Abstract
Membrane fusion is a vital process in key cellular events. The fusion capability of a membrane depends on its elastic properties and varies with its lipid composition. It is believed that as the composition varies, the consequent change in C0 (monolayer spontaneous curvature) is the major factor dictating fusion, owing to the associated variation in GEs (elastic energies) of the fusion intermediates (e.g. stalk). By exploring the correlations among fusion, C0 and Kcp (monolayer bending modulus), we revisit this long-held belief and re-examine the fusogenic contributions of some relevant factors. We observe that not only C0 but also Kcp variations affect fusion, with depression in Kcp leading to suppression in fusion. Variations in GEs and inter-membrane interactions cannot account for the Kcp-fusion correlation; fusion is suppressed even as the GEs decrease with Kcp, indicating the presence of factor(s) with fusogenic importance overtaking that of GE. Furthermore, analyses find that the C0 influence on fusion is effected via modulating GE of the pre-fusion planar membrane, rather than stalk. The results support a recent proposition calling for a paradigm shift from the conventional view of fusion and may reshape our understanding to the roles of fusogenic proteins in regulating cellular fusion machineries.
Membrane fusion is vital for living organisms. Many cellular events, such as the release of neurotransmitters, the invasion of enveloped viruses, the intracellular trafficking of proteins and the conception for sexual reproduction, involve membrane fusion1,2. Complete of the fusion process sees two membrane-bound entities merge into a single one, with the initially discrete membranes and the enclosed contents mixed together. Cellular implementation of fusion requires the concerted action of an intricate machinery consisting of lipids, fusogenic proteins and fusion-triggering stimulants (e.g., Ca2+)3,4. While the wide diversity of the lipids, proteins and other biomolecules involved in cellular fusion often complicates the attempts to explore the inner working shared by various fusion machineries, protein-free model membranes with defined lipid compositions [e.g., liposome, also known as unilamellar vesicle (ULV), a hollow spherical structure bound with a single lipid bilayer] have been proven an indispensable tool in uncovering the universal mechanism for all sorts of fusion3,5.
It is known from model membrane studies that initiating and advancing the fusion process demand the overcoming of several energy barriers; recognizing these barriers has provided insight on how proteins regulate cellular fusion machineries3,5. The first energy barrier arises from the need to bring two fusion-destined membranes into close proximity to initiate fusion6,7. The barrier, an inter-membrane interaction known as hydration repulsion, results from the resistance to removing inter-membrane water needed for shortening the inter-membrane distance8. Once fusion is initiated, the next energy barriers are related to the structural transformations of the cis-monolayers (the proximal leaflets of the lipid bilayers undergoing fusion)4,9. Theoretical studies predict that the monolayers transform from the initial planar conformation to two fusion intermediate structures, hemifusion stalk and hemifusion diaphragm (HD), before forming a fusion pore (FP) to complete the process. The hemifusion structures are highly curved. Analogous to the well-studied lamellar ↔ nonlamellar phase transitions of lipid dispersions10, the transition from planar monolayers to the hemifusion structures also entails the membrane deformations that implicate the monolayer elastic energy density, gE,
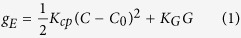 |
where Kcp is the monolayer bending modulus, C the total curvature, C0 the monolayer spontaneous curvature (the molar-weighted average of the C0s of the constituting lipids), KG the Gaussian modulus and G the Gaussian curvature11,12. When free of any external constraint, a monolayer of a specific C0 tends to form a structure with C = C0. Any deviation of C from C0 increases gE, destabilizes the monolayer structure possessing the given C and raises the energy barrier to the formation of this structure. Accordingly, a monolayer having |C0| >> 0 exhibits a high tendency of forming nonlamellar structures, with the sign of the C0 indicating its preference for normal (positive) or inverted (negative) nonlamellar structures, while monolayers with |C0| ≈ 0 prefer a planar conformation.
The energy barriers, and thus the fusion capability, can be reduced/raised by tuning the membrane composition. Experiments show that adjusting the composition of a ULV alters its fusion capability considerably, which is attributable to the consequent changes in gE and/or hydration repulsion6,13. Conventionally, the former is considered as the major factor affecting fusion and is induced by introducing to membranes the lipids of negative C0s, because hemifusion stalk, whose existence has been experimentally confirmed14, is a structure with negative Cs for the cis-monolayers (Fig. 1). Nevertheless, varying C0 is merely one approach to change gE; varying Kcp can be another, as manifested in Equation (1). While many efforts have been dedicated to exploring the effects of varying C0 on fusion and their underlying mechanisms, few, if any, take on the issue of how, or even whether, the change in Kcp affects the energy barriers and thus fusion of a membrane, even though such investigations will surely provide further insight. Moreover, a recent X-ray study demonstrated the need to shift the conventional “C0-centered” view on fusion because hydration repulsion was proven as energetically important as gE, if not more so, to fusion6. It is therefore of great value to re-examine the fusogenic importance of elastic mechanics and explore the fusogenic relevance of other factors.
Figure 1. Hemifusion stalk.
Color encodes the origins of the lipid molecules. In this fusion stage, the lipid molecules of the two cis-monolayers have intermixed while the trans-monolayers and the contents are still separated.
This study aspires to address these issues and provide further insight on the fusion mechanisms of protein-free lipid membranes, in the hope that the learned knowledge will advance our understanding to the working principles general to fusogenic proteins. Several experimental techniques (e.g., X-ray diffraction, the electron density reconstruction and fluorescence spectroscopy) have been employed and an experimental scheme (specifically for the Kcp measurements) developed to measure 1) the C0s and Kcps of several lipid species [i.e., the species carrying 18 carbon-long tails of various saturation degrees: dioleoylphosphatidylethanolamine (DOPE or 18:1 PE), 18:2 PE, 18:3 PE, dioleoylphosphatidylcholine (DOPC or 18:1 PC), 18:2 PC and 18:3 PC] and 2) the fusion efficiencies of ~150-nm ULVs (i.e., large unilamellar vesicles, LUVs) as functions of C0 and Kcp via tuning the lipid composition. To the best of our knowledge, the measurements are the first systematic investigation on the correlations among C0, Kcp, the hydrocarbon (CH) chain saturation of a lipid and fusion. Energetic analyses on the C0- and Kcp-dependences of fusion reveal the fusogenic relevance of a factor(s) other than elastic mechanics and inter-membrane interactions, whose fusogenic importance even overtakes that of C0 and GE, and also provide a new energetic perspective on how fusion is promoted by making C0 more negative. The results complement the earlier studies by expanding the known effects of changing the lipid composition on the fusion energetics, and may reshape the current understanding to the fusion mechanism.
