Abstract
Dinitrogen (N2)-fixation by cyanobacteria living in symbiosis with pleurocarpous feather mosses (for example, Pleurozium schreberi and Hylocomium splendens) represents the main pathway of biological N input into N-depleted boreal forests. Little is known about the role of the cyanobacterial community in contributing to the observed temporal variability of N2-fixation. Using specific nifH primers targeting four major cyanobacterial clusters and quantitative PCR, we investigated how community composition, abundance and nifH expression varied by moss species and over the growing seasons. We evaluated N2-fixation rates across nine forest sites in June and September and explored the abundance and nifH expression of individual cyanobacterial clusters when N2-fixation is highest. Our results showed temporal and host-dependent variations of cyanobacterial community composition, nifH gene abundance and expression. N2-fixation was higher in September than June for both moss species, explained by higher nifH gene expression of individual clusters rather than higher nifH gene abundance or differences in cyanobacterial community composition. In most cases, ‘Stigonema cluster' made up less than 29% of the total cyanobacterial community, but accounted for the majority of nifH gene expression (82–94% of total nifH expression), irrespective of sampling date or moss species. Stepwise multiple regressions showed temporal variations in N2-fixation being greatly explained by variations in nifH expression of the ‘Stigonema cluster'. These results suggest that Stigonema is potentially the most influential N2-fixer in symbiosis with boreal forest feather mosses.
Introduction
Dinitrogen (N2)-fixation by filamentous heterocystous cyanobacteria living in symbiosis with pleurocarpous feather mosses (for example, Pleurozium schreberi and Hylocomium splendens) has been shown to provide nitrogen (N) to their mosses (Bay et al., 2013), and this serves as a major input of N into boreal forests (DeLuca et al., 2002; Turetsky et al., 2012; Jonsson et al., 2014), and this input can be as high as 3.5 kg N ha−1 per year (Zackrisson et al., 2004; Lindo et al., 2013). Several studies have revealed that N2-fixation by these cyanobacteria can vary greatly across stands of different successional ages (Zackrisson et al., 2004; Lagerström et al., 2007; Gundale et al., 2010), within and between years (DeLuca et al., 2007; Gundale et al., 2012b), and among moss species (Leppänen et al., 2013; Lindo et al., 2013). P. schreberi and H. splendens often dominate different microhabitats where P. schreberi occupies a wider range of environments while H. splendens dominates in mesic and more productive sites (Cronberg et al., 1997; Zackrisson et al., 2009). Even when the two mosses coexist in the same microhabitat, the N2-fixation rates have been shown to differ among the species (Zackrisson et al., 2009; Gundale et al., 2012a; Leppänen et al., 2013; Stuiver et al., 2015). The role of different extrinsic environmental factors in driving the observed spatial and temporal variability of N2-fixation rates have been studied (Gentili et al., 2005; Gundale et al., 2009, 2012a, 2012b; Jackson et al., 2011; Jean et al., 2012; Sorensen et al., 2012), whereas little is known about the role of the cyanobacterial community contributing to this variability. A better knowledge of this issue will contribute to a more complete understanding of the mechanisms underpinning N inputs and dynamics of boreal forest. The cyanobacterial communities living in symbiosis with feather mosses have been characterized primarily through morphological observations and culture-dependent studies, and the association has been regarded as a promiscuous symbiotic association consisting of multiple cyanobacterial species belonging to the genera Nostoc, Calothrix, Fischerella, Cylindrospermum and Stigonema (Gentili et al., 2005; Houle et al., 2006; Zackrisson et al., 2009). In the study by Ininbergs et al. (2011), the nifH gene (encoding the iron-protein component of the nitrogenase complex) was used as a molecular marker for investigating the diversity of the cyanobacterial community inhabiting P. schreberi and H. splendens. They showed that the cyanobacterial community is host-specific and composed of five different clusters that were represented by nifH phylotypes belonging to the genera previously known to live on these feather mosses. Moreover, Ininbergs et al. (2011) revealed that the diversity of nifH phylotypes was negatively correlated with N2-fixation rates, suggesting that N2-fixation might be performed by a few dominant cyanobacterial clusters. However, no attempts have been made to quantify the abundance or the N2-fixation activity of individual cyanobacteria clusters occurring within the community.
