Abstract
Study Objectives:
Prolonged exposure to blue wavelength light has been shown to have an alerting effect, and enhances performance on cognitive tasks.
A small number of studies have also shown that relatively short exposure to blue light leads to changes in functional brain responses during the period of exposure. The extent to which blue light continues to affect brain functioning during a cognitively challenging task after cessation of longer periods of exposure (i.e., roughly 30 minutes or longer), however, has not been fully investigated.
Methods:
A total of 35 healthy participants (18 female) were exposed to either blue (469 nm) (n = 17) or amber (578 nm) (n = 18) wavelength light for 30 minutes in a darkened room, followed immediately by functional magnetic resonance imaging (fMRI) while undergoing a working memory task (N-back task).
Results:
Participants in the blue light condition were faster in their responses on the N-back task and showed increased activation in the dorsolateral (DLPFC) and ventrolateral (VLPFC) prefrontal cortex compared to those in the amber control light condition. Furthermore, greater activation within the VLPFC was correlated with faster N-back response times.
Conclusions:
This is the first study to suggest that a relatively brief, single exposure to blue light has a subsequent beneficial effect on working memory performance, even after cessation of exposure, and leads to temporarily persisting functional brain changes within prefrontal brain regions associated with executive functions. These findings may have broader implication for using blue-enriched light in a variety of work settings where alertness and quick decision-making are important.
Citation:
Alkozei A, Smith R, Pisner DA, Vanuk JR, Berryhill SM, Fridman A, Shane BR, Knight SA, Killgore WD. Exposure to blue light increases subsequent functional activation of the prefrontal cortex during performance of a working memory task. SLEEP 2016;39(9):1671–1680.
Keywords: blue light, amber light, working memory, functional magnetic resonance imaging, fMRI, prefrontal cortex, PFC, N-back task
Significance.
This study shows that exposure to thirty minutes of blue wavelength light in the morning subsequently leads to faster response times on a cognitive working memory task and greater functional brain responses within the prefrontal cortex than comparable exposure to amber light. This is the first study to show that a short, single exposure to blue light during the daytime can lead to enduring measurable changes in brain activation and speed of performance during subsequent completion of a cognitively challenging task. While these findings may have important implications for using blue light in occupational settings, future research will be necessary to establish whether these findings generalize to naturalistic settings.
INTRODUCTION
Exposure to light has important effects on human physiology that are independent of visual perception. These non-image forming effects of light include the regulation of circadian rhythms, melatonin production, changes in core body temperature, sleep propensity, and alertness.1,2 Many of these effects of light are due to activation of retinal ganglion cells, which are maximally sensitive to light within the short wavelength (∼480 nm; blue light). These cells transmit irradiance signals to hypothalamic nuclei (e.g., the suprachiasmatic nucleus [SCN]), which are responsible for regulating circadian rhythms and melatonin production.1,2 Exposure to blue light in the evening or at night has been shown to increase alertness and improve performance on reaction time tasks, most likely as a result of the suppression of the evening onset of melatonin, which leads to a phase delay of the circadian rhythm.3–6 In a similar vein, morning blue light exposure suppresses melatonin in the early part of the day and leads to a phase advance of the circadian rhythm by inducing the onset of plasma melatonin earlier in the evening.7 In addition, blue light, and bright white light exposure more generally, during the day, has also been shown to have beneficial effects on alertness in a number of studies. One study compared the effects of bright (5,000 lux) versus dim light (< 10 lux) exposure during the day (between 12:00 and 16:00) and at night (between 00:00 and 04:00), and found that participants reported lower levels of sleepiness and fatigue, and greater energy during bright versus dim light exposure, regardless of time of day.8 In addition, the effects of daytime blue light exposure appear to have beneficial effects over longer periods of exposure. In a work place office setting, participants who were exposed to blue-enriched white light during the work day for 4 weeks reported increased subjective alertness, performance, positive mood, and concentration, in comparison to 4 weeks of white light exposure.9 Further evidence suggests that blue light can also be superior to caffeine for sustaining performance on tasks requiring psychomotor functioning.10
While the alerting effects of nighttime exposure to blue light appear to be produced predominantly by the suppression of melatonin, the increases in daytime alertness after blue light exposure are thought to be largely due to effects other than melatonin regulation.11 In particular, the daytime alerting effect of blue light may come from the indirect effects of melanopsin photosensitive retinal ganglion cells, which also project to brain regions other than the hypothalamus. For example, these cells can also indirectly influence activation of the locus coeruleus (LC),12 which in turn releases norepinephrine broadly throughout the cerebral cortex,13 leading to increases in alertness.14 Such downstream influences may explain some of the effects of blue light on alertness during the daytime, independent of the effects of melatonin. In fact, a functional magnetic resonance imaging (fMRI) study of in-scanner acute light exposure demonstrated that short 50-second bursts of blue light increased activation within the middle frontal gyrus and the brainstem, in comparison to violet light, while participants completed an auditory working memory task.15 While precise identification of brainstem nuclei is difficult using fMRI techniques, the location of the activation was consistent with the general stereotaxic coordinates of the LC.15 Thus, it appears plausible that blue light exposure may result in increased noradrenergic influence over cortical regions involved in controlled cognitive processing.
The aforementioned research suggests that blue light exposure activates brain networks that underlie many aspects of cognitive performance. One especially important cognitive function that may benefit from blue light exposure is working memory. Working memory comprises a set of cognitive processes that allow information to be actively held in mind in order to guide decision-making.16 Studies of healthy populations as well as patients with brain lesions have shown that working memory performance is associated with increased activation within the prefrontal cortex (PFC), and especially the dorsolateral PFC (DLPFC) and ventrolateral PFC (VLPFC).16,17 Since blue light exposure appears to influence the LC, which can increase the release of norepinephrine and lead to subsequent neural activation within the PFC, this may plausibly influence neural processes associated with working memory.
Neurocomputational models suggest that decision-making processes, such as those supported by working memory, require a trade-off between speed and accuracy. In this case, either a lot of time is spent to accumulate evidence for “safe and slow” decision-making, or less time is spent for “fast but risky” decision-making.18 These models also suggest that changes in baseline activation levels, as opposed to changes in the decision-threshold itself, may control this trade-off. For example, increases in baseline activation levels would decrease the distance from threshold, leading to faster but less reliable choices.18 One might therefore predict that blue light exposure would lead to faster response times within this type of task by increasing baseline activation levels. However, the few studies that have actually examined the effects of blue wavelength light during working memory performance (e.g., an auditory N-back task and an oddball task) have not found significant effects in terms of response time or accuracy when compared to non-blue wavelengths, despite significant increases in the activation of arousal and working memory systems of the brain.15,19,20 It should be noted that the duration of blue light exposure was considerably longer in those behavioral studies9 mentioned above (where a significant effect on performance was observed) compared to those fMRI studies finding no effect15,19,20 (one to several hours of blue light exposure in behavioral studies in comparison to 50 seconds up to 21 minutes in fMRI studies). Importantly, a recent review has suggested that the performance-enhancing effects of blue light at night as well as during the day usually occur with an exposure duration of roughly 30 minutes or longer.21 It is therefore possible that the shorter durations (e.g., 18 minutes) of blue light exposure applied in prior fMRI studies may not have been long enough to induce measurable behavioral changes. Furthermore, it has not been investigated in detail whether blue light exposure has the ability to affect functional brain responses and working memory performance after cessation of a single dose of daytime blue light exposure. While it has been shown that self-reported sleepiness is reduced after blue light exposure at nighttime,3 it is unclear whether a single dose of daytime blue light exposure can lead to enduring effects in terms of cognitive performance and functional brain responses. It is also possible that the lack of findings in previous fMRI studies may have been the result of participants completing the working memory task during light exposure, and not afterwards.