Results
Elastic properties of DOPE and DOPC
The C0 and Kcp of DOPE are measured to compare against the published data to establish credibility for our experimental protocol and data. The C0 of DOPE measured here, C0,DOPE = −0.0334 ± 0.0001 Å−1 [=1/(−29.9 ± 0.1) Å−1] at 20 °C (Table 1), agrees well with the literature [e.g., C0,DOPE = 1/(−30.2 ± 0.8) Å−1 at 20 °C; C0,DOPE = 1/(−30) Å−1 at 20 °C]15,16. The thermal variation rate of C0,DOPE, −0.00014 ± 0.00004 Å−1 °C−1, is also consistent with the reports by refs 15,17 (−0.00017 Å−1 °C−1 and −0.00013 ± 0.00004 Å−1 °C−1, respectively). A similar consistency is also observed for Kcp, where Kcp,DOPE = 0.57 ± 0.12 × 10−19 J (≈13.9 ± 2.9 KBT) at 25 °C conforms to, e.g., Kcp,DOPE = 0.46 × 10−19 J (≈11.2 KBT) at 20 °C in ref. 16 and Kcp,DOPE = 0.53 × 10−19 J (≈12.9 KBT) at 22 °C in ref. 18.
Table 1. C0 and Kcp of the studied lipids and LUVs.
|
C0 (Å−1) |
Kcp (×10−19 J) | |||||||
|---|---|---|---|---|---|---|---|---|
| Species | 15 °C | 20 °C | 25 °C | 30 °C | 35 °C | 40 °C | 25 °C | |
| DOPE | −0.0329 ± 0.0001 | −0.0334 ± 0.0001 | −0.0341 ± 0.0002 | −0.0347 ± 0.0003 | −0.035 ± 0.0002 | −0.0366 ± 0.0001 | 0.57 ± 0.12 | |
| 18:2 PE | −0.0345 ± 0.00004 | −0.0350 ± 0.00005 | −0.0356 ± 0.00002 | −0.0361 ± 0.00005 | −0.0365 ± 0.00007 | −0.0379 ± 0.0002 | 0.33 | |
| 18:3 PE | −0.0337 ± 0.00006 | −0.0347 ± 0.00006 | −0.0355 ± 0.00006 | −0.0362 ± 0.00006 | −0.0367 ± 0.00006 | −0.0383 ± 0.00006 | — | |
| DOPC | −0.0094 ± 0.0005 | −0.0102 ± 0.0007 | −0.0106 ± 0.0008 | −0.0111 ± 0.0010 | −0.0116 ± 0.0009 | −0.0120 ± 0.0011 | — | |
| 18:2 PC | −0.0106 ± 0.0006 | −0.0112 ± 0.0008 | −0.0117 ± 0.0008 | −0.0123 ± 0.0009 | −0.0128 ± 0.0009 | −0.0132 ± 0.0013 | 0.14 | |
| 18:3 PC | −0.0086 ± 0.0007 | −0.0095 ± 0.0014 | −0.0099 ± 0.0015 | −0.0104 ± 0.0017 | −0.0109 ± 0.0019 | −0.0113 ± 0.0021 | 0.14 | |
| DOPE/DOPC LUVs (mol% of DOPE) | 0 | — | — | −0.0106 ± 0.0008 | — | — | — | 0.40 |
| 25 | — | — | −0.0165 ± 0.0006 | — | — | — | 0.44 | |
| 30 | — | — | −0.0177 ± 0.0006 | — | — | — | 0.45 | |
| 40 | — | — | −0.02 ± 0.0005 | — | – | — | 0.47 | |
| 50 | — | — | −0.0224 ± 0.0004 | — | — | — | 0.49 | |
| 67 | — | — | −0.0264 ± 0.0003 | — | — | — | 0.51 | |
| Equimolar DOPE/PC LUVs (PC species) | 18:1 | — | — | −0.0224 ± 0.0008 | — | — | — | 0.49 |
| 18:2 | — | — | −0.0229 | — | — | — | 0.35 | |
| 18:3 | — | — | −0.0220 | — | — | — | 0.36 | |
The C0 of DOPC, derived from the linear correlation between C0 and lipid composition (see Methods), is measured as C0,DOPC = −0.0111 ± 0.001 Å−1 [=1/(−90.1 ± 8.1) Å−1] at 30 °C and C0,DOPC = −0.0116 ± 0.0009 Å−1 [=1/(−86.2 ± 6.7) Å−1] at 35 °C. These are comparable to C0,DOPC = 1/(−110.0 ± 9.7) Å−1 at 35 °C in ref. 17 and C0,DOPC = 1/(−87.3) Å−1 at 32 °C in ref. 18. (Note that the uncertainties for C0,DOPCs in this study and ref. 17 are intrinsic to the C0-determination method and do not arise from experimental errors.) The thermal variation rate of C0,DOPC, −0.00010 ± 0.00002 Å−1 °C−1, is also agreeable with −0.00011 ± 0.00006 Å−1 °C−1 in ref. 17. The consistency with the published data validates the protocol used here and establishes credibility for the data below.
Elastic properties of the polyunsaturated PEs and PCs
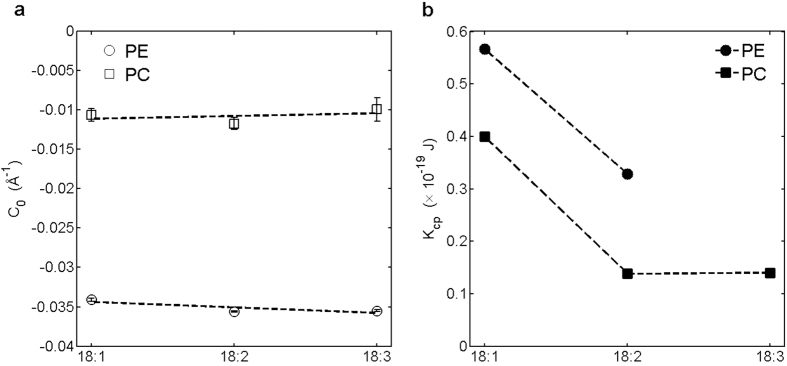
The C0 measurements for the unsaturated PEs and PCs show that carrying tails with different saturation degrees has only marginal effects on the C0s of the lipids, with the C0s barely shifting from C0,PE = −0.0341 ± 0.0002 Å−1 [=1/(−29.3 ± 0.2) Å−1] to −0.0355 ± 0.0001 Å−1 [=1/(−28.2 ± 0.1) Å−1] and from C0,PC = −0.0106 ± 0.0008 Å−1 [=1/(−94.3 ± 7.1) Å−1] to −0.0099 ± 0.0015 Å−1 [=1/(−101.0 ± 15.3) Å−1] when the CH chains go from monounsaturated to triunsaturated at 25 °C (Fig. 2a). This is unexpected, since CH chains with higher unsaturation degrees are more flexible and shall be more capable of assuming the splaying-out conformations, making the lipid molecules more cone-shaped and the C0s more negative, as in raising temperature.