The aim of the present study was to provide insights into how and to what extent N2-fixation rates performed by cyanobacteria in symbiosis with P. schreberi and H. splendens are related to the abundance and composition of the cyanobacterial community and whether this is consistent over the growing season. Specifically, we investigated, for each moss species, whether temporal variation in N2-fixation rates is related to variation in cyanobacterial abundance and community composition. Further, for each moss species and over the growth season, we aimed to explore how N2-fixation activity (as shown through nifH expression) of individual cyanobacterial clusters within the community contributes to overall N2-fixation rates. Thus, specific nifH primers targeting individual cyanobacterial clusters in the community were designed and used to quantify their abundance and expression by quantitative PCR (qPCR). Firstly, we hypothesized that the cyanobacterial abundance and community composition (that is, the relative abundances of different individual clusters) differ between coexisting moss species (that is, P. schreberi versus H. splendens), and that these will change over the growing season for both species. Secondly, we hypothesized that the relative nifH expression of specific clusters within the cyanobacterial community varies between moss species as well as across the growing season for both moss species. Finally, we hypothesized that the majority of cyanobacterial N2-fixation will be performed by only a few of the clusters present, and that N2-fixation rates therefore will be well correlated with the activity of those clusters alone.
Materials and methods
Sampling sites and moss collection
The gametophytes of P. schreberi and H. splendens used in this experiment were randomly collected from nine forest stands in Northern Sweden (65°35′–65°60′N and 18°14′–19°10′E) on 15 June and 10 September 2010. These two periods correspond to the main N2-fixation seasonal peaks of moss–cyanobacteria symbioses (DeLuca et al., 2002; Lagerström et al., 2007). In each stand, we set up three main plots (each 10 m in diameter and at a distance of 15 m from each other) along a 60 m transect. In each plot, we collected a bundle of gametophytes of both moss species from about 30 randomly chosen microspots over the whole area, and all gametophytes from within each main plot were pooled to result in three within-stand replicates. With this approach, we were able to account for within-plot variation in microhabitat properties. Sample collection was performed only further than 2 m from overstory trees and in patches that were devoid of dense shrub vegetation. All forest stands are open-canopy late post-fire successional forest stands that have not been affected by fire over the last 300 years. All stands were dominated by coniferous trees (Pinus sylvestris and Picea abies), with the understory mainly composed of ericaceous shrubs (Vaccinium myrtillus, V. vitis-idaea and Empetrum hermaphroditum), and a thick ground layer of feather mosses (P. schreberi and H. splendens). Similarly to other boreal regions, N deposition has been shown to be very low in this area (<2 kg ha−1 year; Lagerström et al., 2007).
N2-fixation activity measurements
The N2-fixation rates of each moss species (that is, P. schreberi and H. splendens) were indirectly measured by acetylene reduction assay (Hardy et al., 1968), for each of the three plots in each of the nine forest stands at the two sampling times (that is, June and September), making a total of 54 measurements for each moss species. For each measurement, either five H. splendens gametophytes or 10 P. schreberi gametophytes were placed in a 22-ml glass sealed vial (Gundale et al., 2010; Ininbergs et al., 2011). As P. schreberi gametophytes generally have half the mass of those of H. splendens, the amounts of material added per vial were similar for both species (that is, mean±s.e. of 0.03±0.0028 g dry weight for P. schreberi and 0.03±0.0032 g dry weight for H. splendens). Only the upper green part (ca. 3–5 cm) of the moss gametophytes was used as this is where most of the N2-fixation activity occurs (Solheim et al., 2004). After field collection but prior to N2-fixation analysis, all samples were kept outdoors for 72 h in a natural forest site near the laboratory in Umeå, Sweden (63°49'N—20°15'E) and misted with deionized water to keep both cyanobacteria and mosses hydrated until further experimentation. Measurements of the levels of ethylene produced after 24-h incubation were performed as described by Ininbergs et al. (2011). A ratio of three moles of ethylene per mol of N was used to convert acetylene reduction rates to μg N fixed g−1 fresh weight moss d−1 (DeLuca et al., 2002; Zackrisson et al., 2004; Lagerström et al., 2007).
