Abstract
Rho-associated kinases play an important role in a variety of cellular functions. Although Rho-associated kinase activity has been shown to be an independent predictor for future cardiovascular events in a general population, there is no information on Rho-associated kinase activity in patients with acute coronary syndrome. We evaluated leukocyte Rho-associated kinase activity by Western blot analysis in 73 patients with acute coronary syndrome and 73 age- and gender-matched control subjects. Rho-associated kinase activity within 2 hours of acute coronary syndrome onset was higher in patients with acute coronary syndrome than in the control subjects (0.95±0.55 versus 0.69±0.31; P<0.001). Rho-associated kinase activity promptly increased from 0.95±0.55 to 1.11±0.81 after 3 hours and reached a peak of 1.21±0.76 after 1 day (P=0.03 and P=0.03, respectively) and then gradually decreased to 0.83±0.52 after 7 days, 0.78±0.42 after 14 days, and 0.72±0.30 after 6 months (P=0.22, P=0.29, and P=0.12, respectively). During a median follow-up period of 50.8 months, 31 first major cardiovascular events (death from cardiovascular causes, myocardial infarction, ischemic stroke, and coronary revascularization) occurred. After adjustment for age, sex, cardiovascular risk factors, and concomitant treatment with statins, increased Rho-associated kinase activity was associated with increasing risk of first major cardiovascular events (hazard ratio, 4.56; 95% confidence interval, 1.98–11.34; P<0.001). These findings suggest that Rho-associated kinase activity is dramatically changed after acute coronary syndrome and that Rho-associated kinase activity could be a useful biomarker to predict cardiovascular events in Japanese patients with acute coronary syndrome.
Keywords: acute coronary syndrome, biological markers, cardiovascular diseases, myocardial infarction, stroke
Rho-associated kinase (ROCK) is one of the first downstream targets of the small guanosine triphosphate–binding protein RhoA and plays a pivotal role in the regulation of vascular smooth muscle contraction, endothelial function, and a variety of cellular functions, including cell proliferation, migration, adhesion, and apoptosis.1–7 ROCK has been shown to contribute to atherosclerotic lesion formation, neointimal formation, vascular remodeling, and cardiac hypertrophy in vivo.8–11 In addition, several lines of clinical evidence have shown that activation of ROCK is associated with angina pectoris, vasospastic angina, pulmonary hypertension, heart failure, stroke, and even smoking.12–18 We previously showed that leukocyte ROCK activity substantially correlated with endothelial function and Framingham risk score19 and could predict future cardiovascular events in subjects with cardiovascular risk factors, as well as in healthy subjects.20 ROCK may be intimately involved in the pathogenesis of cardiovascular events.
Acute coronary syndrome (ACS) is well known as a critical disease among numerous cardiovascular diseases that lead to fatal outcomes. Therefore, it is clinically important to prevent fatal prognosis of cardiovascular complications in patients with ACS. It is postulated that ACS is also associated with increased ROCK activity. Indeed, several investigators have shown that ROCK is activated in experimental models of acute myocardial infarction (AMI), that ROCK activity plays an important role in left ventricular function and remodeling after AMI, and that inhibition of ROCK activity limits infarct size in these models.21–27
However, there is no information on ROCK activity under the condition of ACS, including AMI, in humans. Therefore, we evaluated leukocyte ROCK activity in Japanese patients with ACS within 2 hours of ACS onset and at 3 hours, 24 hours, 7 days, 14 days, and 6 months after ACS onset and the prognostic value of ROCK activity for future cardiovascular events in patients with ACS.
Methods
Subjects
Between August 2008 and January 2011, we enrolled a total of 73 patients with ACS (60 men and 13 women; mean age, 66±11 years) who were admitted to the Coronary Care Unit of Hiroshima City Asa Hospital and 73 age- and gender-matched control subjects (59 men and 14 women; mean age, 66±11 years) who underwent health screening examinations at Hiroshima University Hospital. ACS included AMI and unstable angina requiring hospitalization, as previously defined.28 All patients underwent angiography to document the responsible coronary lesions within 24 hours after the onset of chest pain. The definition of hypertension was based on a self-reported diagnosis from a doctor or systolic blood pressure of >140 mm Hg or diastolic blood pressure of >90 mm Hg, in a sitting position, on at least 3 different occasions. Diabetes mellitus was defined according to the American Diabetes Association.29 Dyslipidemia was defined according to the third report of the National Cholesterol Education Program.30 We defined smokers as those who had ever smoked. The ethical committees of our institutions approved the study protocol. Written informed consent for participation in the study was obtained from all of the subjects.
ROCK Activity and Cardiovascular Events During the Follow-Up Period
ROCK activity was measured at the following time points: within 2 hours of ACS onset and at 3 hours, 24 hours, 7 days, 14 days, and 6 months after ACS onset. Additional details are available in the online-only Data Supplement.
Measurement of ROCK Activity
ROCK activity was assayed in peripheral blood leukocytes as the amount of phospho-Thr853 in the myosin-binding subunit (MBS) of myosin light-chain phosphatase, because MBS of myosin light-chain phosphatase is one of the downstream targets of ROCK. Blood was collected at room temperature in heparinized tubes (20 U/mL). Additional details are available in the online-only Data Supplement.
Statistical Analysis
Results are presented as mean±SD for continuous variables and as percentages for categorical variables. Statistical significance was set at a level of P<0.05. Comparison of continuous variables between 2 groups was performed using the Mann–Whitney U test or the χ2 test for categorical data. Changes of ROCK activity after ACS were analyzed by ANOVA for repeated measures by the Bonferroni correction for multipaired comparisons. The receiver operating characteristic (ROC) curve analyses were performed to assess the sensitivity and specificity of measurement of ROCK activity for predicting first major cardiovascular events. Time-to-event end point analyses were performed using the Kaplan–Meier method. A log-rank test was used to compare survival in the groups. Multivariable Cox proportional hazard regression analysis was performed to assess the association between ROCK activity and cardiovascular events in addition to univariate analysis. The data were processed using the software package Stata, version 9 (Stata Co, College Station, TX).