Figure 2.
Dependences on the CH chain saturation of (a) the C0s and (b) the Kcps of PE and PC. Note the Kcp,DOPC is adopted from refs 17,18.
On the contrary, Kcp is dependent on the saturation degree. The Kcps of PE and PC are smaller for the lipids carrying tails with higher unsaturation degrees (Fig. 2b). A monolayer is thus softer when constituted by di- or triunsaturated lipids than would be when constituted by the monounsaturated counterparts. Together with Kcp,DOPC = 0.42 ± 0.05 × 10−19 J at 18 °C from ref. 19 and Kcp,DOPC = 0.40 ± 0.04 × 10−19 J at 30 °C from ref. 20, Kcp,PC varies from Kcp,PC = ~0.4 × 10−19 J (≈9.7 KBT) to 0.14 × 10−19 J (≈3.4 KBT) when the number of the cis-double bonds in a CH chain goes from 1 to 3 (Fig. 2b). A similar trend is observed for PE: Kcp,PE decreases from Kcp,PE = 0.57 × 10−19 J (≈13.9 KBT) to 0.33 × 10−19 J (≈8 KBT) when the chain saturation goes from monounsaturated to diunsaturated. Overall, variations by a factor of ~2 are observed for PE and PC when the CH chains go from monounsaturated to polyunsaturated.
It is noted that our results for Kcp,PC are consistent with ref. 19: The Kcp,PC in the reference (measured with the micropipette-aspiration technique) declined sharply from Kcp,PC = 0.42 ± 0.05 × 10−19 J (≈10.2 ± 1.2 KBT) to 0.22 ± 0.04 × 10−19 J (≈5.3 ± 1 KBT) as the chains go from monounsaturated to diunsaturated, but the Kcp,PC changed only modestly when the chains go further to triunsaturated. (Note that the property measured in ref. 19 was the bilayer bending modulus. Following ref. 21, this is converted to its monolayer counterpart by dividing it by 2, with the assumption of no coupling between the two monolayers). Even with different experimental techniques and an oversimplified assumption of no inter-monolayer coupling, the discrepancy between the Kcps obtained here and in ref. 19 is still remarkably small. The consistency warrants the application of the experimental scheme developed here (see Methods) to measuring the Kcps of the lamellar-forming lipids.
C 0 and K cp dependences of fusion
Based on the C0 and Kcp measurements, we manage to tune the composition of a monolayer so that one of the two properties varies while the other remains constant. This objective is achieved satisfyingly for varying Kcp but only with limited success for C0. With such a composition control, we study the correlation between fusion and Kcp (or C0) for LUVs. Two series of LUVs are prepared: one series composed of DOPE and DOPC with the molar fraction varied; the other composed of equimolar DOPE and PC with the PC selected from among DOPC, 18:2 PC or 18:3 PC. Fusion is initiated by PEG 8000 and detected/quantified fluorescently (fluorescence is emitted after fusion due to the complexation of the dyes, DPA and TbCl3, initially encapsulated in separate LUVs; see Methods)22.
The C0 dependence of fusion is studied by examining fusion between the DOPE/DOPC LUVs. The monolayer spontaneous curvature of the LUVs, C0,LUV, varies from −0.0106 ± 0.0008 Å−1 [=1/(−94.3 ± 7.1) Å−1)] to −0.0264 ± 0.0003 Å−1 [=1/(−37.9 ± 0.4) Å−1], while the monolayer bending modulus, Kcp,LUV (assumed to be the molar-weighted average of Kcp,DOPE and Kcp,DOPC), is kept at a narrower range of 0.4 × 10−19 J (≈9.7 KBT) to 0.51 × 10−19 J (≈12.2 KBT), when DOPE rises from 0 mol% to 67 mol% (Table 1). As the DOPE fraction rises, the extent of fusion for the LUVs also rises (Fig. 3a). A comparison where the relative changes [relative change = 100% × {(property determined at a given [DOPE]) – (property determined at [DOPE] = 50 mol%)}/{property determined at [DOPE] = 50 mol%}] in C0, Kcp and fusion are plotted against DOPE fraction demonstrates the expected correlation between fusion and C0,LUV reported in the literature (Fig. 3c): It was reported that ULVs with higher contents in lipids of negative C0s, or prepared with lipids of more negative C0s, displayed a higher propensity for fusion13,23, while introducing to ULVs lipids of strongly positive C0s could inhibit fusion24, whether the systems were protein-free or contained fusogenic proteins. However, it has to be stressed that the variation in Kcp,LUV may still be substantial enough to sway fusion, which is further discussed below.
Figure 3.
Fusion behavior for (a) the DOPE/DOPC and (b) the equimolar DOPE/PC LUVs. The relative changes in fusion, C0,LUV and Kcp,LUV as functions of (c) DOPE fraction and (d) PC chain saturation display the correlation among fusion, C0 and Kcp. The dashed lines are to guide the eyes; the solid lines in (c,d) mark the 0% and 70% changes.
The Kcp dependence of fusion is studied on the DOPE/PC LUVs. Substituting 18:2 PC or 18:3 PC for DOPC in the LUVs varies the Kcp,LUV from 0.49 × 10−19 J (≈11.8 KBT) to 0.35 × 10−19 J (≈8.6 KBT) or 0.36 × 10−19 J (≈8.6 KBT) but retains the C0,LUV at ~−0.0220 Å−1 [=1/(−45.5) Å−1] (Table 1). Surprisingly, the fluorescence intensity emitted by the dye complexes decreases drastically when the CH chains of PC go from monounsaturated to polyunsaturated; in some instances, the fluorescence for the DOPE/18:3 PC LUVs is even nearly undetectable (Fig. 3b). This sharp decline in fluorescence may originate from 1) a failure to load the dyes into the LUVs, 2) a failure to form LUVs or 3) a depression in fusion of the LUVs. The first two scenarios are excluded because (I) the fluorescence was fully recovered when the structures self-assembled by DOPE/polyunsaturated PC (whether they were LUVs or not) were disrupted by Triton X-100 (a detergent), indicating that the dyes had initially been sequestered by the self-assembled structures; and (II) the self-assembled structures were ~150 nm in size, as with the LUVs made for the rest of the study, suggesting the self-assembled structures being LUVs. Hence, we consider the reduction in fusion as the most probable cause for the sharp decline in fluorescence when the Kcp,LUV decreases, thereby establishing a correlation between fusion and Kcp,LUV (Fig. 3d).