Diurnal N2-fixation activity
As Ruttjeheden was the site where the highest variation in N2-fixation rate between June and September was observed (Figure 1), the site was selected for further study of nifH abundance and gene expression using qPCR analysis. To identify the time of the day when the N2-fixation rates peak and the samples for RNA isolation should be sampled, a diurnal study was conducted on P. schreberi and H. splendens collected from Ruttjeheden the day following the acetylene reduction assay measurements for the nine forest stands described above. On 17 June and 12 September, five H. splendens gametophytes and 10 P. schreberi gametophytes were placed in each of 36 vials as described above, and the vials were arranged into three independent replicate blocks. Therefore, each block consisted of 12 vials, with 6 vials for each moss species. Every 4 h, one vial from each moss species from each block was sealed and incubated with acetylene for 4 h (that is, 6 vials × 4 h incubation each=24 h). For each vial, the measurement of N2-fixation through acetylene reduction was performed directly after the 4 h incubation period, as described above. Each sample was then immediately snap-frozen and kept in liquid N to prevent DNA/RNA degradation until the start of extractions. All incubations with acetylene occurred in a natural forest site near the laboratory in Umeå, Sweden (63°49′N—20°15′E), where light intensity and temperature were recorded every 2 h.
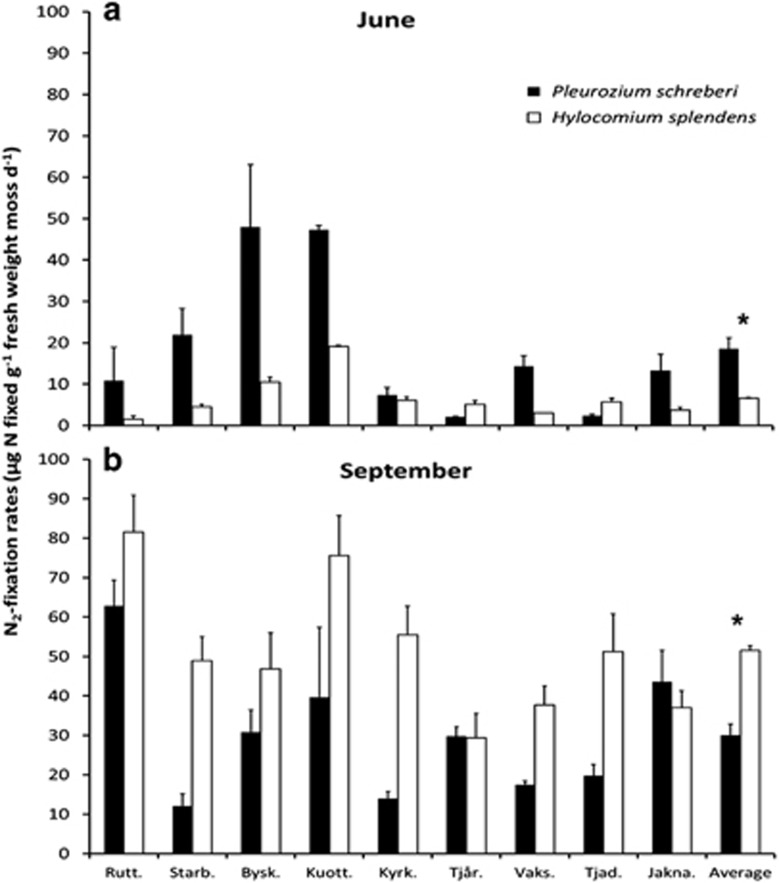
Figure 1.
N2-fixation rates (mean±s.e.) for P. schreberi and H. splendens collected in June (a) and September (b) 2010 expressed as μg N fixed g−1 fresh weight moss d−1, at each of nine late successional boreal forest stands in northern Sweden (n=3). The ‘average' presented is the mean of N2-fixation rates measured at the nine sites with each forest stand as independent replicates (n=9) for each moss species at each sampling date. ANOVA results are presented in Supplementary Table 1. Forest sites: Rutt.=Ruttjeheden, Starb.=Starbetjvare, Bysk.=Byskeälven, Kuott.=Kuottavare, Tjår.=Tjårre, Vaks.=Vaksliden, Tjad.=Tjadnes, Jakna.=Jaknapuoda. *indicates significant differences between average N2-fixation rates measured on each moss species (α<0.05).