Results
ROCK Activity in Patients With ACS
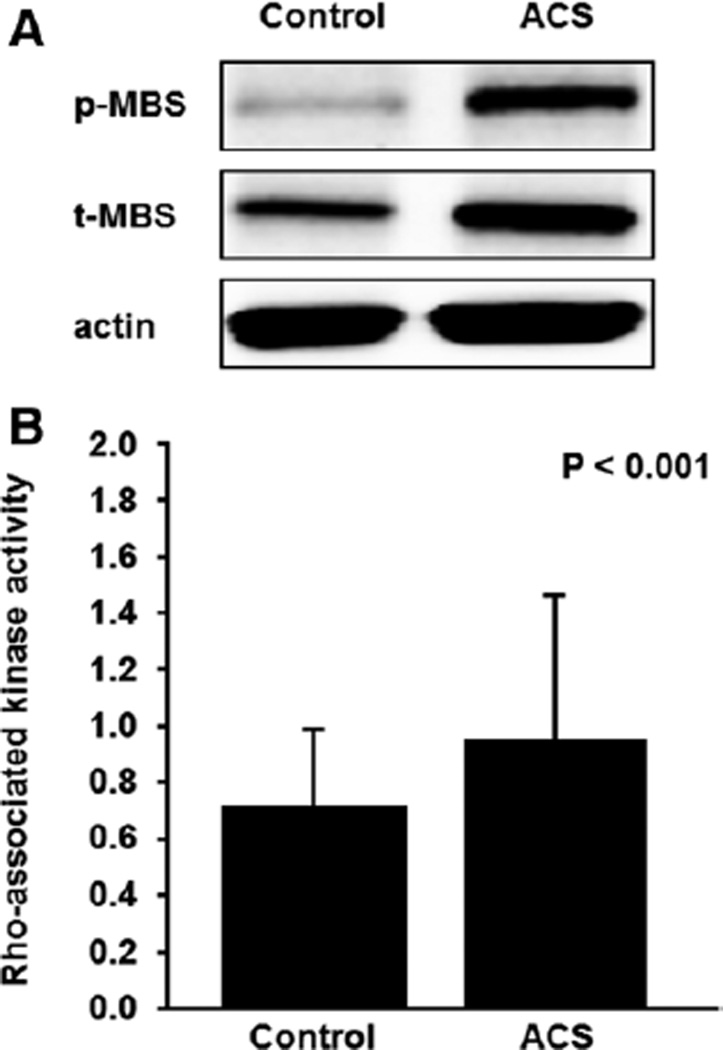
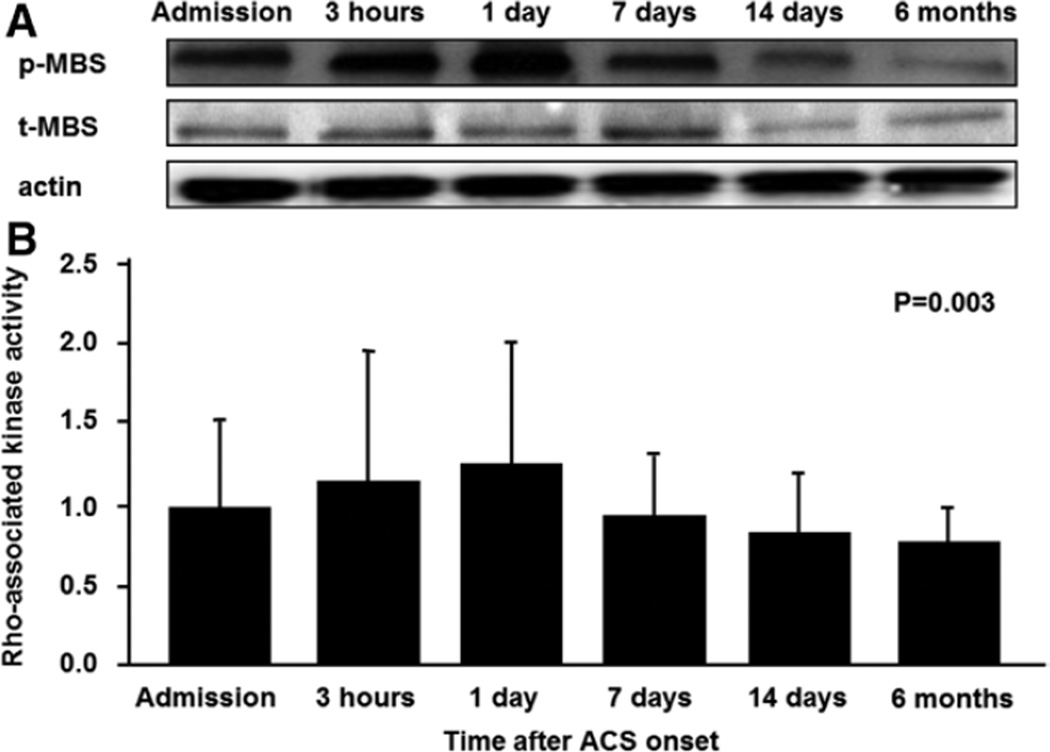
The baseline clinical characteristics of control subjects and patients with ACS are summarized in Table 1. ROCK activity within 2 hours of ACS onset was higher in patients with ACS than in the control subjects (0.95±0.55 versus 0.69±0.31, P<0.001; Figure 1; Figure S1A in the online-only data Supplement). ROCK activity promptly increased from 0.95±0.55 to 1.11±0.81 after 3 hours and reached a peak of 1.21±0.76 after 1 day (P=0.03 and P=0.03, respectively) and then gradually decreased to 0.83±0.52 after 7 days, 0.78±0.42 after 14 days, and 0.72±0.30 after 6 months (P=0.22, P=0.29, and P=0.12, respectively). The expression of total MBS was similar in the 2 groups and in all of the follow-up periods in patients with ACS (Figure 2, Figure S1B).
Table 1.
Clinical Characteristics of the Control Subjects and Patients with ACS
| Variable | Control Subjects, n=73 | Patients With ACS, n=73 | P Value |
|---|---|---|---|
| Age, y | 66±11 | 66±11 | 0.70 |
| Sex, men/women | 59/14 | 60/13 | 0.83 |
| Body mass index, kg/m2 | 23.8±4.1 | 23.9±3.1 | 0.93 |
| Systolic blood pressure, mm Hg | 133±21 | 144±28 | 0.007 |
| Diastolic blood pressure, mm Hg | 75±13 | 80±18 | 0.09 |
| Heart rate, bpm | 70±11 | 78±19 | 0.003 |
| Medical history, n (%) | |||
| Hypertension | 56 (76.7) | 55 (75.3) | 0.85 |
| Dyslipidemia | 50 (68.5) | 52 (71.2) | 0.72 |
| Diabetes mellitus | 30 (41.1) | 29 (39.7) | 0.87 |
| Smoker | 54 (74.0) | 57 (78.1) | 0.56 |
| Laboratory determinations | |||
| Total cholesterol, mmol/L | 5.17±1.06 | 5.35±1.06 | 0.23 |
| Triglycerides, mmol/L | 1.44±0.96 | 1.58±0.86 | 0.14 |
| High-density lipoprotein cholesterol, mmol/L | 1.44±0.41 | 1.32±0.36 | 0.12 |
| Low-density lipoprotein cholesterol, mmol/L | 3.10±0.88 | 3.47±0.88 | 0.02 |
| Glucose, mmol/L | 6.83±3.55 | 9.27±3.50 | <0.001 |
| Medications, n (%) | |||
| Antiplatelets | 10 (13.7) | 9 (12.3) | 0.81 |
| Calcium-channel blockers | 27 (37.0) | 29 (39.7) | 0.73 |
| Renin–angiotensin system inhibitors | 26 (35.6) | 30 (41.1) | 0.50 |
| Statins | 17 (23.3) | 13 (17.8) | 0.41 |
| Medically treated diabetes mellitus | |||
| Any | 12 (16.4) | 17 (23.3) | 0.30 |
| Insulin dependent | 2 (2.7) | 2 (2.7) | 1.0 |
All results are presented as means±SD. ACS indicates acute coronary syndrome.