Discussion
The rise in G E of the planar monolayer, rather than the drop in G E of stalk, is responsible for the fusion promotion induced by making C 0 more negative
The fusion promotion by making C0 more negative has long been associated with the energetics of the fusion intermediate structures, especially hemifusion stalk3. Due to its hourglass shape, stalk is more energetically favorable, while a planar conformation is less favored, if the C0s of the underlying monolayers are more negative25. Indeed, experiments show that using the lipid of highly negative C0, diphytanoyl phosphatidylcholine (DPhPC), to prepare oriented bilayers allowed researchers to determine the first X-ray diffraction structure for stalk14; and the difference between the curvature energies [the first term in Equation (1)] of stalk and planar monolayers dropped triply when the composition of the oriented bilayers changed from DOPC alone to equimolar DOPE/DOPC, contributing to the lowering of the osmotic stress minimally needed for stabilizing stalk6. However, it has not been explicitly verified from experimental data whether the preference for stalk is mainly due to a rise in the elastic energy of planar monolayers or a drop in that of stalk, even though the latter is implicitly assumed to be the case in many studies. To resolve the question, we calculate the elastic energy density of a LUV, gE,LUV, for the DOPE/DOPC LUVs via Equation (1). With the measured diameter of ~150 nm (see Methods) and the common bilayer thickness of ~4 nm for the LUVs, the Cs of the inner and outer leaflets are −(1/740 + 1/740) Å−1 and + (1/760 + 1/760) Å−1, respectively, while the Gaussian curvatures (1/740 × 1/740 Å−2 and 1/760 × 1/760 Å−2) are negligible. (With the dimensions, the bilayer can be regarded as locally flat). Based on this and the C0/Kcp data, gE,LUV = gE,inner leaflet + gE,outer leaflet is determined (Table 2). The gE,LUV steadily rises along with the DOPE fraction, leading to its positive correlation with fusion (the Pearson correlation coefficient is 0.8889, Fig. 4), i.e., the higher the gE,LUV is, the more the LUVs are inclined to fusing. The variation in C0 alone accounts for >75% of the change in gE,LUV. Thus, the result establishes a correlation among C0, gE,LUV and fusion: Making C0 more negative elevates gE,LUV, which in turn promotes fusion. More specifically, making C0 more negative aggravates the energetic penalty of keeping the monolayers in the planar conformation, causing the transformation to stalk more appealing in comparison.
Table 2. Calculated elastic energy densities of the LUVs.
| Elastic energy density (× 10−3 KBT/Å2) | ||||
|---|---|---|---|---|
| Species | gE,inner leaflet | gE,outer leaflet | gE,LUV | |
| DOPE/DOPC LUVs (mol% of DOPE) | 0 | 0.3 | 0.9 | 1.2 |
| 25 | 1.0 | 2.0 | 3.0 | |
| 30 | 1.2 | 2.3 | 3.5 | |
| 40 | 1.7 | 2.9 | 4.6 | |
| 50 | 2.3 | 3.7 | 6.0 | |
| 67 | 3.5 | 5.2 | 8.7 | |
| Equimolar DOPE/PC LUVs (PC species) | 18:1 | 2.3 | 3.7 | 6.0 |
| 18:2 | 1.8 | 2.8 | 4.6 | |
| 18:3 | 1.6 | 2.6 | 4.2 | |
Figure 4.
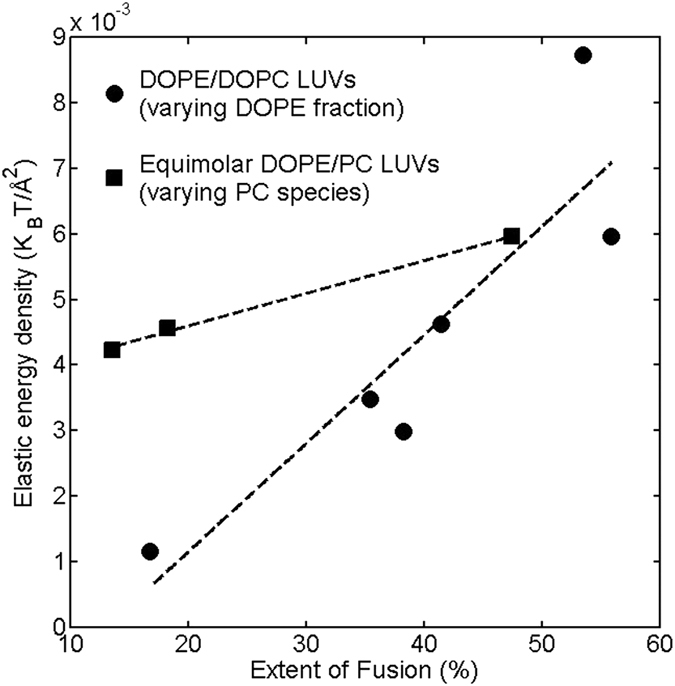
gE,LUV versus fusion for the DOPE/DOPC LUVs with the DOPE fraction varied, and for the equimolar DOPE/PC LUVs with the PC species changed. The dashed lines are to guide the eyes.
The remaining question is: Does making C0 more negative also reduce the elastic energy of stalk? We deduce the elastic energies (defined against the reference point, the elastic energy of a monolayer with C0 = 0 and in the planar conformation), GEs, of the cis-monolayers in the planar and stalk conformations. The difference, ΔGE,ps, between GEs of the two conformations can be expressed as6,
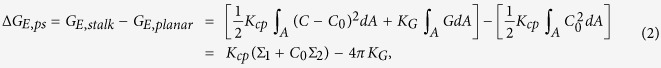 |
with
 |
(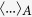 is the average over surface area A) and,
is the average over surface area A) and,
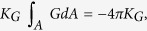 |
for stalk. Based on our C0/Kcp/gE,LUV data, (Σ1, Σ2) = (7.84, 207.6) for DOPC alone and (Σ1, Σ2) = (6.81, 200.5) for equimolar DOPE/DOPC6 and KG ≈ 8 KBT26, ΔGE,ps is determined to be ~155 KBT and ~128 KBT for the cis-monolayers made of DOPC alone and equimolar DOPE/DOPC, respectively. The shrink in the ΔGE,ps upon introducing DOPE is consistent with what is seen for gE,LUV. By adopting from ref. 6 the surface areas of ~7,600 Å2 for monolayers made of DOPC alone and ~8,700 Å2 for equimolar DOPE/DOPC, GE of the cis-monolayers in the planar conformation is determined as GE,planar = gE,outer leaflet × 7,600 = 6.5 KBT (or 0.27 × 10−19 J) for DOPC alone and 32.0 KBT (or 1.32 × 10−19 J) for equimolar DOPE/DOPC. Again, the result is consistent with the observation for gE,LUV. We deduce GE of stalk by summing the corresponding ΔGE,ps and GE,planar. Strikingly, the deduced GE,stalk is ~160 KBT for the cis-monolayers made of both DOPC alone and equimolar DOPE/DOPC. In contrast, GE,planar differs by a factor of 5 or ~26 KBT upon the same change in the composition.