DNA/RNA extractions
As the diurnal study revealed that the highest N2-fixation activity occurred between 16:00 and 20:00 in June for both moss species, and between 08:00 and 12:00 in September for both moss species (Figure 2), frozen moss samples corresponding to those times of the day (three replicates for each moss species) were used for qPCR analysis. Moss samples were homogenized in liquid N and approximately 250 mg of each sample were further processed for DNA/RNA co-purification using the NucleoSpin RNA Plant and NucleoSpin RNA/DNA Buffer Set (Macherey-Nagel, Düren, Germany) following the manufacturer's recommendations, but with slight modifications to improve cell lysis using the FastPrep Instrument (MP Biomedicals, Santa Ana, CA, USA). For DNA samples, the yield was quantified with a NanoDrop 1000 spectrophotometer (Thermo Scientific, Waltham, MA, USA). For RNA samples, genomic DNA contamination was removed using the Ambion DNA-free kit (Life Technologies, Carlsbad, CA, USA) following the manufacturer's recommendations. After DNase treatment, the total amount of RNA was quantified with a NanoDrop 1000 spectrophotometer. Complementary DNA was generated from 100 ng of RNA pooled samples using the qScript Flex cDNA Kit (Quanta Biosciences Inc., Gaithersburg, MD, USA), following the manufacturer's instructions for gene-specific priming of the cyanobacterial nifH gene (Olson et al., 1998) and the housekeeping gene coding for cyanobacterial 16S rRNA (Nübel et al., 1997). We used DNA, cDNA and negative controls (minus-RT and water) as templates for quantitative PCR assays.
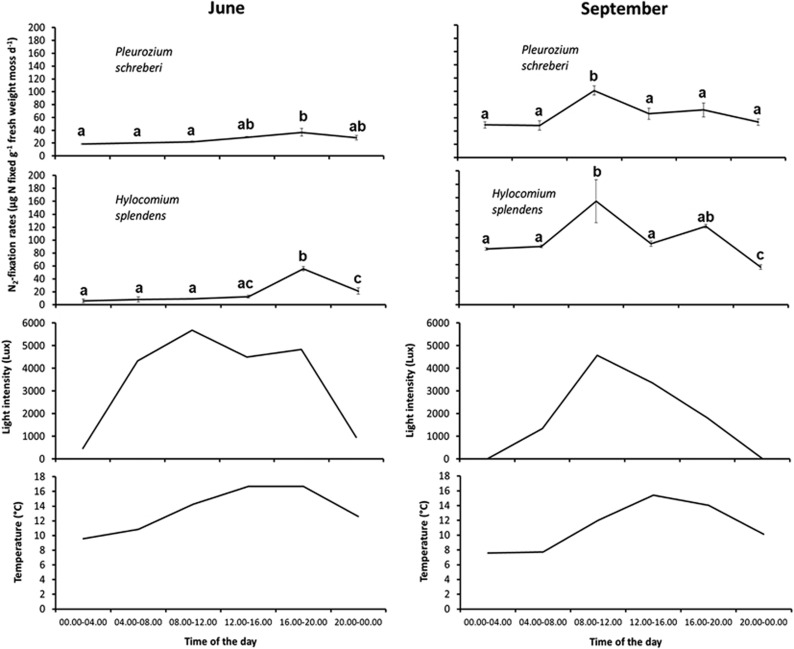
Figure 2.
Diurnal variation of cyanobacterial N2-fixation rates (mean±s.e.) for P. schreberi and H. splendens sampled from Ruttjeheden in June and September, and of light and temperature at the incubation site. Data are obtained after consecutive acetylene reduction assay measurements performed after 4 h incubations. In each panel, different letters above data points indicate statistically significant differences between N2-fixation rates measured at different times of the day for each moss species at each sampling date (ANOVA followed by Tukey's HSD test, α<0.05). ANOVA results are presented in Supplementary Table 2.
Primer design
On the basis of the alignment of nifH sequences carried out by Ininbergs et al. (2011), specific primers targeting each cyanobacterial cluster were designed (that is, ‘Nostoc cluster I', ‘Nostoc cluster II', ‘Stigonema cluster' and ‘nifH2 cluster', respectively; Table 1). Primer sequences were subjected to BLASTN (Altschul et al., 1997) to confirm that they were targeting each cyanobacterial cluster in the GenBank database. To reconfirm the specificity of the individual primer sets, the qPCR products were purified using the QIAquick PCR Purification Kit (Qiagen GmbH, Minden, Germany) and sequenced using the respective forward primer from each product. The retrieved sequences were included in a phylogenetic analysis using the same nucleotide sequences as Ininbergs et al. (2011). Nucleotide sequences were aligned in Ugene (Okonechnikov et al., 2012), using the MUSCLE algorithm and a phylogenetic dendrogram was inferred. Branch support was obtained with 100 bootstraps replicates.
