [PubMed] [Google Scholar]
- 32.Bayraktar UD, de Lima M, Saliba RM, et al. Ex vivo T cell-depleted versus unmodified allografts in patients with acute myeloid leukemia in first complete remission. Biol Blood Marrow Transplant. 2013;19:898–903. doi: 10.1016/j.bbmt.2013.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hobbs GS, Hamdi A, Hilden PD, et al. Comparison of outcomes at two institutions of patients with ALL receiving ex vivo T-cell-depleted or unmodified allografts. Bone Marrow Transplant. 2015;50:493–498. doi: 10.1038/bmt.2014.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pasquini MC, Devine S, Mendizabal A, et al. Comparative outcomes of donor graft CD34+ selection and immune suppressive therapy as graft-versus-host disease prophylaxis for patients with acute myeloid leukemia in complete remission undergoing HLA-matched sibling allogeneic hematopoietic cell transplantation. J Clin Oncol. 2012;30:3194–3201. doi: 10.1200/JCO.2012.41.7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holtick U, Albrecht M, Chemnitz JM, et al. Bone marrow versus peripheral blood allogeneic haematopoietic stem cell transplantation for haematological malignancies in adults. Cochrane Database Syst Rev. 2014;4:CD010189. doi: 10.1002/14651858.CD010189.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stem Cell Trialists' Collaborative G. Allogeneic peripheral blood stem-cell compared with bone marrow transplantation in the management of hematologic malignancies: an individual patient data meta-analysis of nine randomized trials. J Clin Oncol. 2005;23:5074–5087. doi: 10.1200/JCO.2005.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]