The goal of the present study was therefore to examine how 30 minutes of continuous blue wavelength light exposure would affect subsequent working memory performance and associated functional brain responses after cessation of the light exposure. We hypothesized that the enduring effects of blue wavelength light exposure would be associated with greater activation during a working memory task (N-back task) within areas usually recruited by such tasks, specifically the DLPFC and VLPFC, and that this increased activation would be associated with faster response times during the task, in comparison to a control exposure of amber light under the same conditions.
METHODS
Participants
Thirty-five healthy 18- to 32-year olds (18 female; 17 male) took part in the study. Participants completed an average of 12.5 years of education, were all right handed, primary English speaking, free from psychiatric, neurological, and substance use disorders, and reported a regular sleep schedule of going to bed between 22:00 and 01:00 and waking between 06:00 and 09:00.
Materials
Light Exposure
Participants underwent the controlled light exposure while sitting in an otherwise completely darkened room. All participants began with a blue light Washout Period (described in more detail under Procedure) that involved sitting in a dark room while only exposed to two amber light devices (described below) mounted on a desk at a distance of approximately 80 cm from the participant's nasion, with each light centered at a 45-degree angle from midline (see Figure 1A). Actual distance and angle of the light devices were adjusted manually until the pair of amber devices used during the initial washout period resulted in a 20-lux reading as measured by a light meter (Digital Lux Meter LX1330B) on each side of the participant's nose. During the Exposure Period, light was administered by a similar configuration of 4 light devices, also centered at 45 degrees to each side of the participant with a distance of approximately 80 cm from the participant's nasion (see Figure 1B). During the Exposure Period, the light devices were either blue or amber depending on random assignment. Blue light exposure utilized an array of commercially available Philips goLITE BLU Energy Light devices (Model HF3321/60; Philips Electronics, Stamford, CT). Each device consisted of a plastic table-mounted chassis with a 10 × 6 array of light emitting diodes (LEDs), encased in 1 × 1 cm cubical projection elements and a translucent plastic window cover. The goLITE BLU Energy Light is commercially available and has a narrow bandwidth (peaking at λ = 469 nm, at 214 lux, and panel irradiance [mW/cm2] = 1.23 at 20 cm). The amber devices were provided by the manufacturer for research purposes and were essentially identical to the goLITE BLU devices, with the exception that they were fitted with amber LEDs (peaking at λ = 578 nm, at 188 lux, and total irradiance [mW/cm2] = 0.35).
Figure 1.
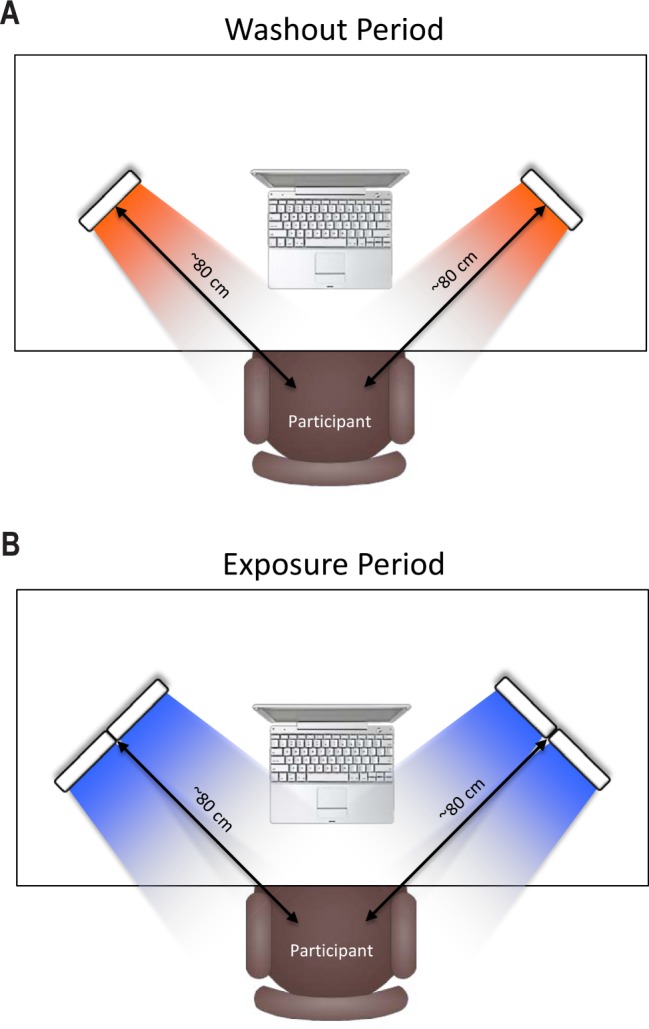
Diagram of light device placements. Participants were seated at a desk in a darkened room with light devices placed at a 45-degree angle to the right and left of center at a distance of approximately 80 cm. (A) During the 30-minute blue light Washout Period, 2 devices (one on the left and one on the right) fitted with amber light emitting diodes (LEDs; λ = 578 nm) were directed toward the participant, with each device centered at a 45-degree angle). (B) During the 30-minute Exposure Period, 4 devices (2 on the left and 2 on the right) were directed toward the participant, with each pair centered at a 45-degree angle. Devices were fitted with either blue (λ = 469 nm) or amber (λ = 578 nm) LEDs, according to random assignment.
N-back Task
This task was used during functional neuroimaging. The N-back task is a widely used task for assessing working memory22 and is typically applied in either auditory or visual modalities. In the present study, we employed a widely used visual variant of the task whereby participants viewed and responded to a series of letters appearing in serial order on the screen.16 Participants were presented with white letters appearing one letter at a time centered on a black screen. The N-back task included 3 conditions of varying cognitive load. During the control condition (i.e., “zero-back”), participants were asked to identify by button press whether each letter on the screen matched a predetermined letter (e.g., “P”) by pressing “yes” with their middle finger or “no” with the index finger of their right hand. In the “one-back” condition, participants responded with a button press using their right hand to indicate whether the letter presented in the current trial was identical to the letter presented in the immediately preceding trial. In the same way, during the “two-back” condition, participants indicated whether the letter shown in the current trial was identical to the letter presented 2 letter trials previously. Each cognitive load condition was presented as a block lasting 42 seconds. These blocks each consisted of a 6-s instruction screen followed by 16 trials (trial = stimulus displayed for 500 ms + 1,750 ms blank screen, ISI = 2,250 ms). Each cognitive load block was presented 3 times in pseudo-random order for a total of 9 blocks (3 “zero-back”; 3 “one-back”; 3 “two-back”) throughout the task. The task began and ended with a 10-s crosshair image requiring only visual fixation, and each block was also separated by a 10-s crosshair fixation image, for a total task run of 478 seconds (7 min 58 sec). Prior to neuroimaging, participants underwent a practice version of the task outside of the scanner. This involved completing each cognitive load condition once (i.e., 16 trials each) with immediate visual feedback on each trial to ensure that they understood the task before completing it in the scanner. Verbal instructions were given to participants while in the scanner and they were encouraged to ask any questions before beginning the task.