Table 1. Primers used to amplify cyanobacterial DNA and cDNA in this study.
| Primer | Sequence (5′ → 3′) | References |
|---|---|---|
| Ncl I-F | CACCGTTCTTCACCTCGCC | This study |
| Ncl I-R | TGCATACATCGCCATCATTTC | This study |
| Stig-F | TTCACCTCGCTGCTGAACGTGG | This study |
| Stig-R | TCCAAGAAGTTGATAGCGGTAAT | This study |
| Ncl II-F | GCTGACCGGTTTCCGGGATGT | This study |
| Ncl II-R | CAACATCTTTGTAAGCACCG | This study |
| nifH2-F | AACCCGGTGTTGGTTGCGCT | This study |
| nifH2-R | ATGGCCATCATCTCGCCGGA | This study |
| nifH cyano-R | GCATACATCGCCATCATTTCACC | Olson et al., 1998 |
| 16S cyano-F | CGGACGGGTGAGTAACGCGTGA | Nübel et al., 1997 |
| 16S cyano-R | GACTACTGGGGTATCTAATCCCATT | Nübel et al., 1997 |
Abbreviations: Ncl I, Nostoc cluster I; Ncl II, Nostoc cluster II; nifH2, nifH2 cluster; Stig, Stigonema cluster.
Cyanobacterial 16S rRNA primer pair (Nübel et al., 1997) was evaluated using the TestPrime web tool (http://www.arb-silva.de/search/testprime). Primer specificity and coverage for the most commonly observed cyanobacteria genera on P. schreberi and H. splendens (Nostoc, Calothrix, Fischerella, Cylindrospermum and Stigonema) and cross-reactivity with non-cyanobacteria sequences was determined with ARB software package (Ludwig et al., 2004) using the SILVA RefNR SSU-123 sequences (Quast et al., 2013).
Quantitative PCR and quantitative reverse transcription PCR
All qPCR analyses were carried out in a mixture of 20 μl containing 0.3 μM of each primer, 10 μl of SYBR Green, 1 μl of master mix (Roche Diagnostics Scandinavia AB, Bromma, Sweden), 4 mM MgCl2 (Qiagen) and 3% DMSO (Thermo Scientific) using LightCycler 480 (Roche Diagnostics Scandinavia AB). Four microliters of DNA (dilution 1/5) and cDNA (dilution 1/10) were used as template. The PCR conditions comprised a denaturation step of 95 °C for 15 min, followed by 40 cycles of denaturation at 95 °C for 20 s, annealing at 64–68 °C for 40 s and no extension step to avoid non-specific amplification. Annealing temperatures for the different cyanobacterial clusters were optimized empirically. No amplifications were detected from reverse transcriptase-free control reactions or from reactions including water instead of cDNA. Melting curve analysis of the PCR products was conducted following each assay to confirm that the fluorescence signal was derived from specific PCR products and not from primer-dimers or other artifacts. Each qPCR analysis was performed in three technical replicates and additionally repeated on two independent plates. Fold changes in gene abundance and expression were calculated by the comparative 2−ΔΔCT method, using the housekeeping gene coding for cyanobacterial 16S rRNA as the normalizer for nifH gene abundance and expression; both were corrected for the PCR efficiency (Livak and Schmittgen, 2001) using LightCycler 480 software release 1.5.1 (Roche).
Statistical analysis
A repeated measures analysis of variance (ANOVA) was performed to test for the effects of moss species, forest site, sampling date (as the repeated measures factor) and their interactions on N2-fixation rates. Repeated measure ANOVA was also used to test for the effects of time of the day, moss species, sampling date (as the repeated measures factor) and their interactions on N2-fixation. Tukey's HSD post hoc tests were then used to help identify the time of the day at which N2-fixation rates are highest.
To determine the transcriptional efficiency of each individual cyanobacterial cluster, we calculated the ratio between the nifH expression and abundance (defined as the nifH transcriptional efficiency of the individual cyanobacterial clusters in the population). Repeated measure split-plot ANOVAs were used to test for the effects of moss species (as the main plot factor), individual cyanobacterial cluster (as the subplot factor) and sampling date (as the repeated measure factor), and their interactions, on nifH gene abundance, nifH gene expression and nifH transcriptional efficiency. Whenever ANOVAs indicated that significant differences were present, Tukey's HSD post hoc tests were performed to compare treatment means.