The invariability of GE,stalk may suggest that GE,stalk is a conserved property for monolayers and irrespective of the composition, at least for the two compositions considered here. Given the intimacy between GE and the overall geometry of a structure, this may further imply that the overall geometry of stalk is also universal among monolayers of various compositions. Indeed, experimental and computational studies have shown that the overall geometry of stalk is highly conserved among monolayers of diverse compositions6, as well as among various coarse-grained models used in the computational studies9. Given the energetic/geometric invariability of stalk and the variation in GE,planar upon changing C0, we argue that the fusion promotion by making C0 more negative is effected mainly via a rise in GE,planar, rather than a drop in GE,stalk.
Some may wonder: Equation (1) demands gE,stalk to vary with C0 if C is constant (as seemingly entailed by the geometric invariability of stalk). Thus, how can GE,stalk remains unchanged when the composition varies? One has to realize that the conservation in geometry is not equivalent of the conservation in dimension, or more specifically, in C. C of stalk may vary with the composition so that GE,stalk is unchanged. Indeed, the X-ray diffraction structures of stalk indicate that 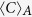 of stalk is ~1/36.6 Å−1 for DOPC alone and ~1/43.4 Å−1 for equimolar DOPE/DOPC6.
of stalk is ~1/36.6 Å−1 for DOPC alone and ~1/43.4 Å−1 for equimolar DOPE/DOPC6.
Changes in the inter-membrane interactions and in G E s of stalk, HD and FP cannot account for the fusion reduction upon raising the chain unsaturation
Several factors may account for the apparent Kcp,LUV-fusion correlation (Fig. 3d). Among these factors is the modulation of the inter-membrane repulsion arising from the membrane undulational motion (i.e., the out-of-plane fluctuation of membrane); enhancing the repulsion would prevent the LUVs from shortening the inter-membrane distances and thus stymie the fusion initiation. The undulation repulsion is entropic in nature, and proportional to temperature while inversely proportional to Kcp27; the inverse proportionality to Kcp is consistent with the observed Kcp,LUV-fusion correlation. To explore the fusogenic relevance of the undulation repulsion, we examine the sizes of the equimolar DOPE/PC LUVs before and after the addition of PEG 8000 with dynamic light scattering. Interestingly, while the DOPE/DOPC LUVs increase their diameters from ~150 nm to ~600 nm, as expected for fusion, the diameter of DOPE/18:2 PC LUVs also expands considerably from ~150 nm to >600 nm (Supplementary Fig. S5). Together with the result of the content-mixing fusion assays for the latter LUV species (Fig. 3b), we infer that raising the chain unsaturation does not prevent the LUVs from approaching one another but still considerably compromises their capabilities of completing the fusion process. Therefore, enhancement of the inter-membrane repulsions is not expected to be responsible for the correlation between chain saturation and fusion.
The apparent Kcp,LUV-fusion correlation may alternatively arise from the influence on GE of varying Kcp. We again deduce GE,planar and ΔGE,ps to explore the underlying mechanism in the context of stalk formation. The surface area of the cis-monolayers is assumed to remain ~8,700 Å2, even though the PC of the equimolar DOPE/PC monolayers is either 18:2 PC or 18:3 PC rather than DOPC (the interfacial areas of lipids with the same headgroup but distinct chains differ marginally when they are in the same phase at a given temperature)28,29. The deduced GE,planars are 32.0 KBT, 24.4 KBT and 22.8 KBT for DOPE/DOPC, DOPE/18:2 PC and DOPE/18:3 PC, respectively. The variation is modest in comparison with the case where the DOPE fraction (and C0) of the DOPE/DOPC monolayers is varied. On the other hand, we expect the energetic/geometric invariability of stalk to be valid for the DOPE/PC monolayers and GE,stalk to remain ~160 KBT, because the stalk geometry is highly conserved among monolayers made of a wide variety of lipids (including DPhPC, which carries bulky CH chains)6. Hence, ΔGE,ps is ~136.5 KBT for both DOPE/18:2 PC and DOPE/18:3 PC. Thus, varying Kcp by ~27%, while steadying C0 at ~−0.0220 Å−1, only raises ΔGE,ps from ~128 KBT to ~136.5 KBT. Interestingly, this modest rise by ~8.5 KBT is sufficient to reduce fusion by 70% (Fig. 3d), while the same extent of decline in fusion is reached only when ΔGE,ps increases by ~27 KBT for the case of varying the DOPE fraction (Fig. 3c). The large discrepancy between the two cases indicates that an extra factor(s), other than the elastic energy involved in the planar-to-stalk transformation, may act to dictate fusion. Indeed, as shown in Fig. 4, the variation in fusion is less correlated with gE,LUV for varying the PC species than for varying the DOPE fraction.
In addition to the one related to stalk, GEs of HD and FP may also contribute to the apparent Kcp,LUV-fusion correlation. To examine the significance of these contributions, we numerically estimate how the GEs would vary with Kcp,LUV upon substituting 18:2 PC for DOPC through,
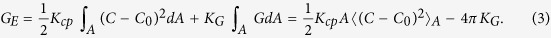 |
KGs of the LUVs are estimated by multiplying their Kcps with the ratio of –KG/Kcp, which is about 0.84 for DOPC, DOPE and even their mixtures with other lipids (e.g., DOPC/sphingomyelin/cholesterol)30. Integrating G over a given surface area also yields −4π for both HD and FP due to the identity of their topological genus to that of stalk31. Due to the lack of information on how Cs of HD and FP vary locally and with the composition, we adopt a wide range of Cs, −0.05 ≤ C ≤ + 0.018, for our estimation to include all possible variations in C, with the lower limit more negative than any known negative C0 for a phospholipid17 and the upper limit even greater than Cs of the most curved region of FP32. The result shows that GE,HD and GE,FP would be constantly higher for DOPE/DOPC than for DOPE/18:2 PC, however C varies locally and with the composition (Table 3). This result contradicts with the observation that the DOPE/DOPC LUVs fuse more readily than the DOPE/18:2 PC LUVs (Fig. 3b), thus suggesting the irrelevance of GE,HD and GE,FP to the observed difference in fusion.