Stanford Sleepiness Scale (SSS)
The Stanford Sleepiness Scale (SSS)23 is a one-item measure to assess participants' current level of sleepiness on a 1–7 point scale, ranging from “feeling active, vital, alert, or wide awake” to “no longer fighting sleep, sleep onset soon, having dreamlike thoughts.” Higher scores on the SSS indicate higher levels of sleepiness.
Procedure
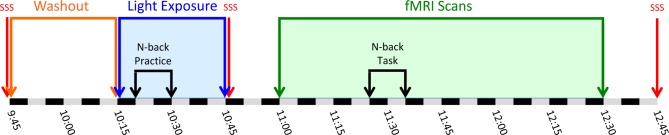
Participants completed the study on an individual basis, but all participants were run at the same time each day to control for circadian time of day effects. To ensure that participants were not in caffeine withdrawal during the procedure, they were asked to consume their normal levels of morning caffeine before arrival for the study. Participants arrived for the study at 07:45 and were escorted to the laboratory. For the first portion of the day, participants completed the informed consent process, and completed some basic information questionnaires and cognitive tasks. Participants were randomized to receive either 30 min of blue (n = 17) or amber (n = 18) light exposure. At approximately 09:15, participants were then fitted with wrap around polycarbonate blue light-blocking glasses (to minimize extraneous blue light exposure) and were escorted to the neuroimaging center at the University of Arizona Department of Medical Imaging. At 09:45, participants then completed the SSS and immediately underwent a “blue light washout” period for 30 min to ensure that residual effects of outdoor and ambient lighting had dissipated before the beginning of the light exposure period. During this washout period, participants were seated comfortably in a darkened room and then removed the light-blocking glasses. Ambient lighting was provided by 2 amber light devices (see Materials), which were activated on the desk in front of the participant and located 45° to the left and right of center, approximately 80 cm from the participant's nasion (see Figure 1A). The amount of light exposure was measured and the lights were adjusted for each participant to ensure that 20 lux of amber light was registered on each side of the nose. Participants were instructed not to look directly at the light devices, and to relax with their eyes open and maintain a generally forward gaze. At 10:15, the 2 Washout Period light devices were replaced with the 4 Exposure Period devices (see Figure 1B). Then the 30-min Exposure Period was initiated by engaging the 2 pairs of light devices (either blue or amber, depending on condition), with each pair mounted side by side on the desk in front of the participant, centered at the same location as the Washout Period amber lights. During the 30-min Exposure and Washout Periods, participants maintained a forward gaze and completed several computerized practice tasks to prepare them for their time in the scanner. The laptop monitors were shielded by an amber colored Plexiglas panel, which was acquired from www.lowbluelights.com, to block blue wavelength light. These computerized practice tasks ranged from 5 to 10 min each and were interspersed with 5-min rest breaks that involved sitting silently and maintaining a forward gaze at a crosshair on the wall facing the participant. At the completion of the Exposure Period (10:45), participants again donned their blue light-blocking glasses, were escorted to the MRI scanner, and again completed the SSS. Once in the scanner, the scanner room lights were dimmed and the glasses were removed. While we have no measurement of the lux levels in the scanner due to the incompatibility of lux meters within the magnetic field of the scanner, the light conditions were held constant across participants. The scanning sequence was initiated at 11:00, and the N-back task was started at approximately 11:25, and the scan was completed by 12:30. At the conclusion of the scan, participants exited the scanner and completed one last SSS (10:45) and were released. Figure 2 details the timeline of the study procedure.
Figure 2.
Timeline detailing the study procedure. Between 09:45 and 10:15, participants underwent 30 minutes of “washout” amber light exposure. Immediately following the washout period, participants either received 30 minutes of amber placebo light or blue light exposure (i.e., between 10:15 and 10:45). During this time, at 10:20, participants received instructions on the N-back task and completed one practice run, lasting 10 minutes in total. At 11:00, participants began the fMRI scan, and the N-back task was initiated at 11:25 and ended at 11:33. The scan ended at 12:30. Participants completed the Stanford Sleepiness Scale (SSS) 3 times during the procedure, including just before the start of the washout period, immediately after the light exposure, and at 12:45 after exiting the fMRI scanner.
Ethical Considerations
The research protocol was reviewed and approved by the Institutional Review Board of the University of Arizona and the U.S. Army Human Research Protections Office.
Neuroimaging Methods
Participants underwent fMRI immediately after completion of the 30-min exposure to either blue or amber light. Neuroimaging scans were collected on a Skyra 3T scanner (Siemens, Erlangen, Germany) using a 32-channel head coil. Structural images were first acquired using a T1-weighted 3D MPRAGE sequence (TR / TE / flip angle = 2.1 s / 2.33 ms / 12 degree) over 176 sagittal slices (256 × 256) and a slice thickness of 1.00 mm (voxel size = 1 × 1 × 1). T2*-weighted functional MRI scans were collected over 32 transverse slices and a slice thickness of 2.5 mm using an interleaved sequence (TR / TE / flip angle = 2.0 s / 25.0 ms / 90 degree) with 239 images collected per slice. Data were collected with a 22.0 cm field of view and a 64 × 64 acquisition matrix.
Image Processing
Processing and analysis of neuroimaging scans was conducted in SPM12 (Wellcome Department of Cognitive Neurology, London, UK; http://www.fil.ion.ucl.ac.uk/spm). Raw functional images were first preprocessed by realigning and unwarping the functional images, and then co-registering the newly created mean functional image to each subject's structural T1 scan. Forward deformation fields were used to normalize the images from subject native space to Montreal Neurological Institute (MNI) coordinate space. Finally, the images were spatially smoothed (6 mm full-width at half maximum), and resliced to 2 × 2 × 2 mm voxels. A high pass filter with a 128-s cutoff period was used to remove low frequency confounds. The standard canonical hemodynamic response function in SPM was employed, and serial autocorrelation was corrected with an autoregressive model of 1 (+white noise). Motion artifacts exceeding 3 SD in mean global intensity and scan-to-scan motion that exceed 1.0 mm were regressed out using the Artifact Detection Tool (http://www.nitrc.org/projects/artifact_detect/).