Figure 1.
Rho-associated kinase activity in peripheral blood leukocytes from control patients and patients with acute coronary syndrome (ACS). A, Western blot analysis for phospho myosin-binding subunit (p-MBS), total myosin-binding subunit (t-MBS), and actin in peripheral blood leukocytes. B, Rho-associated kinase activity (p-MBS/t-MBS) in control patients and patients with ACS.
Figure 2.
Rho-associated kinase activity in peripheral blood leukocytes from patients with acute coronary syndrome (ACS) within 2 hours of ACS onset (admission) and at 3 hours, 1 day, 7 days, 14 days, and 6 months after ACS onset. A, Western blot analysis for phospho myosin-binding subunit (p-MBS), total myosin-binding subunit (t-MBS), and actin in peripheral blood leukocytes within 2 hours of ACS onset (admission) and at 3 hours, 1 day, 7 days, 14 days, and 6 months after ACS onset. B, Rho-associated kinase activity (p-MBS/t-MBS) in patients with ACS within 2 hours of ACS onset (admission) and at 3 hours, 1 day, 7 days, 14 days, and 6 months after ACS onset.
Predictive Value of ROCK Activity for First Major Cardiovascular Events
During a median period of 50.8 months (interquartile range, 41.6–56.6 months) of follow-up, 5 patients died (4 from cardiovascular causes), 4 patients had myocardial infarction, 29 had coronary revascularization, 3 had ischemic stroke, and 4 had been hospitalized for heart failure (Table 2). As shown in Figure S2, maximum ROCK activity within 24 hours after arrival at the hospital predicts first major cardiovascular events with an area under curve of 0.77. ROC curve analysis indicated that maximum ROCK activity for predicting first major cardiovascular events was 1.05 (sensitivity of 87.1% and specificity of 59.6%).
Table 2.
Clinical Outcomes of Patients With Acute Coronary Syndrome on the Basis of Maximum Rho-Associated Kinase Activity
| Variable | Low Group, n=37 | High Group, n=36 | P Value |
|---|---|---|---|
| First major cardiovascular event, n (%) | 9 (24.3) | 22 (61.1) | 0.001 |
| Death from cardiovascular disease, n (%) | 2 (5.4) | 2 (5.6) | 0.98 |
| Acute myocardial infarction, n (%) | 3 (8.1) | 1 (2.8) | 0.31 |
| Ischemic stroke, n (%) | 1 (2.7) | 2 (5.6) | 0.54 |
| Coronary revascularization, n (%) | 9 (24.3) | 20 (55.6) | 0.006 |
| Hospitalization for heart failure, n (%) | 1 (2.7) | 3 (8.3) | 0.28 |
| Death from any cause, n (%) | 3 (8.1) | 2 (5.6) | 0.66 |
First major cardiovascular events include cardiovascular death, acute myocardial infarction, ischemic stroke, and coronary revascularization. Low group indicates maximum Rho-associated kinase activity of <1.14, and high group indicates ≥1.14.
We next performed ROC curve analysis to evaluate the prognostic value of maximum creatine kinase (CK) and ejection fraction for future cardiovascular events. ROC curve analysis indicated that maximum CK and ejection fraction predict cardiovascular events with areas under the curve of 0.64 and 0.55, respectively (Figure S3).
ROCK Activity and Clinical Events
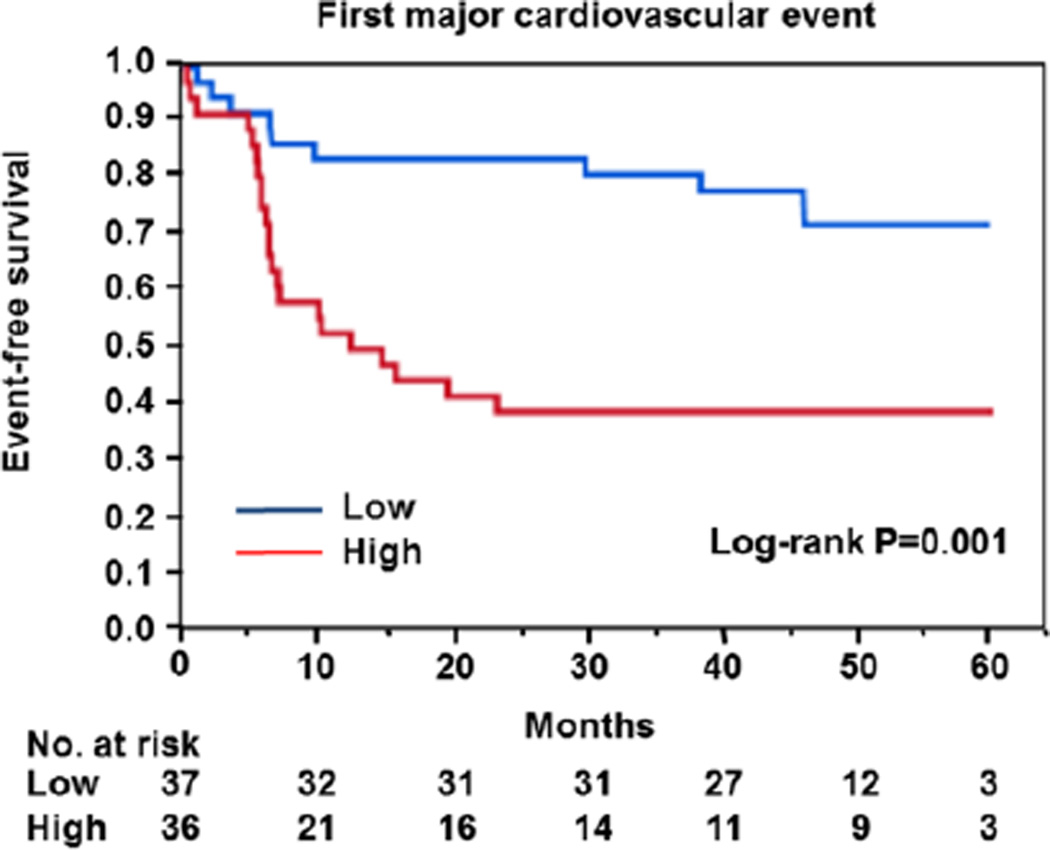
Because there is no standard value for ROCK activity, we divided into 2 groups based on ROCK activity identified by the median value of 1.14. The low group had maximum ROCK activity of <1.14, and the high group had maximum ROCK activity of ≥1.14. Clinical characteristics of patients with ACS on the basis of maximum ROCK activity are summarized in Table S1. Maximum CK was significantly higher in the high group than in the low group. There was no significant difference in other parameters and medications, culprit vessel, or percutaneous coronary intervention methods between the 2 groups. Time-course graphs of the actual ROCK activity values in the low and high groups are presented (Figure S4). In patients with high levels of ROCK activity, ROCK activity promptly increased from 