Table 3. Elastic energy estimations for the monolayers of HD and FP in different compositions.
| Curvature energy term | Gaussian term | GE | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Lipid composition | Kcp (×10−19 J) | C (Å) | C0 (Å) | KcpA(C − C0)2/2 (×10−19 J) | KG | ∫GdA | KG∫GdA (×10−19 J) | (×10−19 J) | |
| DOPE/DOPC | Negative C | 0.49 | −0.05 ~ −0.022 | −0.022 | 1.67 ~ 0 | −0.41 | −4π | 5.15 | 6.82 ~ 5.15 |
| −0.022 ~ 0 | 0 ~ 1.03 | 5.15 ~ 6.18 | |||||||
| Positive C | 0 ~ 0.018 | 1.03 ~ 3.41 | 6.18 ~ 8.56 | ||||||
| DOPE/18:2PC | Negative C | 0.35 | −0.05 ~ −0.022 | −0.022 | 1.19 ~ 0 | −0.29 | −4π | 3.64 | 4.84 ~ 3.64 |
| −0.022 ~ 0 | 0 ~ 0.74 | 3.64 ~ 4.38 | |||||||
| Positive C | 0 ~ 0.018 | 0.74 ~ 2.44 | 4.38 ~ 6.08 | ||||||
Conclusion
Overall, neither the modulation of inter-membrane interactions nor the changes in ΔGE,ps, GE,stalk, GE,HD and GE,FP (GE,HD and GE,FP decrease even when fusion is suppressed!) can account for the apparent Kcp,LUV-fusion correlation. This may indicate the presence of an unrecognized factor that is subject to Kcp and dictates fusion. It is known from Fig. 3c,d that fusion is depressed by ~70% when one either decreases Kcp by ~17% plus C0 by >50%, or simply decreases Kcp by ~27% without varying C0. It seems that depressing Kcp to enhance this unrecognized factor alone is sufficient to suppress fusion to a great extent; if Kcp is slightly less depressed, the same extent of fusion suppression is achieved only when C0 is simultaneously changed by a much larger degree. It is thus tempting to claim that this factor is at least as important as C0 and GE in dictating fusion, if not more so. Another possibility is that the variation in Kcp is only a consequence of changing the LUV composition and does not directly affects fusion; it is another effect arising from the composition change that is responsible for the differential fusion capabilities. One such candidate is the variation in the tilt modulus, which is associated with the CH chain stretching and tilting when the chains are arranged to form stalk and HD33,34. Indeed, raising the chain unsaturation increases the tilt modulus35,36 and may thus aggravate the related energetic penalties of maintaining stalk and HD34. Another candidate is the variation in the hydrophobic interactions arising from exposing the CH chains of cis-monolayers to water during the stalk formation34. Nevertheless, quantifying the contributions of the two effects is out of the scope of the paper. Further studies are still desired to quantify the effects or to identify other responsible factor(s).
Conventionally, upon introducing to membranes lipids of highly negative or positive C0s, the change in fusion is often attributed to the change in C0 (and consequently in GE), while the accompanying variations in Kcp and/or other potential factors are overlooked. By examining the apparent Kcp,LUV-fusion correlation, we have demonstrated that a factor(s) other than the variations in C0 and GE, which is affected by or accompanies the Kcp variation, plays a key role in dictating fusion when the membrane composition is changed; the fusogenic importance of the factor(s) may even overtake the importance of C0 or membrane elastic energetics in general. We therefore suggest that caution shall be used when one interprets the influence on fusion of varying C0 via membrane composition changes. This understanding, along with the conclusion that promoting fusion by making C0 more negative is effected via a rise in GE,planar, may reshape the energetic considerations on fusion, particularly the roles of fusogenic proteins in regulating cellular fusion machineries.
This study also made two intriguing observations: (1) Modulating the chain saturation (with the length fixed) of a lipid from monounsaturated to triunsaturated has virtually no effect on its C0. The observation falsifies the expectation that raising the unsaturation degree always makes C0 more negative; (2) our developed scheme enables the application of the osmotic stress method to the Kcp measurements for lipids not preferring the formation of the hexagonal HII phase (formation of the phase is a prerequisite for the conventional osmotic stress method), providing an extra means for the Kcp determination.
Methods
C 0 Measurement
Excessive buffer containing 10 mM HEPES with pH ≈ 7.4 was used to suspend a dried mixture of lipid and tetradecane. The dispersion was shuffled between two glass syringes for >100 runs and underwent >10 freeze-thaw cycles for homogenization. Each sample had a fixed lipid composition and contained 16 wt% tetradecane. The measurements by X-ray diffraction were carried out with Cu Kα or synchrotron radiation (BL13A1 and BL23A1 of NSRRC, Hsinchu, Taiwan). The diffraction images were recorded with a Pilatus 100 K pixel (Dectris, Switzerland), a Mar165 CCD and a Pilatus 1MF pixel detectors for Cu Kα, BL13A and BL23A1, respectively.
Data were collected at 15 °C to 40 °C with a 5 °C interval. The reduced, azimuthally integrated and background-subtracted 1-D diffraction profiles of the lipids forming the HII phase were used to reconstruct the electron density profiles through 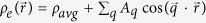 and
and 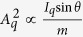 , where Iq is the diffraction intensity, sin θ the Lorentz correction and m the multiplicity factor37. Following the method in ref. 15, the radial distances, Rps, between the HII center and the pivotal plane were extracted from the profiles and experimentally determined the C0s of the HII-forming PEs. For the PCs, which preferred the formation of lamellar phases, a series of binary DOPE/PC mixtures were prepared; the molar fractions were controlled such that HII was the sole stable phase in excess water and tetradecane and the mixture C0s were determined as for DOPE alone. The C0s showed linear correlations with the PC fraction (Supplementary Fig. S1). Extrapolating the correlations to pure PC yielded its C0. A sample, with a fixed lipid composition, only contributed to a data point in Supplementary Fig. S1.
, where Iq is the diffraction intensity, sin θ the Lorentz correction and m the multiplicity factor37. Following the method in ref. 15, the radial distances, Rps, between the HII center and the pivotal plane were extracted from the profiles and experimentally determined the C0s of the HII-forming PEs. For the PCs, which preferred the formation of lamellar phases, a series of binary DOPE/PC mixtures were prepared; the molar fractions were controlled such that HII was the sole stable phase in excess water and tetradecane and the mixture C0s were determined as for DOPE alone. The C0s showed linear correlations with the PC fraction (Supplementary Fig. S1). Extrapolating the correlations to pure PC yielded its C0. A sample, with a fixed lipid composition, only contributed to a data point in Supplementary Fig. S1.