Statistical Analysis
On an individual basis, a general linear model (GLM) was spec-ified to contrast activation between the two-back > zero-back condition. These contrast images were entered into a second-level independent samples t-test analysis with light group (blue versus amber) as the independent variable. Based on our a priori hypotheses and previous findings from a large meta-analysis of normative functional neuroimaging studies using the N-back task,16 spheres of a 10 mm radius centered on stereotaxic coordinates derived from the previous meta-analysis were placed in areas of the DLPFC and VLPFC. The Talairach coordinates reported in Owen et al.16 were transformed to MNI coordinates using the MNI2TAL online program from Lacadie et al.24 (http://sprout022.sprout.yale.edu/mni2tal/mni2tal.html). The following MNI coordinates were used: DLPFC (x = 41, y = 31, z = 30; x = −37, y = 45, z = 21; x = −46, y = 19, z = 22), and VLPFC (x = −31, y = 21, z = 4; x = 34, y = 23, z = 1). Analyses were thresholded at P < 0.001 (uncorrected) and subjected to small volume correction for multiple comparisons within each search territory, family wise error (FWE) corrected at P < 0.05, and k (extent) ≥ 10 contiguous voxels.
In addition to the primary analysis of our hypothesized effects, we also conducted an exploratory whole brain analysis to provide complete data for future hypothesis generation. Here, we used a slightly more liberal height threshold of P < 0.005, while protecting against type I error through a cluster-corrected extent threshold of 201 voxels, which represents an FWE correction of P < 0.05.25 Because this analysis was exploratory, we had no a priori hypothesis and merely present these supplemental findings for completeness and to obviate bias in reporting.
RESULTS
Descriptive Statistics
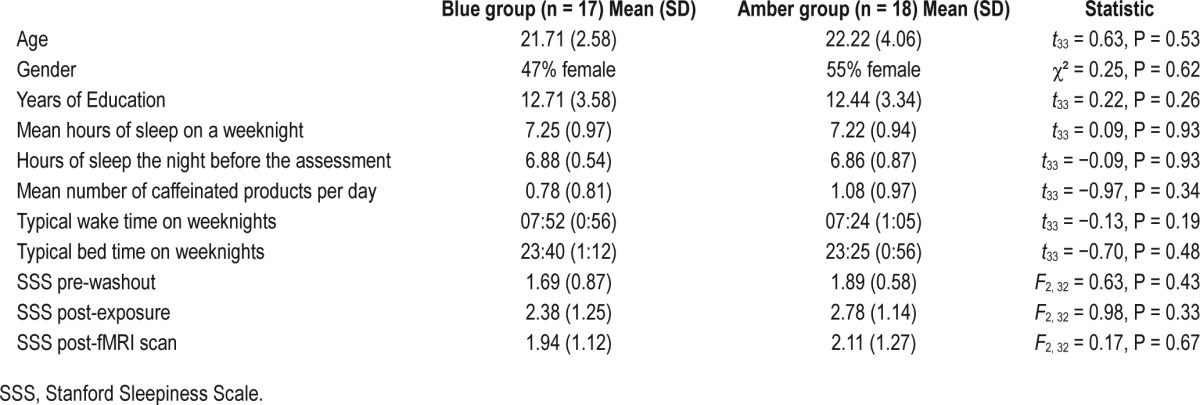
According to self-report, participants slept on average 7.2 h (SD 0.94) per night, and obtained 6.8 h (SD 0.72) of sleep the night before the assessment. Participants reported going to bed on average at 23:32 (SD 1 h 4 min) and waking at 07:37 (SD 1 h 2 min) on weekdays. Participants reported drinking an average of 0.93 (SD 0.89) caffeinated products per day, and 8 participants (4 in each group) reported having had one caffeinated product prior to the assessment, which was consistent with their normal morning consumption patterns. Groups did not differ on age, gender, years of education, mean number of hours slept on weeknights, number of hours slept the previous night, mean number of caffeinated products per day, and waking and bed times (see Table 1).
Table 1.
Sample characteristics.
Behavioral Results
A repeated-measures ANOVA of the SSS scores showed no interaction between light color and session (pre-washout, post light exposure, and post-fMRI) (F2, 31 = 0.12, P = 0.88). An analysis of simple effects showed no difference between light color groups at each of the 3 sessions (see Table 1).
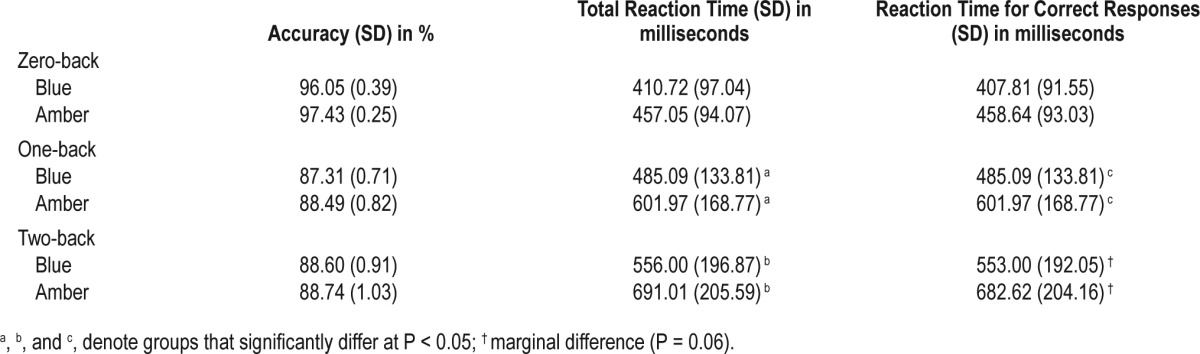
There was no difference in accuracy and response time between the blue and amber groups for the zero-back condition, but participants in the blue group responded faster during the one- (t33 = −2.26, P = 0.03) and two-back conditions (t33 = −1.98, P = 0.05) than participants in the control group (see Table 2).
Table 2.
Mean accuracy and reaction times for the N-back task.
Neuroimaging Results
Hypothesis Testing
For the two-back > zero-back contrast, individuals in the blue light group showed significantly greater activation in a cluster within the left DLPFC (k = 29; PFWE = 0.03; t = 4.12; x = −50, y = 14, z = 22, small volume corrected) and a cluster within the right VLPFC (k = 17, PFWE = 0.006, t = 4.83; x = 34, y = 20, z = −6, small volume corrected) than individuals who were exposed to the amber control light (see Figure 3). There were no regions within the brain where amber light exposure was associated with greater functional brain responses than blue light exposure.
Figure 3.