1.22±0.64 to 1.51±0.96 after 3 hours and reached a peak of 1.59±0.15 after 1 day (P=0.04 and P=0.057, respectively) and then gradually decreased to 0.99±0.13 after 7 days, 0.93±0.25 after 14 days, and 0.74±0.31 after 6 months (P=0.16, P=0.18, and P=0.08, respectively). In patients with low levels of ROCK activity, ROCK activity did not differ between the time points (admission, 0.69±0.26; 3 hours, 0.71±0.29; 1 day, 0.74±0.05; 7 days, 0.70±0.07; 14 days, 0.58±0.14; and 6 months, 0.70±0.31). First major cardiovascular events were significantly more frequent among patients with high levels of ROCK activity than among those with low levels (P=0.001; Figure 3). There were significant differences between the Kaplan–Meier curves for coronary revascularization (P=0.003; Figure S5), but there were no significant differences between the Kaplan–Meier curves for death from cardiovascular disease (P=0.96), AMI (P=0.33), ischemic stroke (P=0.5), hospitalization for heart failure (P=0.28), and death from any cause (P=0.69; Figure S5). The causes of death included sudden death (n=1), AMI (n=1), ischemic stroke (n=1), heart failure (n=1), and cancer (n=1; Table S2). In addition, patients were divided into 2 groups based on serum CK levels identified by the median value of 1474 IU/L. The low group had a peak serum CK level of <1474 IU/L, and the high group had a peak serum CK level of ≥1474 IU/L. There was no significant difference in parameters and medications, culprit vessel, or percutaneous coronary intervention methods between the 2 groups (Table S3). There was also no significant difference between the 2 groups in Kaplan–Meier curves for first major cardiovascular events (P=0.26; Figure S6) or for death from cardiovascular disease, AMI, ischemic stroke, coronary revascularization, hospitalization for heart failure, and death from any cause (Figure S7).
Figure 3.
Kaplan–Meier curves of cumulative event-free survival of first major cardiovascular events (death from cardiovascular causes, myocardial infarction, ischemic stroke, and coronary revascularization), according to the median value of maximum Rho-associated kinase activity. Low indicates maximum Rho-associated kinase activity <1.14, and high indicates maximum Rho-associated kinase activity ≥1.14.
After adjustment for age, sex, cardiovascular risk factors, and maximum creatine kinase value, the associations between increasing levels of ROCK activity and increasing risk of first major cardiovascular events (hazard ratio, 3.52; 95% confidence interval, 1.46–8.98; P=0.005) and coronary revascularization (hazard ratio, 2.90; 95% confidence interval, 1.19–7.43; P=0.02) remained significant (Table S4). After adjustment for age, sex, cardiovascular risk factors, and concomitant treatment with stains, the associations between increasing levels of ROCK activity and increasing risk of first major cardiovascular events (hazard ratio, 4.56; 95% confidence interval, 1.98–11.34; P<0.001) and coronary revascularization (hazard ratio, 3.79; 95% confidence interval, 1.63–9.49; P=0.002) remained significant (Table S4).
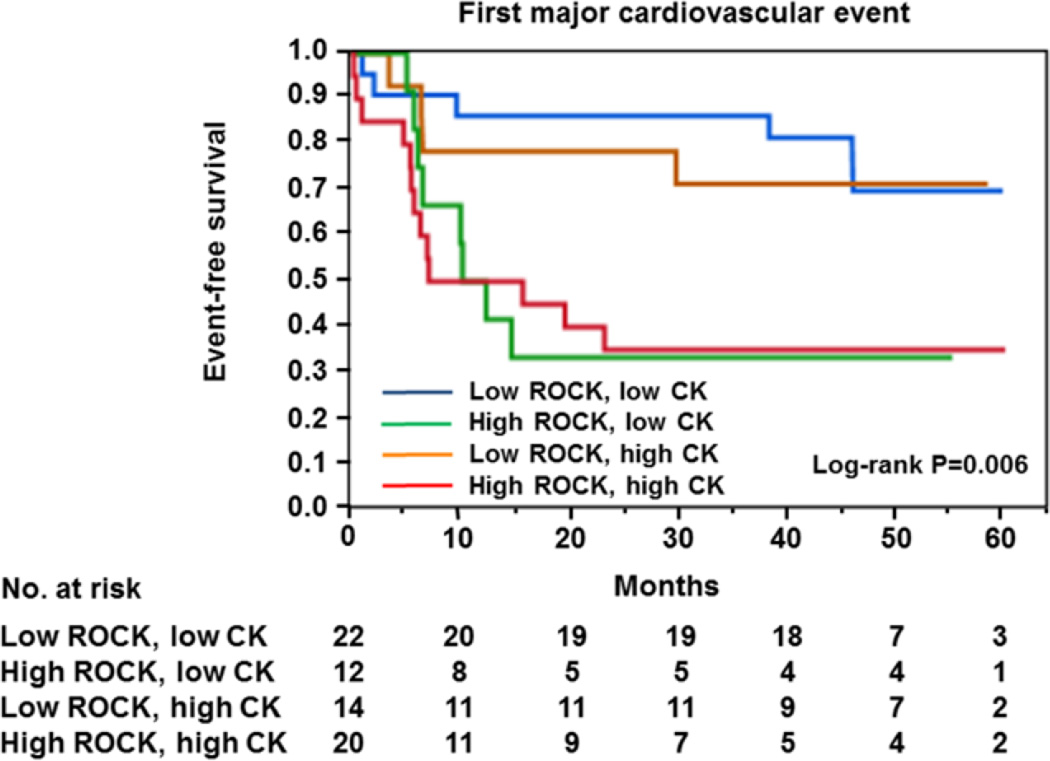
We categorized ACS patients into 4 groups according to the median value of maximum ROCK activity and the median value of the peak serum CK level after ACS onset. There were significant differences between the Kaplan–Meier curves for first major cardiovascular events (P=0.006; Figure 4). The rate of first major cardiovascular events for patients who had ROCK activity of ≥1.14 and CK of ≥1474 or <1474 IU/L was significantly different from the rate for patients who had ROCK activity of <1.14 and CK of ≥1474 or <1474 IU/L, but there was no significant difference between the rates of first major cardiovascular events for patients who had CK of ≥1474 IU/L and patients who had CK of <1474 IU/L (Figure 4).