K cp Measurement
Except that the buffer contained PEG 8000 and the data were taken at 25 °C only, the samples were prepared similarly as above. Given the Flory radius of ~8 nm, PEG molecules could not enter the HII water core (<4 nm) and thus applied an osmotic stress on HII. The consequent structural deformation of HII was given by 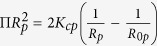 , where Π is the osmotic stress and Rop = 1/C016. The slopes of the
, where Π is the osmotic stress and Rop = 1/C016. The slopes of the 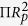 -versus-1/Rp relation gave the Kcps of the HII-forming PEs. An experimental scheme was developed to measure the Kcps of the lamellar-forming PCs. A series of binary DOPE/PC mixtures were prepared and individually subjected to various osmotic stresses when HII was the sole stable phase in excessive buffer and tetradecane. Supplementary Fig. S2 exemplifies the data thereby collected. Based on such data, we obtained the
-versus-1/Rp relation gave the Kcps of the HII-forming PEs. An experimental scheme was developed to measure the Kcps of the lamellar-forming PCs. A series of binary DOPE/PC mixtures were prepared and individually subjected to various osmotic stresses when HII was the sole stable phase in excessive buffer and tetradecane. Supplementary Fig. S2 exemplifies the data thereby collected. Based on such data, we obtained the 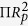 -versus-1/Rp relations for DOPE/18:3 PC and DOPE/18:2 PC of various molar ratios (Supplementary Fig. S2b) and extracted the Kcps of the mixtures. (Note each data point in supplementary Fig. S2 corresponds to an independently prepared sample.) We then established the Kcp-composition relations for the mixtures, which were mostly linear (Supplementary Fig. S3) and could determine the Kcps of the PCs as pure substances. 16 independent samples were in average used to determine the Kcp of a PC, which is thus statistically sound and representative.
-versus-1/Rp relations for DOPE/18:3 PC and DOPE/18:2 PC of various molar ratios (Supplementary Fig. S2b) and extracted the Kcps of the mixtures. (Note each data point in supplementary Fig. S2 corresponds to an independently prepared sample.) We then established the Kcp-composition relations for the mixtures, which were mostly linear (Supplementary Fig. S3) and could determine the Kcps of the PCs as pure substances. 16 independent samples were in average used to determine the Kcp of a PC, which is thus statistically sound and representative.
Fusion Assay
Two populations of ULVs encapsulating either TbCl3 or DPA (fluorescent dyes) were prepared with extrusion38. A dried mixture of DOPE, PC and DOPA was prepared similarly as above, with the DOPA fixed at 4 mol% and no tetradecane added. (Adding DOPA was to introduce negative surface charges to facilitate the ULV formation; owing to the tiny, fixed amount and C0,DOPA ≈ C0,DOPC the DOPA contribution was negligible15,39). Buffer containing either (a) 2.5 mM TbCl3, 50 mM sodium citrate and 10 mM HEPES (pH = 7.4) or (b) 50 mM DPA and 10 mM HEPES (pH = 7.4) was used to suspend the lipid mixtures and the final lipid concentration was 5 mg/ml. Extrusion was carried out with Mini-Extruder system (Avanti) and a polycarbonate membrane at 40 °C. Unloaded dyes were removed with dialysis (3.5K MWCO, SnakeSkin dialysis tubing). The lipid concentrations were unchanged throughout the dialysis. Removal of unloaded dyes was confirmed by the lack of fluorescence from mixing the two ULV populations in the absence of PEG 8000. Small-angle X-ray scattering confirmed the ULV formation, rather than MLVs (Supplementary Fig. S4). The ULV size was measured with dynamic light scattering at 25 °C. Regardless of the composition, all our ULV samples had the average diameter of ~150 nm with narrow size distributions (Supplementary Fig. S5, upper panels).
Fusion was quantified with fluorescence from the DPA/TbCl3 complexes formed upon fusion of the LUVs. The assay was carried out by mixing the solutions of (a) DPA-loading LUVs, (b) TbCl3-loading LUVs, and (c) 50 wt% PEG 8000 and 1 mM EDTA, in the ratio of 100:100:800 μl. PEG 8000 initiated fusion by modifying the water chemical potential and hydration repulsion. The emission at 545 nm upon fusion was excited at 276 nm in a spectrofluorometer at 25 °C. The extent of fusion was determined as: Extent of fusion (%) = 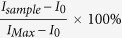 , where Isample and I0 were the intensities of the sample at equilibrium and before fusion, respectively; and IMax was the maximally possible intensity and obtained by adding 0.1% (v/v) Triton X-100, which disrupted the LUVs and released the dyes to maximize the number of the complexes formed. In measuring Isample, EDTA was present outside the LUVs to sequester leaked TbCl3 so that the detected emission was from fusion.
, where Isample and I0 were the intensities of the sample at equilibrium and before fusion, respectively; and IMax was the maximally possible intensity and obtained by adding 0.1% (v/v) Triton X-100, which disrupted the LUVs and released the dyes to maximize the number of the complexes formed. In measuring Isample, EDTA was present outside the LUVs to sequester leaked TbCl3 so that the detected emission was from fusion.
Additional Information
How to cite this article: Fan, Z.-A. et al. Revisit the Correlation between the Elastic Mechanics and Fusion of Lipid Membranes. Sci. Rep. 6, 31470; doi: 10.1038/srep31470 (2016).
Supplementary Material
Acknowledgments
This study is supported by the Ministry of Science and Technology (Grant no.: MOST 104-2221-E-008-113-MY2) and by National Central University. We thank the scientific and technical supports from the staff scientists of Beamlines BL13A and BL23A of NSRRC.
Footnotes
Author Contributions Z.-A.F. and K.-Y.T. contributed equally to the work. Y.-F.C. initiated and laid out the strategy for the work. Z.-A.F., K.-Y.T. and Y.-F.C. designed the experiments. Z.-A.F., K.-Y.T. and S.-H.C. performed the experiments. Z.-A.F., K.-Y.T. and Y.-F.C. processed, analyzed and drew conclusions from the data. Y.-F.C. supervised the work and wrote the paper. All authors reviewed the manuscript.