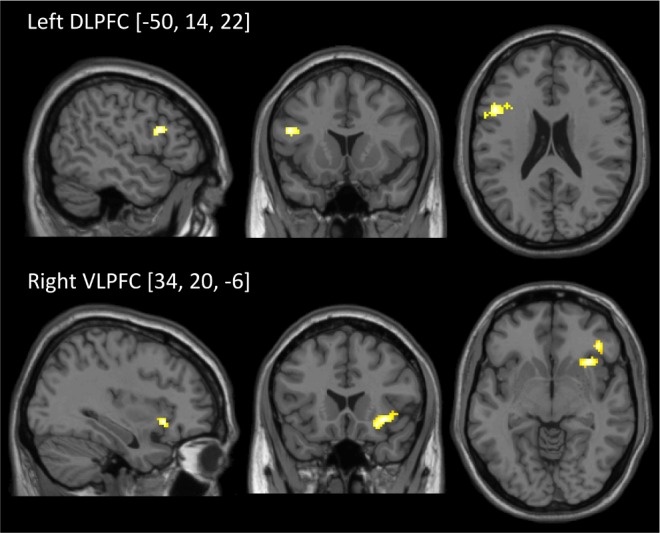
SPM images showing the clusters of significant activation where Blue > Amber for the N-Back task (two-back > zero-back). Based on the a priori regions of interest, this comparison revealed that the blue light condition was associated with significantly greater activation within the left dorsolateral prefrontal cortex (DLPFC) and the right ventrolateral prefrontal cortex (VLPFC) when compared to the amber light condition during complex working memory. Clusters are significant at P < 0.05, FWE corrected, but are displayed at P < 0.005 for ease of visualization.
In order to investigate the association between regional activation and behavioral responses, we extracted the activation for the unadjusted cluster eigenvariate for both brain regions and conducted Pearson correlations between the eigenvariate and response time and performance metrics during the two-back condition. There was a negative correlation between VLPFC activation and response time (r = −0.35, P = 0.04). This correlation was present among the sample as a whole and was not driven by one group in particular (Figure 4). No significant associations with accuracy were found. In addition, no significant associations were found between activation within the DLPFC and performance on the working memory task.
Figure 4.
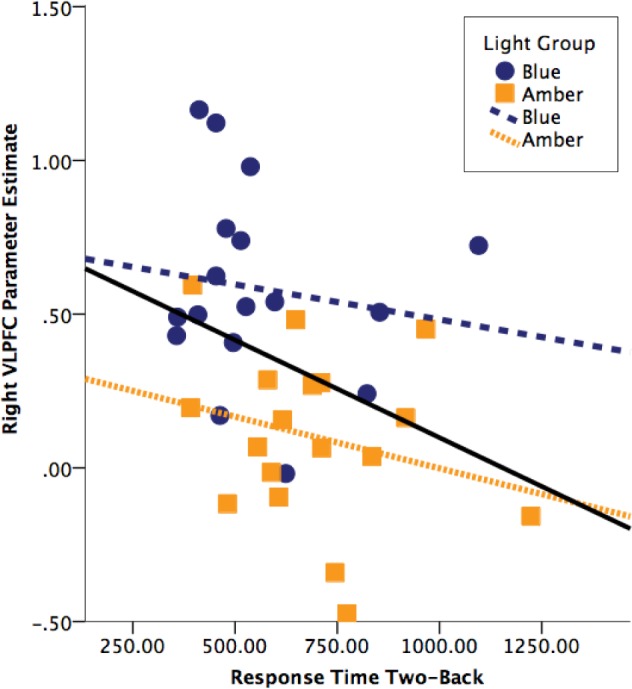
The scatterplots illustrate the association between the activation within the right ventrolateral prefrontal cortex (VLPFC) and reaction time during the two-back condition for the blue and amber light groups, and the sample as a whole.
To investigate whether participants were more “efficient” with increases in working memory (i.e., the number of correct responses per second), a measure of cognitive throughput was calculated ([Accuracy × (1 / RT) * 1,000]).26 Throughput provides a quantitative metric of the speed versus accuracy tradeoff. While there was no difference in throughput between the 2 groups in the zero-back condition (t33 = −1.60, P = 0.19), participants in the blue group showed enhanced throughput in the one-back (t33 = −2.57, P = 0.01), and marginally higher throughput in the two-back condition (t33 = −1.92, P = 0.06). In other words, participants in the blue light group provided a greater number of correct responses per unit of time than participants in the amber control group (Figure 5). Given that the groups were essentially equivalent with regard to accuracy, this difference suggests that exposure to blue light led to faster response times with no loss in accuracy.
Figure 5.
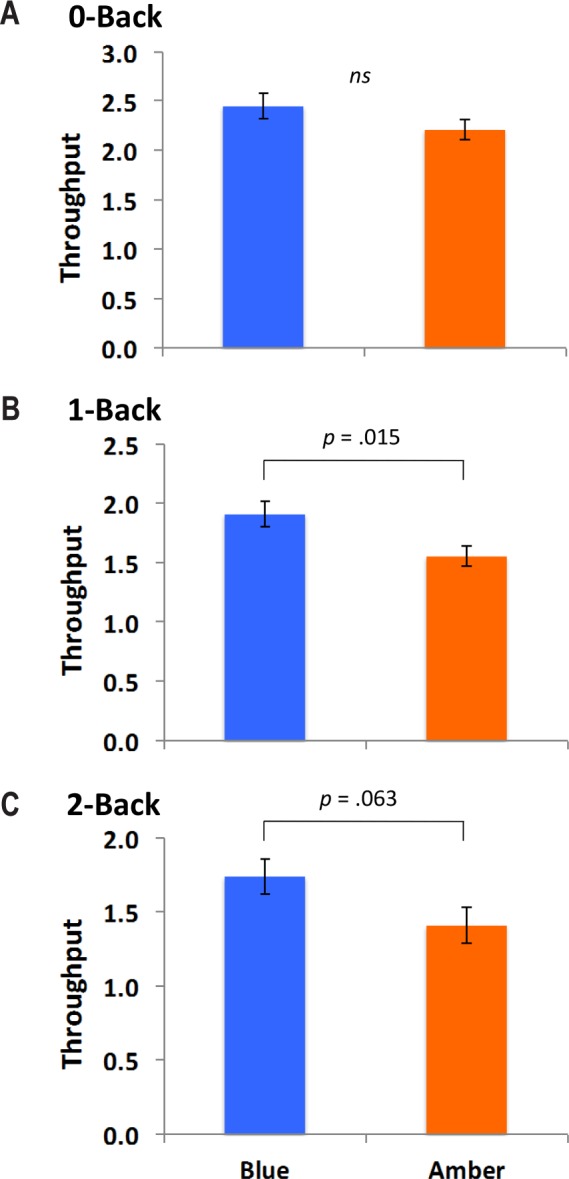
The figure shows group differences in working memory cognitive throughput (Accuracy × [1 / RT] * 1,000), which is a measure of the speed × accuracy trade-off. (A) There was no difference between the blue and amber groups with regard to throughput performance on the zero-back task. (B) On the one-back task, the blue light group showed significantly enhanced throughput performance compared to the amber control group. (C) On the two-back task, there was a marginally significant trend toward greater throughput for the blue compared to the amber control group.