Figure 4.
Kaplan–Meier curves of cumulative event-free survival of first major cardiovascular events (death from cardiovascular causes, myocardial infarction, stroke, and coronary revascularization) in subgroups of subjects categorized as being above or below the median values for maximum Rho-associated kinase (ROCK) activity and peak creatine kinase. Low ROCK indicates maximum ROCK activity <1.14; high ROCK, maximum ROCK ≥1.14; low CK, peak creatine kinase <1474 IU/L; and high CK, peak creatine kinase ≥1474 IU/L.
We next categorized ACS patients into 2 groups based on ROCK activity evaluated them at 6 months after ACS onset by the median value of 0.70. The low group had ROCK activity of ≤0.70, and the high group had ROCK activity of >0.70. Clinical characteristics of the subjects on the basis of ROCK activity are summarized in Table S5. There was no significant difference between the 2 groups in Kaplan–Meier curves for first major cardiovascular events (Figure S8), death from cardiovascular disease, AMI, ischemic stroke, coronary revascularization, hospitalization for heart failure, and death from any cause (Figure S9).
Discussion
In this study, ROCK activity was dramatically changed after ACS. Leukocyte ROCK activity reached a peak at ≈1 day after ACS onset and gradually decreased for 2 weeks. We also confirmed the prognostic impact of maximum ROCK activity during the initial 24 hours after admission for first major cardiovascular events.
We found that the maximum ROCK activity is an independent predictor of first major cardiovascular events in patients with ACS. It has been shown that inhibition of ROCK activity prevents the development and maintenance of cardiovascular damage in experimental animal models.6,31,32 Inhibition of ROCK activity limits infarct size in experimental AMI models through augmentation of Akt activation and nitric oxide synthesis in the myocardium.21–23 In this study, we found that ROCK activity differed between the time points in the high ROCK group in contrast to the low ROCK group. These findings suggest that elevation of ROCK activity after ACS onset may be associated with an increased risk of subsequent cardiovascular disease. In addition, several investigators including us confirmed that pharmacological therapy, such as administration of statins and antihypertensive agents, decreased ROCK activity in patients with dyslipidemia and essential hypertension.33,34 We have recently shown that cumulative cardiovascular risk factors increase ROCK activity and that ROCK activity is an independent predictor of future cardiovascular events in a general population.19,20 These findings suggest that ROCK activity may be a useful biomarker as a therapeutic target for future cardiovascular events and that inhibition of the Rho/ROCK pathway may lead to reduction of cardiovascular events in patients with ACS.
ROCK activity was significantly higher in patients with ACS than in controls within 2 hours of ACS onset. Interestingly, ROCK activity promptly increased and reached a peak at 24 hours after admission and then gradually decreased for 2 weeks in patients with ACS. Sanada et al24 reported that ROCK activity in the myocardium of an experimental model of ACS increased by ≈3-fold after 60 minutes of ischemia. Hamid et al23 reported an ≈2-fold increase in ROCK activity in rat injured myocardium after 10 minutes of reperfusion. These findings suggest that activation of ROCK occurs at a early stage of ACS. In addition, it has been shown that inhibition of ROCK activity limits infarct size in experimental AMI models.21–23 Therefore, it is expected that early administration of a ROCK inhibitor after ACS onset will have a beneficial cardioprotective effect. Further investigation is needed to determine the effects of acute and long-term administration of a ROCK inhibitor on left ventricular function and remodeling and on clinical outcomes after ACS in a clinical setting.
The peak CK level after ACS onset is routinely measured as indices of the volume of infarcted myocytes and the extent of myocardial necrosis. It is well known that increased peak CK level is associated with an increased risk of subsequent mortality.35 It is expected that the combination of ROCK activity and CK would be a better predictor of cardiovascular events than ROCK activity alone and CK alone. However, in this study, measurement of peak CK did not improve the predictive value of future cardiovascular events. We also found that area under the curve value of the ROC curve for ROCK activity to predict future cardiovascular events was higher than that for peak CK. Although ROCK activity has low specificity for predicting first major cardiovascular events, it is likely that ROCK activity is superior to peak CK for discrimination capacity of future cardiovascular events.
In this study, there was no association between ROCK activity measured at 6 months after ACS onset and increasing risk of cardiovascular events. Although the precise mechanism remains unclear, this might be because of the effects of medications. Indeed, several investigators including us have reported that antihypertensive drugs and lipid-lowering drugs could inhibit ROCK activity in patients with atherosclerosis.33,34 In addition, at 6 months after ACS onset, ROCK activity was decreased to levels of those in control subjects. These findings suggest that ROCK activity is reversible in patients with ACS.
Several investigators have shown that inhibition of ROCK activity limits infarct size in experimental AMI models.21–23 It is thought that leukocyte ROCK activity also reflects infarction size in patients with ACS. In this study, we found that peak CK was significantly higher in the high maximum ROCK activity group than in the low maximum ROCK activity group and that there was a significant correlation between ROCK activity and peak CK (r=0.29; P=0.01). These findings suggest that leukocyte ROCK activity may be a biomarker of myocardial infarct size during ACS.
Several investigators have shown that ROCK is activated in experimental models of ACS, including AMI.21–27 ROCK in peripheral leukocytes was also activated in patients with ACS. ACS-induced ROCK activation would lead to left ventricular dysfunction and remodeling after ACS. Indeed, several lines of evidence have shown that ROCKs play an important role in cardiac hypertrophy and ventricular remodeling through modulation of cardiac gene expression.36–41 It is postulated that several downstream mediators of ROCKs contribute to the process of cardiomyocyte hypertrophy and ventricular remodeling. RhoA is a downstream target of angiotensin II, endothelin-1, and phenylephrine in the heart.32,42,43 These neurohumoral factors increase the expression of Rho family members, including RhoA, Rac1, and Cdc42.43 There is growing evidence that RhoA is also a key mediator of cardiomyocyte hypertrophy and ventricular remodeling through activation of ROCKs.32,42,43 Rho and Ras play an important role in modulation of the expression of cardiac genes, including β-myosin heavy chain and atrial natriuretic factor during hypertrophy and remodeling, that are induced by signaling through Gq.36,43,44 Yanazume et al45 recently reported that the Rho/ROCK pathway is linked to downstream GATA4 through activation of extracellular signal–related kinase 1/2 during myocardial cell hypertrophy and remodeling. These findings suggest that the Rho/ROCK pathway, which is activated by several neurohormonal factors such as angiotensin II, endothelin-1, and phenylephrine, is involved in left ventricular dysfunction and remodeling in response to expression of specific genes and an increase in protein synthesis. These findings suggest that activation of the Rho/ROCK pathway may contribute to cardiovascular events in patients with ACS.