References
- Jahn R. & Fasshauer D. Molecular machines governing exocytosis of synaptic vesicles. Nature 490, 201–207 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly S. A., Jackson J. O., Jardetzky T. S. & Longnecker R. L. Fusing structure and function: a structural view of the herpesvirus entry machinery. Nat. Rev. Microbiol. 9, 369–381 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernomordik L. V. & Kozlov M. M. Mechanics of membrane fusion. Nat. Struct. Mol. Biol. 15, 675–683 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner W. & Schekman R. Membrane fusion. Nat. Struct. Mol. Biol. 15, 658–664 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden H. R., Tomatsu I. & Kros A. Model systems for membrane fusion. Chem. Soc. Rev. 40, 1572–1585 (2011). [DOI] [PubMed] [Google Scholar]
- Aeffner S., Reusch T., Weinhausen B. & Salditt T. Energetics of stalk intermediates in membrane fusion are controlled by lipid composition. Proc. Natl. Acad. Sci. USA 109, E1609–E1618 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khattari Z., Köhler S., Xu Y., Aeffner S. & Salditt T. Stalk formation as a function of lipid composition studied by X-ray reflectivity. Biochim. Biophys. Acta 1848, 41–50 (2015). [DOI] [PubMed] [Google Scholar]
- Rand R. P. & Parsegian V. A. Hydration forces between phospholipid bilayers. Biochim. Biophys. Acta 988, 351–376 (1989). [Google Scholar]
- Fuhrmans M., Marelli G., Smirnova Y. G. & Müller M. Mechanics of membrane fusion/pore formation. Chem. Phys. Lipids 185, 109–128 (2015). [DOI] [PubMed] [Google Scholar]
- Siegel D. P. & Epand R. M. The mechanism of lamellar-to-inverted hexagonal phase transitions in phosphatidylethanolamine: implications for membrane fusion mechanisms. Biophys. J. 73, 3089–3111 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seddon J. M. Structure of the inverted hexagonal (HII) phase, and non-lamellar phase transitions of lipids. Biochim. Biophys. Acta 1031, 1–69 (1990). [DOI] [PubMed] [Google Scholar]
- Gruner S. M. Stability of lyotropic phases with curved interfaces. J. Phys. Chem. 93, 7562–7570 (1989). [Google Scholar]
- Churchward M. A. et al. Specific lipids supply critical negative spontaneous curvature—an essential component of native Ca2+-triggered membrane fusion. Biophys. J. 94, 3976–3986 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L. & Huang H. W. Observation of a membrane fusion intermediate structure. Science 297, 1877–1879 (2002). [DOI] [PubMed] [Google Scholar]
- Chen Y. F., Tsang K. Y., Chang W. F. & Fan Z. A. Differential dependencies on [Ca2+] and temperature of the monolayer spontaneous curvatures of DOPE, DOPA and cardiolipin: effects of modulating the strength of the inter-headgroup repulsion. Soft Matter. 11, 4041–4053 (2015). [DOI] [PubMed] [Google Scholar]
- Fuller N., Benatti C. R. & Rand R. P. Curvature and bending constants for phosphatidylserine-containing membranes. Biophys. J. 85, 1667–1674 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollmitzer B., Heftberger P., Rappolt M. & Pabst G. Monolayer spontaneous curvature of raft-forming membrane lipids. Soft Matter. 9, 10877–10884 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z. & Rand R. P. The influence of cholesterol on phospholipid membrane curvature and bending elasticity. Biophys. J. 73, 267–276 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawicz W., Olbrich K. C., McIntosh T., Needham D. & Evans E. Effect of chain length and unsaturation on elasticity of lipid bilayers. Biophys. J. 79, 328–339 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y. & Nagle J. F. Diffuse scattering provides material parameters and electron density profiles of biomembranes. Phys. Rev. E 69, 040901 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh D. Elastic curvature constants of lipid monolayers and bilayers. Chem. Phys. Lipids 144, 146–159 (2006). [DOI] [PubMed] [Google Scholar]
- Käsbauera M., Lasicb D. D. & Winterhalterc M. Polymer induced fusion and leakage of small unilamellar phospholipid vesicles: effect of surface grafted polyethylene-glycol in the presence of free PEG. Chem. Phys. Lipid 86, 153–159 (1997). [Google Scholar]
- Chang J. et al. Fusion step-specific influence of cholesterol on SNARE-mediated membrane fusion. Biophys. J. 96, 1839–1846 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchward M. A., Rogasevskaia T., Höfgen J., Bau J. & Coorssen J. R. Cholesterol facilitates the native mechanism of Ca2+ -triggered membrane fusion. J. Cell Sci. 118, 4833–4848 (2005). [DOI] [PubMed] [Google Scholar]
- Katsov K., Müller M. & Schick M. Field theoretic study of bilayer membrane fusion. I. Hemifusion mechanism. Biophys. J. 87, 3277–3290 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel D. P. The Gaussian curvature elastic energy of intermediates in membrane fusion. Biophys. J. 95, 5200–5215 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leckband D. & Israelachvili J. Intermolecular forces in biology. Q. Rev. Biophys. 34, 105–267 (2001). [DOI] [PubMed] [Google Scholar]
- Kucerka N., Tristram-Nagle S. & Nagle J. F. Structure of fully hydrated fluid phase lipid bilayers with monounsaturated chains. J. Membr. Biol. 208, 193–202 (2005). [DOI] [PubMed] [Google Scholar]
- Kucerka N. et al. Structure of fully hydrated fluid phase DMPC and DLPC lipid bilayers using X-ray scattering from oriented multilamellar arrays and from unilamellar vesicles. Biophys. J. 88, 2626–2637 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M., Briguglio J. J. & Deserno M. Determining the Gaussian curvature modulus of lipid membranes in simulations. Biophys. J. 102, 1403–1410 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel D. P. & Kozlov M. M. The gaussian curvature elastic modulus of N-monomethylated dioleoylphosphatidylethanolamine: relevance to membrane fusion and lipid phase behavior. Biophys. J. 87, 366–374 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M. B. Minimum membrane bending energies of fusion pores. J. Membr. Biol. 231, 101–115 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlovsky Y. & Kozlov M. M. Stalk model of membrane fusion: solution of energy crisis. Biophys. J. 82, 882–895 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryham R. J., Klotz T. S., Yao L. & Cohen F. S. Calculating Transition Energy Barriers and Characterizing Activation States for Steps of Fusion. Biophys. J. 110, 1110–1124 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablin M. S., Akabori K. & Nagle J. F. Experimental support for tilt-dependent theory of biomembrane mechanics. Phys. Rev. Lett. 113, 248102 (2014). [DOI] [PubMed] [Google Scholar]
- Jablin M. S., Akabori K. & Nagle J. F. Experimental Assessment of Tilt-Dependent Thermal Fluctuations in Lipid Bilayers. Biophys. J. 108 (suppl. 1), p84a (2015). [Google Scholar]
- Harper P. E., Mannock D. A., Lewis R. N., McElhaney R. N. & Gruner S. M. X-ray diffraction structures of some phosphatidylethanolamine lamellar and inverted hexagonal phases. Biophys. J. 81, 2693–2706 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapinski M. M., Castro-Forero A., Greiner A. J., Ofoli R. Y. & Blanchard G. J. Comparison of liposomes formed by sonication and extrusion: rotational and translational diffusion of an embedded chromophore. Langmuir 23, 11677–11683 (2007). [DOI] [PubMed] [Google Scholar]
- Israelachvili J. & Wennerström H. Entropic forces between amphiphilic surfaces in liquids. J. Phys. Chem. 96, 520–531 (1992). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.