Exploratory Analysis
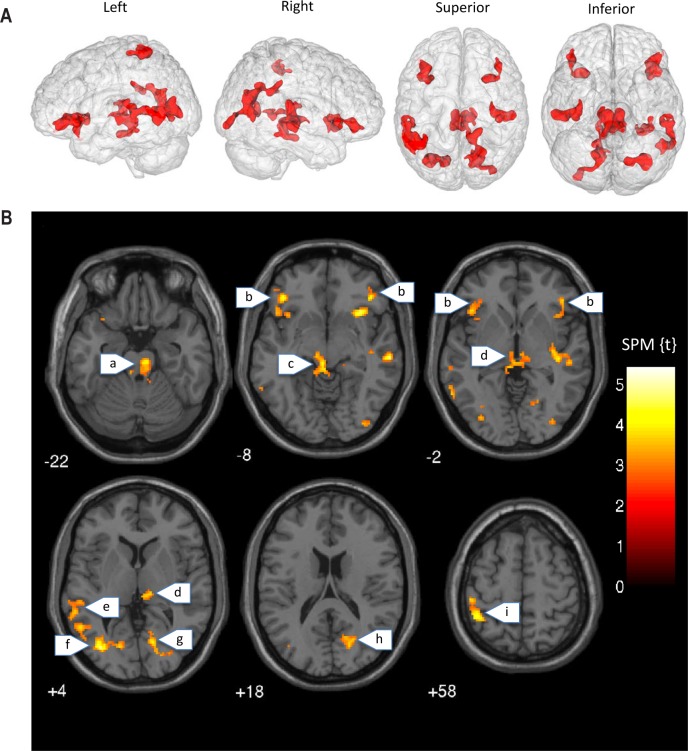
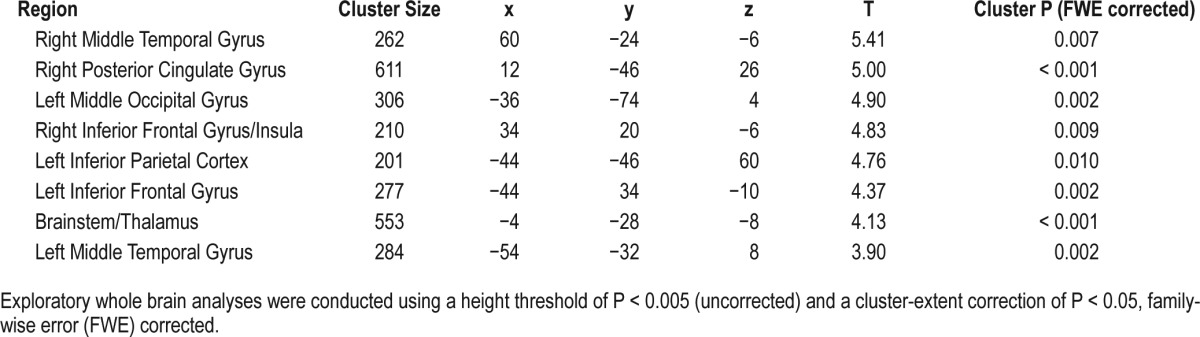
Finally, exploratory whole brain analysis was undertaken for the purpose of facilitating future hypothesis generation, with a peak height threshold of P < 0.005, and cluster-corrected extent threshold of P < 0.05 (FWE corrected). Again comparing the two-back > zero-back contrast, we found that the blue-wavelength light exposure group showed significantly greater activation than the amber control group within several distributed regions including left and right VLPFC (i.e., inferior frontal gyrus/insula), left and right middle temporal gyrus, right posterior cingulate gyrus, left middle occipital cortex, brainstem, and thalamus (Figure 6). Table 3 lists the cluster maxima for these exploratory analyses. There were no regions in the brain showing greater activation for the amber control light group compared to blue light group during the working memory task.
Figure 6.
Whole brain exploratory analysis (height P < 0.005, cluster corrected P < 0.05, FWE). (A) The “glass brain” figures show the location of significant clusters of brain activation where Blue > Amber for the N-Back Task (two-back > zero-back). (B) The axial slices show the aforementioned clusters in greater anatomical detail. Blue light was associated with greater activation than amber control within: (a) pons, (b) inferior frontal gyrus, (c) superior brainstem, (d) thalamus, (e) middle temporal gyrus, (f) middle occipital gyrus, (g) lingual gyrus, (h) calcarine cortex, (i) inferior parietal lobule.
Table 3.
Cluster maxima for whole brain exploratory analysis of blue > amber light conditions.
DISCUSSION
The goal of the present study was to examine the effects of 30 minutes of controlled blue wavelength (469 nm) light exposure compared to amber placebo light (578 nm) exposure on subsequent functional brain responses and performance during an N-back working memory task among healthy non-sleep deprived individuals. We found that exposure to 30 minutes of blue wavelength light produced greater activation within regions of the DLPFC and VLPFC and faster response times during a subsequent working memory task than exposure to amber wavelength light under otherwise identical conditions. Moreover, greater activation in the VLPFC for both groups combined was significantly correlated with faster response times during the working memory task, consistent with this region's role in executive functioning. Finally, while blue light effects were observed for brain activation and response time, there were no group differences in accuracy on the working memory task. Together, these findings suggest that a relatively brief exposure to blue light has an enhancing effect on speeded cognition and brain function that may persist for at least 40 minutes after cessation of the light.
It is well established that both the DLPFC and VLPFC are critically involved in the encoding, retention, and retrieval of information during working memory tasks.16,27,28 Our findings suggest that a single, relatively short exposure to blue wavelength light of only 30 minutes can increase neural activation over the subsequent 40-minute period within those prefrontal areas most critical for successful working memory performance. Prior work has shown that even short bursts of blue light for periods lasting from 50 seconds to 20 minutes are effective at activating similar regions of the DLPFC and VLPFC during auditory working memory tasks.15,19,29 While previous studies have found increases within the prefrontal regions during exposure,15,19 this study shows that brain activation and improved working memory task performance as a result of light exposure can substantially endure well beyond the exposure period and adds to emerging work suggesting that prolonged blue light exposure (30 minutes or more) may continue to affect brain function even after termination of the light.30 Although previous studies suggest that light-induced changes in functional brain responses may decline within 10 minutes after the end of the exposure period,20 it is important to consider that the duration of the light exposure was considerably shorter, roughly 10 to 29 minutes less time than in the present study.15,19,20 It is therefore possible that the longer light exposure may have contributed to these differences in findings. However, it should be also pointed out that some of these previous studies may have employed shorter periods of light exposure (e.g., 50 second bursts of exposure15) in order to prevent the confounding effects of variations in alertness and performance on the N-back task. Future studies comparing varying durations of light exposure, and employing different tasks, will therefore be necessary to determine the extent of the persisting effects of light on subsequent performance. It is conceivable that this prolonged effect may be a result of sustained noradrenergic activation. Prior research has shown that blue light exposure leads to greater activation within the LC, which in turn releases norepinephrine throughout the cortex.13 If blue light exposure in our study promoted increased noradrenergic influence within the PFC (leading to an increase in baseline regional activation), this could plausibly explain the increased prefrontal BOLD responses and improved response times that we observed.