Limitations
First, the number of subjects in this study was relatively small. Recently, we reported that leukocyte ROCK activity was associated with death from cardiovascular disease and stroke in a general population.20 In this study, leukocyte ROCK activity was not associated with death from cardiovascular disease and stroke. This discrepancy might be because of the small sample size. However, we found that maximum ROCK activity in acute phase was an independent predictor of first major cardiovascular events after adjustment of cardiovascular risk factors. A more specific conclusion about the role of ROCK activity in cardiovascular events in patients with ACS could be drawn by investigation using a larger number of subjects.
Second, peak ROCK activity occurred at about 24 hours after ACS onset. However, we cannot exclude the possibility that the actual peak occurred somewhere between 3 hours and 1 day or between 1 day and 7 days. Measurement of more time points would indicate more exactly when peak ROCK activity occurs. In addition, all of the patients underwent angiography and subsequent primary percutaneous coronary intervention immediately after admission. We cannot deny the possibility that percutaneous coronary intervention alters the time-dependent changes in ROCK activity after ACS onset.
Third, all of the subjects were Japanese and were enrolled at a single center. These points could limit the generalization of the conclusion.
Fourth, several investigators have previously measured leukocyte ROCK activity by Western blot analysis.19,20,33,34,46–48 However, measurement of leukocyte ROCK activity by Western method may vary between laboratories and evaluators. A more clinically relevant assay, such as ELISA, to assess ROCK activity is required in clinical setting.
Finally, we selected measurement of leukocyte phospho MBS (p-MBS)/total MBS (t-MBS) as a noninvasive method for assessing ROCK activity because peripheral leukocytes are able to be simply and feasibly obtained. Measurement of p-MBS/t-MBS in myocardiocytes from humans would enable more specific conclusions about the role of ROCK activity in ACS to be drawn. Unfortunately, we cannot easily obtain these samples by myocardial biopsies under the condition of ACS. Several lines of evidence have shown that measurement of leukocyte p-MBS/t-MBS, as well as vascular response to the ROCK inhibitor fasudil, is useful for assessing ROCK activity.46–48 Therefore, we expect that measurement of leukocyte p-MBS/t-MBS will become an index of myocardial ROCK activity.
Perspectives
Leukocyte ROCK activity is activated in patients with ACS. Leukocyte ROCK activity may be a novel and useful biomarker for cardiovascular events after ACS. Inhibition of ROCK activity, especially at the early phase of ACS, may prevent cardiovascular and cardiomyocyte injury in patients with ACS.
Supplementary Material
Novelty and Significance.
What Is New?
Rho-associated kinase activity was dramatically changed after acute coronary syndrome. Increased Rho-associated kinase activity was associated with future cardiovascular events in acute coronary syndrome.
What Is Relevant?
Inhibition of leukocyte Rho-associated kinase activity may be a therapeutic target for prevention of cardiovascular events in acute coronary syndrome.
Summary
Rho-associated kinase activity could be a useful biomarker to predict cardiovascular events in patients with acute coronary syndrome.
Acknowledgments
We thank Megumi Wakisaka, Ki-ichiro Kawano, and Satoko Michiyama for their excellent secretarial assistance.
Sources of Funding
This study was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, and Culture of Japan (1859081500 and 21590898) and the National Institutes of Health (HL052233).
Footnotes
The online-only Data Supplement is available with this article at http://hyper.ahajournals.org/lookup/suppl/doi:10.1161/HYPERTENSIONAHA.115.05587/-/DC1.
Disclosures
None.
References
- 1.Uehata M, Ishizaki T, Satoh H, Ono T, Kawahara T, Morishita T, Tamakawa H, Yamagami K, Inui J, Maekawa M, Narumiya S. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997;389:990–994. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- 2.Amano M, Chihara K, Kimura K, Fukata Y, Nakamura N, Matsuura Y, Kaibuchi K. Formation of actin stress fibers and focal adhesions enhanced by Rho-kinase. Science. 1997;275:1308–1311. doi: 10.1126/science.275.5304.1308. [DOI] [PubMed] [Google Scholar]
- 3.Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 4.Verdecchia P, Porcellati C, Reboldi G, Gattobigio R, Borgioni C, Pearson TA, Ambrosio G. Left ventricular hypertrophy as an independent predictor of acute cerebrovascular events in essential hypertension. Circulation. 2001;104:2039–2044. doi: 10.1161/hc4201.097944. [DOI] [PubMed] [Google Scholar]
- 5.Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, Yamamori B, Feng J, Nakano T, Okawa K, Iwamatsu A, Kaibuchi K. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase) Science. 1996;273:245–248. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- 6.Noma K, Rikitake Y, Oyama N, Yan G, Alcaide P, Liu PY, Wang H, Ahl D, Sawada N, Okamoto R, Hiroi Y, Shimizu K, Luscinskas FW, Sun J, Liao JK. ROCK1 mediates leukocyte recruitment and neointima formation following vascular injury. J Clin Invest. 2008;118:1632–1644. doi: 10.1172/JCI29226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Noma K, Kihara Y, Higashi Y. Striking crosstalk of ROCK signaling with endothelial function. J Cardiol. 2012;60:1–6. doi: 10.1016/j.jjcc.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 8.Mallat Z, Gojova A, Sauzeau V, Brun V, Silvestre JS, Esposito B, Merval R, Groux H, Loirand G, Tedgui A. Rho-associated protein kinase contributes to early atherosclerotic lesion formation in mice. Circ Res. 2003;93:884–888. doi: 10.1161/01.RES.0000099062.55042.9A. [DOI] [PubMed] [Google Scholar]
- 9.Sawada N, Itoh H, Ueyama K, Yamashita J, Doi K, Chun TH, Inoue M, Masatsugu K, Saito T, Fukunaga Y, Sakaguchi S, Arai H, Ohno N, Komeda M, Nakao K. Inhibition of rho-associated kinase results in suppression of neointimal formation of balloon-injured arteries. Circulation. 2000;101:2030–2033. doi: 10.1161/01.cir.101.17.2030. [DOI] [PubMed] [Google Scholar]
- 10.Kataoka C, Egashira K, Inoue S, Takemoto M, Ni W, Koyanagi M, Kitamoto S, Usui M, Kaibuchi K, Shimokawa H, Takeshita A. Important role of Rho-kinase in the pathogenesis of cardiovascular inflammation and remodeling induced by long-term blockade of nitric oxide synthesis in rats. Hypertension. 2002;39:245–250. doi: 10.1161/hy0202.103271. [DOI] [PubMed] [Google Scholar]
- 11.Rikitake Y, Oyama N, Wang CY, Noma K, Satoh M, Kim HH, Liao JK. Decreased perivascular fibrosis but not cardiac hypertrophy in ROCK1+/− haploinsufficient mice. Circulation. 2005;112:2959–2965. doi: 10.1161/CIRCULATIONAHA.105.584623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Masumoto A, Hirooka Y, Shimokawa H, Hironaga K, Setoguchi S, Takeshita A. Possible involvement of Rho-kinase in the pathogenesis of hypertension in humans. Hypertension. 2001;38:1307–1310. doi: 10.1161/hy1201.096541. [DOI] [PubMed] [Google Scholar]
- 13.Vicari RM, Chaitman B, Keefe D, Smith WB, Chrysant SG, Tonkon MJ, Bittar N, Weiss RJ, Morales-Ballejo H, Thadani U Fasudil Study Group. Efficacy and safety of fasudil in patients with stable angina: a double-blind, placebo-controlled, phase 2 trial. J Am Coll Cardiol. 2005;46:1803–1811. doi: 10.1016/j.jacc.2005.07.047. [DOI] [PubMed] [Google Scholar]
- 14.Masumoto A, Mohri M, Shimokawa H, Urakami L, Usui M, Takeshita A. Suppression of coronary artery spasm by the Rho-kinase inhibitor fasudil in patients with vasospastic angina. Circulation. 2002;105:1545–1547. doi: 10.1161/hc1002.105938. [DOI] [PubMed] [Google Scholar]
- 15.Ishikura K, Yamada N, Ito M, Ota S, Nakamura M, Isaka N, Nakano T. Beneficial acute effects of rho-kinase inhibitor in patients with pulmonary arterial hypertension. Circ J. 2006;70:174–178. doi: 10.1253/circj.70.174. [DOI] [PubMed] [Google Scholar]
- 16.Kishi T, Hirooka Y, Masumoto A, Ito K, Kimura Y, Inokuchi K, Tagawa T, Shimokawa H, Takeshita A, Sunagawa K. Rho-kinase inhibitor improves increased vascular resistance and impaired vasodilation of the forearm in patients with heart failure. Circulation. 2005;111:2741–2747. doi: 10.1161/CIRCULATIONAHA.104.510248. [DOI] [PubMed] [Google Scholar]
- 17.Shibuya M, Hirai S, Seto M, Satoh S, Ohtomo E Fasudil Ischemic Stroke Study Group. Effects of fasudil in acute ischemic stroke: results of a prospective placebo-controlled double-blind trial. J Neurol Sci. 2005;238:31–39. doi: 10.1016/j.jns.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 18.Noma K, Goto C, Nishioka K, Hara K, Kimura M, Umemura T, Jitsuiki D, Nakagawa K, Oshima T, Chayama K, Yoshizumi M, Higashi Y. Smoking, endothelial function, and Rho-kinase in humans. Arterioscler Thromb Vasc Biol. 2005;25:2630–2635. doi: 10.1161/01.ATV.0000189304.32725.bd. [DOI] [PubMed] [Google Scholar]
- 19.Soga J, Noma K, Hata T, Hidaka T, Fujii Y, Idei N, Fujimura N, Mikami S, Maruhashi T, Kihara Y, Chayama K, Kato H, Liao JK, Higashi Y ROCK Study Group. Rho-associated kinase activity, endothelial function, and cardiovascular risk factors. Arterioscler Thromb Vasc Biol. 2011;31:2353–2359. doi: 10.1161/ATVBAHA.111.227892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kajikawa M, Noma K, Maruhashi T, Mikami S, Iwamoto Y, Iwamoto A, Matsumoto T, Hidaka T, Kihara Y, Chayama K, Nakashima A, Goto C, Liao JK, Higashi Y. Rho-associated kinase activity is a predictor of cardiovascular outcomes. Hypertension. 2014;63:856–864. doi: 10.1161/HYPERTENSIONAHA.113.02296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bao W, Hu E, Tao L, Boyce R, Mirabile R, Thudium DT, Ma XL, Willette RN, Yue TL. Inhibition of Rho-kinase protects the heart against ischemia/reperfusion injury. Cardiovasc Res. 2004;61:548–558. doi: 10.1016/j.cardiores.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 22.Wolfrum S, Dendorfer A, Rikitake Y, Stalker TJ, Gong Y, Scalia R, Dominiak P, Liao JK. Inhibition of Rho-kinase leads to rapid activation of phosphatidylinositol 3-kinase/protein kinase Akt and cardiovascular protection. Arterioscler Thromb Vasc Biol. 2004;24:1842–1847. doi: 10.1161/01.ATV.0000142813.33538.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamid SA, Bower HS, Baxter GF. Rho kinase activation plays a major role as a mediator of irreversible injury in reperfused myocardium. Am J Physiol Heart Circ Physiol. 2007;292:H2598–H2606. doi: 10.1152/ajpheart.01393.2006. [DOI] [PubMed] [Google Scholar]
- 24.Sanada S, Asanuma H, Tsukamoto O, et al. Protein kinase A as another mediator of ischemic preconditioning independent of protein kinase C. Circulation. 2004;110:51–57. doi: 10.1161/01.CIR.0000133390.12306.C7. [DOI] [PubMed] [Google Scholar]
- 25.Hattori T, Shimokawa H, Higashi M, Hiroki J, Mukai Y, Tsutsui H, Kaibuchi K, Takeshita A. Long-term inhibition of Rho-kinase suppresses left ventricular remodeling after myocardial infarction in mice. Circulation. 2004;109:2234–2239. doi: 10.1161/01.CIR.0000127939.16111.58. [DOI] [PubMed] [Google Scholar]
- 26.Demiryürek S, Kara AF, Celik A, Babül A, Tarakçioglu M, Demiryürek AT. Effects of fasudil, a Rho-kinase inhibitor, on myocardial preconditioning in anesthetized rats. Eur J Pharmacol. 2005;527:129–140. doi: 10.1016/j.ejphar.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 27.Zhang J, Li XX, Bian HJ, Liu XB, Ji XP, Zhang Y. Inhibition of the activity of Rho-kinase reduces cardiomyocyte apoptosis in heart ischemia/reperfusion via suppressing JNK-mediated AIF translocation. Clin Chim Acta. 2009;401:76–80. doi: 10.1016/j.cca.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 28.Kato M, Dote K, Sasaki S, Kagawa E, Nakano Y, Watanabe Y, Higashi A, Itakura K, Ochiumi Y, Takiguchi Y. Presentations of acute coronary syndrome related to coronary lesion morphologies as assessed by intravascular ultrasound and optical coherence tomography. Int J Cardiol. 2013;165:506–511. doi: 10.1016/j.ijcard.2011.09.032. [DOI] [PubMed] [Google Scholar]