It is important to note that performance on the N-back task, in terms of faster response times, correlated positively with activation within the mid VLPFC. This finding is consistent with previous studies suggesting that an increase in baseline lateral prefrontal activation leads to faster decision-making.31 Neuro-computational models suggest that the higher the baseline activity within a cortical area, the lower the activation needed to reach a response threshold, which can lead to faster response times.18 This increase in baseline activation may in turn be explained by increased release of norepinephrine throughout the frontal cortex, due to stimulation of the LC14 as a result of blue light exposure. It is also notable that blue light improved the speed of responses to the working memory task relative to amber control, but did not lead to an overall improvement in accuracy. Consideration of these data in light of the throughput metric, which quantifies the speed-accuracy tradeoff, suggests that while blue light was associated with an increase in the speed of responding to the working memory items, there was no corresponding loss of accuracy. Thus, blue light exposure was associated with the ability to make a greater number of correct responses per unit of time compared to the amber control light.
While previous studies that investigated the alerting effects of blue light have often employed study designs during nighttime,5 or during prolonged (i.e., 4 hours) daytime exposure to blue light,8 the present findings may have a broader application. Together with findings from previous studies, the results suggest that a relatively short duration (i.e., 30 minutes) of blue light exposure during the day can have a measurable effect on brain functioning and cognitive performance, not only acutely during the period of exposure, but that the effects may also endure for some time after termination of the light. This may have implications for the kind of light that is being used in office spaces, cockpits, and hospitals, in particular for individuals who have to perform their duties during sleep-deprived conditions. While the present study only examined the effects of light exposure under normally rested conditions, it is likely that these effects on brain activation and performance might be even more robust during periods of insufficient sleep. Prior work has suggested that blue wavelength light may be effective at improving some aspects of alertness and cognitive performance during nocturnal sleep loss,32 but this has not been explored using neuroimaging techniques. It should also be pointed out that participants did not report any subjective differences in sleepiness/alertness depending on light condition. It is possible that longer light exposure is necessary to produce subjectively alerting effects of blue light exposure.
While the present findings suggest that blue wavelength light has meaningful effects on brain function and performance that persist beyond the exposure period, there are a number of limitations to be borne in mind. First, we present data on only a single cognitive task in a relatively artificial neuroimaging environment. Light exposure in the “real world” rarely follows these constraints. Further work with more ecologically valid tasks and environments will be necessary to establish the effectiveness of blue or blue-enriched white light in a variety of occupational settings. Some work has demonstrated increases in subjective alertness and performance after four weeks of blue-enriched white light exposure in offices,9 but additional research will be necessary to determine the most effective parameters for administering light for the purpose of enhancing or sustaining performance in occupational settings. In addition, previous neuroimaging studies have employed an auditory, and not a visually presented letter variant of the N-back task as in the present study. It is unclear the extent to which the different variants of the N-back task might have contributed to some of the differences in findings across studies. It has been shown that an auditory N-back task may be more difficult than a visual variant of the N-back task. However, these differences in task type were only apparent at the three-back level.33 The present study and previous fMRI studies investigating the effects of blue light on functional brain responses15,19 have thus far been restricted to the two-back level. Nevertheless, future work that includes both visual and auditory N-back tasks that are more cognitively demanding will be necessary to establish whether blue wavelength light has a differential effect on these separate working memory systems. Furthermore, our sample sizes, while consistent with current practice in much of the neuroimaging literature, remain modest and limited in power, necessitating further replication to establish the reliability of the findings. Our sample was also relatively young and homogeneous in terms of background and health. Some evidence suggests that the effects of blue light on alertness may be attenuated among older individuals.34 It has also been suggested that the effects of light on performance and brain responses may differ depending on genotype and circadian phase of testing.35 Although participants were included if habitual bed and wake times fit within the pre-determined range to reduce variability due to circadian differences, the laboratory experiment started relatively early at 07:45 which may have led to elevated melatonin levels in some participants with later waking times. In addition, we did not collect genetic material in this sample, so examination of the role of genetics on the observed effects will require further study. Lastly, it should be pointed out that participants practiced the N-back task during either the blue light or amber light exposure (depending on condition). During the practice session participants received detailed instructions, were able to ask questions, and completed one trial of each condition (zero-back, one-back, and two-back) with feedback while undergoing light exposure. It is therefore possible that blue light exposure during the practice session influenced participants' ability to learn the task in such a way that they were able to perform better during the actual task. This potential role of light in learning is indeed an intriguing possibility. While the effects of blue light on immediate learning versus its persistent effects on subsequent performance cannot be disentangled here, this will likely be a fruitful question for further research.
CONCLUSIONS
The present findings suggest that daytime exposure to 30 minutes of blue wavelength light in non-sleep-deprived individuals has a beneficial impact on working memory performance and elicits measurable functional brain responses within prefrontal regions associated with executive functions. These results extend previous work by showing that exposure to blue light leads to persistent changes within the brain and performance during the post-exposure period (40 minutes). Additional research is necessary to identify the duration and breadth of these effects and how they may interact with individual difference factors such as gender, age, genotype, and other factors such as sleep debt and circadian influences.
DISCLOSURE STATEMENT
This was not an industry supported study. This study was supported by a U.S. Army US Army MOMRP Grant as well as by an Arizona Health Education Centers (AHEC) Research Grant. The authors have no other conflict of interest. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–3. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- 2.Dijk D-J, Archer SN. Light, sleep, and circadian rhythms: together again. PLoS Biol. 2009;7:1221. doi: 10.1371/journal.pbio.1000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chellappa SL, Steiner R, Blattner P, Oelhafen P, Götz T, Cajochen C. Non-visual effects of light on melatonin, alertness and cognitive performance: can blue-enriched light keep us alert. PloS One. 2011;6:e16429. doi: 10.1371/journal.pone.0016429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cajochen C, Munch M, Kobialka S, et al. High sensitivity of human melatonin, alertness, thermoregulation, and heart rate to short wavelength light. J Clin Endocrinol Metab. 2005;90:1311–6. doi: 10.1210/jc.2004-0957. [DOI] [PubMed] [Google Scholar]
- 5.Phipps-Nelson J, Redman JR, Schlangen LJ, Rajaratnam SM. Blue light exposure reduces objective measures of sleepiness during prolonged nighttime performance testing. Chronobiol Int. 2009;26:891–912. doi: 10.1080/07420520903044364. [DOI] [PubMed] [Google Scholar]
- 6.Lockley SW, Evans EE, Scheer F, Brainard GC, Czeisler CA, Aeschbach D. Short-wavelength sensitivity for the direct effects of light on alertness, vigilance, and the waking electroencephalogram in humans. Sleep. 2006;29:161–8. [PubMed] [Google Scholar]
- 7.Wright HR, Lack LC, Kennaway DJ. Differential effects of light wavelength in phase advancing the melatonin rhythm. J Pineal Res. 2004;36:140–4. doi: 10.1046/j.1600-079x.2003.00108.x. [DOI] [PubMed] [Google Scholar]
- 8.Rüger M, Gordijn MC, Beersma DG, de Vries B, Daan S. Time-of-day-dependent effects of bright light exposure on human psychophysiology: comparison of daytime and nighttime exposure. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1413–20. doi: 10.1152/ajpregu.00121.2005. [DOI] [PubMed] [Google Scholar]
- 9.Viola AU, James LM, Schlangen LJ, Dijk D-J. Blue-enriched white light in the workplace improves self-reported alertness, performance and sleep quality. Scand J Work Environ Health. 2008:297–306. doi: 10.5271/sjweh.1268. [DOI] [PubMed] [Google Scholar]
- 10.Beaven CM, Ekström J. A comparison of blue light and caffeine effects on cognitive function and alertness in humans. PloS One. 2013;8:e76707. doi: 10.1371/journal.pone.0076707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rautkyä E, Puolakka M, Halonen L. Alerting effects of daytime light exposure-a proposed link between light exposure and brain mechanisms. Light Res Technol. 2012;44:238–52. [Google Scholar]
- 12.Aston-Jones G, Chen S, Zhu Y, Oshinsky ML. A neural circuit for circadian regulation of arousal. Nat Neurosci. 2001;4:732–8. doi: 10.1038/89522. [DOI] [PubMed] [Google Scholar]
- 13.Florin-Lechner SM, Druhan JP, Aston-Jones G, Valentino RJ. Enhanced norepinephrine release in prefrontal cortex with burst stimulation of the locus coeruleus. Brain Res. 1996;742:89–97. doi: 10.1016/s0006-8993(96)00967-5. [DOI] [PubMed] [Google Scholar]
- 14.Foote S, Berridge C, Adams L, Pineda J. Electrophysiological evidence for the involvement of the locus coeruleus in alerting, orienting, and attending. Progr Brain Res. 1991;88:521–32. doi: 10.1016/s0079-6123(08)63831-5. [DOI] [PubMed] [Google Scholar]
- 15.Vandewalle G, Schmidt C, Albouy G, et al. Brain responses to violet, blue, and green monochromatic light exposures in humans: prominent role of blue light and the brainstem. PLoS One. 2007;2:e1247. doi: 10.1371/journal.pone.0001247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp. 2005;25:46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kane MJ, Engle RW. Working-memory capacity and the control of attention: the contributions of goal neglect, response competition, and task set to Stroop interference. J Exp Psychol Gen. 2003;132:47. doi: 10.1037/0096-3445.132.1.47. [DOI] [PubMed] [Google Scholar]
- 18.Bogacz R, Wagenmakers E-J, Forstmann BU, Nieuwenhuis S. The neural basis of the speed-accuracy tradeoff. Trend Neurosci. 2010;33:10–6. doi: 10.1016/j.tins.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 19.Vandewalle G, Gais S, Schabus M, et al. Wavelength-dependent modulation of brain responses to a working memory task by daytime light exposure. Cereb Cortex. 2007;17:2788–95. doi: 10.1093/cercor/bhm007. [DOI] [PubMed] [Google Scholar]
- 20.Vandewalle G, Balteau E, Phillips C, et al. Daytime light exposure dynamically enhances brain responses. Curr Biol. 2006;16:1616–21. doi: 10.1016/j.cub.2006.06.031. [DOI] [PubMed] [Google Scholar]
- 21.Vandewalle G, Maquet P, Dijk D-J. Light as a modulator of cognitive brain function. Trend Cogn Sci. 2009;13:429–38. doi: 10.1016/j.tics.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 22.Gevins A, Cutillo B. Neuroelectric evidence for distributed processing in human working memory. Electroencephalogr Clin Neurophysiol. 1993;87:128–43. doi: 10.1016/0013-4694(93)90119-g. [DOI] [PubMed] [Google Scholar]
- 23.Hoddes E, Zarcone V, Dement W. Psychophysiology. New York, NY: Cambridge University Press; 1972. Development and use of Stanford Sleepiness scale (SSS) [Google Scholar]
- 24.Lacadie CM, Fulbright RK, Rajeevan N, Constable RT, Papademetris X. More accurate Talairach coordinates for neuroimaging using nonlinear registration. Neuroimage. 2008;42:717–25. doi: 10.1016/j.neuroimage.2008.04.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Woo CW, Krishnan A, Wager TD. Cluster-extent based thresholding in fMRI analyses: pitfalls and recommendations. Neuroimage. 2014;91:412–9. doi: 10.1016/j.neuroimage.2013.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thorne DR. Throughput: a simple performance index with desirable characteristics. Behav Res Methods. 2006;38:569–73. doi: 10.3758/bf03193886. [DOI] [PubMed] [Google Scholar]
- 27.D'Esposito M, Postle BR, Rypma B. Prefrontal cortical contributions to working memory: evidence from event-related fMRI studies. Exp Brain Res. 2000;133:3–11. doi: 10.1007/s002210000395. [DOI] [PubMed] [Google Scholar]
- 28.Curtis CE, D'Esposito M. Persistent activity in the prefrontal cortex during working memory. Trends Cogn Sci. 2003;7:415–23. doi: 10.1016/s1364-6613(03)00197-9. [DOI] [PubMed] [Google Scholar]
- 29.Vandewalle G, Archer SN, Wuillaume C, et al. Effects of light on cognitive brain responses depend on circadian phase and sleep homeostasis. J Biol Rhythms. 2011;26:249–59. doi: 10.1177/0748730411401736. [DOI] [PubMed] [Google Scholar]
- 30.Alkozei A, Smith R, Killgore WD. Exposure to blue wavelength light modulates anterior cingulate cortex activation in response to ‘uncertain’ versus ‘certain’ anticipation of positive stimuli. Neurosci Lett. 2016;616:5–10. doi: 10.1016/j.neulet.2016.01.034. [DOI] [PubMed] [Google Scholar]
- 31.van Veen V, Krug MK, Carter CS. The neural and computational basis of controlled speed-accuracy tradeoff during task performance. J Cogn Neurosci. 2008;20:1952–65. doi: 10.1162/jocn.2008.20146. [DOI] [PubMed] [Google Scholar]
- 32.Taillard J, Capelli A, Sagaspe P, Anund A, Akerstedt T, Philip P. In-car nocturnal blue light exposure improves motorway driving: a randomized controlled trial. PLoS One. 2012;7:e46750. doi: 10.1371/journal.pone.0046750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jaeggi SM, Buschkuehl M, Perrig WJ, Meier B. The concurrent validity of the N-back task as a working memory measure. Memory. 2010;18:394–412. doi: 10.1080/09658211003702171. [DOI] [PubMed] [Google Scholar]
- 34.Daneault V, Hebert M, Albouy G, et al. Aging reduces the stimulating effect of blue light on cognitive brain functions. Sleep. 2014;37:85–96. doi: 10.5665/sleep.3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gaggioni G, Maquet P, Schmidt C, Dijk DJ, Vandewalle G. Neuroimaging, cognition, light and circadian rhythms. Front Syst Neurosci. 2014;8:126. doi: 10.3389/fnsys.2014.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]