- 29.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37(Suppl 1):S81–S90. doi: 10.2337/dc14-S081. [DOI] [PubMed] [Google Scholar]
- 30.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 31.Pasterkamp G, van Lammeren GW. Pleiotropic effects of statins in atherosclerotic disease. Expert Rev Cardiovasc Ther. 2010;8:1235–1237. doi: 10.1586/erc.10.107. [DOI] [PubMed] [Google Scholar]
- 32.Higashi M, Shimokawa H, Hattori T, Hiroki J, Mukai Y, Morikawa K, Ichiki T, Takahashi S, Takeshita A. Long-term inhibition of Rho-kinase suppresses angiotensin II-induced cardiovascular hypertrophy in rats in vivo: effect on endothelial NAD(P)H oxidase system. Circ Res. 2003;93:767–775. doi: 10.1161/01.RES.0000096650.91688.28. [DOI] [PubMed] [Google Scholar]
- 33.Nohria A, Prsic A, Liu PY, Okamoto R, Creager MA, Selwyn A, Liao JK, Ganz P. Statins inhibit Rho kinase activity in patients with atherosclerosis. Atherosclerosis. 2009;205:517–521. doi: 10.1016/j.atherosclerosis.2008.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fujimura N, Noma K, Hata T, Soga J, Hidaka T, Idei N, Fujii Y, Mikami S, Maruhashi T, Iwamoto Y, Kihara Y, Chayama K, Kato H, Liao JK, Higashi Y ROCK Study Group. Mineralocorticoid receptor blocker eplerenone improves endothelial function and inhibits Rho-associated kinase activity in patients with hypertension. Clin Pharmacol Ther. 2012;91:289–297. doi: 10.1038/clpt.2011.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Halkin A, Stone GW, Grines CL, Cox DA, Rutherford BD, Esente P, Meils CM, Albertsson P, Farah A, Tcheng JE, Lansky AJ, Mehran R. Prognostic implications of creatine kinase elevation after primary percutaneous coronary intervention for acute myocardial infarction. J Am Coll Cardiol. 2006;47:951–961. doi: 10.1016/j.jacc.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 36.Hoshijima M, Sah VP, Wang Y, Chien KR, Brown JH. The low molecular weight GTPase Rho regulates myofibril formation and organization in neonatal rat ventricular myocytes. Involvement of Rho kinase. J Biol Chem. 1998;273:7725–7730. doi: 10.1074/jbc.273.13.7725. [DOI] [PubMed] [Google Scholar]
- 37.Wei L, Imanaka-Yoshida K, Wang L, Zhan S, Schneider MD, DeMayo FJ, Schwartz RJ. Inhibition of Rho family GTPases by Rho GDP dissociation inhibitor disrupts cardiac morphogenesis and inhibits cardiomyocyte proliferation. Development. 2002;129:1705–1714. doi: 10.1242/dev.129.7.1705. [DOI] [PubMed] [Google Scholar]
- 38.Thorburn J, Xu S, Thorburn A. MAP kinase- and Rho-dependent signals interact to regulate gene expression but not actin morphology in cardiac muscle cells. EMBO J. 1997;16:1888–1900. doi: 10.1093/emboj/16.8.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kawamura S, Miyamoto S, Brown JH. Initiation and transduction of stretch-induced RhoA and Rac1 activation through caveolae: cytoskeletal regulation of ERK translocation. J Biol Chem. 2003;278:31111–31117. doi: 10.1074/jbc.M300725200. [DOI] [PubMed] [Google Scholar]
- 40.Chang J, Xie M, Shah VR, Schneider MD, Entman ML, Wei L, Schwartz RJ. Activation of Rho-associated coiled-coil protein kinase 1 (ROCK-1) by caspase-3 cleavage plays an essential role in cardiac myocyte apoptosis. Proc Natl Acad Sci U S A. 2006;103:14495–14500. doi: 10.1073/pnas.0601911103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brown JH, Del Re DP, Sussman MA. The Rac and Rho hall of fame: a decade of hypertrophic signaling hits. Circ Res. 2006;98:730–742. doi: 10.1161/01.RES.0000216039.75913.9e. [DOI] [PubMed] [Google Scholar]
- 42.Aikawa R, Komuro I, Yamazaki T, Zou Y, Kudoh S, Zhu W, Kadowaki T, Yazaki Y. Rho family small G proteins play critical roles in mechanical stress-induced hypertrophic responses in cardiac myocytes. Circ Res. 1999;84:458–466. doi: 10.1161/01.res.84.4.458. [DOI] [PubMed] [Google Scholar]
- 43.Sah VP, Hoshijima M, Chien KR, Brown JH. Rho is required for Galphaq and alpha1-adrenergic receptor signaling in cardiomyocytes. Dissociation of Ras and Rho pathways. J Biol Chem. 1996;271:31185–31190. doi: 10.1074/jbc.271.49.31185. [DOI] [PubMed] [Google Scholar]
- 44.Hines WA, Thorburn A. Ras and rho are required for galphaq-induced hypertrophic gene expression in neonatal rat cardiac myocytes. J Mol Cell Cardiol. 1998;30:485–494. doi: 10.1006/jmcc.1997.0613. [DOI] [PubMed] [Google Scholar]
- 45.Yanazume T, Hasegawa K, Wada H, Morimoto T, Abe M, Kawamura T, Sasayama S. Rho/ROCK pathway contributes to the activation of extracellular signal-regulated kinase/GATA-4 during myocardial cell hypertrophy. J Biol Chem. 2002;277:8618–8625. doi: 10.1074/jbc.M107924200. [DOI] [PubMed] [Google Scholar]
- 46.Nohria A, Grunert ME, Rikitake Y, Noma K, Prsic A, Ganz P, Liao JK, Creager MA. Rho kinase inhibition improves endothelial function in human subjects with coronary artery disease. Circ Res. 2006;99:1426–1432. doi: 10.1161/01.RES.0000251668.39526.c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu PY, Chen JH, Lin LJ, Liao JK. Increased Rho kinase activity in a Taiwanese population with metabolic syndrome. J Am Coll Cardiol. 2007;49:1619–1624. doi: 10.1016/j.jacc.2006.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kikuchi Y, Yasuda S, Aizawa K, Tsuburaya R, Ito Y, Takeda M, Nakayama M, Ito K, Takahashi J, Shimokawa H. Enhanced Rho-kinase activity in circulating neutrophils of patients with vasospastic angina: a possible biomarker for diagnosis and disease activity assessment. J Am Coll Cardiol. 2011;58:1231–1237. doi: 10.1016/j.jacc.2011.05.046. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.