Correlation analysis using Spearman's rho and stepwise multiple regressions with Akaike Information Criterion (AICc) were performed to identify the combination of variables that best predicted N2-fixation rates. We determined which combination of variables (out of sampling date, light intensity, temperature, moss species and nifH expression and abundance for each individual cluster) served as best predictors of N2-fixation rates measured in the diurnal study.
Results
Temporal variation in N2-fixation rates of P. schreberi and H. splendens
The average N2-fixation rate was approximately three times higher in September than in June (Figure 1). Moreover, the N2-fixation rate for P. schreberi in June was 2.8 times higher than that of H. splendens, while in September, the fixation rate of H. splendens was 1.7 times higher than that of P. schreberi (Figure 1). Our ANOVA results showed that sampling date, forest site and all possible interactions involving these two factors and moss species significantly influence N2-fixation rates (Supplementary Table 1).
Our results from the diurnal N2-fixation measurements showed that the N2-fixation rates in June were highest between 16.00 and 20.00 for both moss species, while in September, the highest N2-fixation rate occurred between 08.00 and 12.00 (Figure 2). The ANOVA results showed that sampling date, the time of the day and all possible interactions involving these two factors and moss species significantly influence N2-fixation rates (Supplementary Table 2).
Validation of cluster-specific nifH primers and cyanobacterial 16S rRNA primers
The results from the phylogenetic tree computed with the same nucleotide sequences used to design the primers and sequences obtained by qPCR revealed that all amplified qPCR products belong to their respective clusters (data not shown). Primers targeting ‘Nostoc cluster II' did not give any amplification products, indicating that cyanobacteria belonging to this cluster were not present or were below the level of detection.
In silico testing of the 16S rRNA primer pair using Silva-TestPrime with one allowed mismatch indicated an average of 93.1% coverage for the targeted cyanobacteria genera. Specifically for the two most abundant genera Stigonema and Nostoc, the primers have 100% and 96.4% coverage, respectively. In addition, the 16S rRNA primers cover 0.1% of non-cyanobacterial sequences belonging to the Synergistetes phylum.
Temporal change of the cyanobacterial community composition
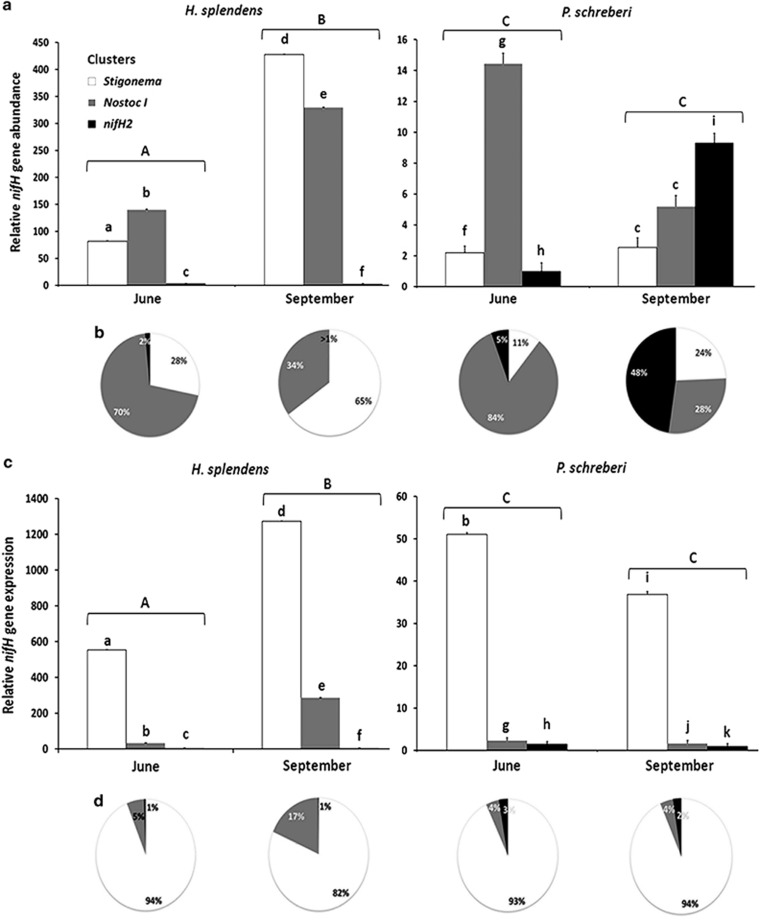
The abundance of nifH genes on H. splendens was overall 28 times higher than on P. schreberi and 3.3 times higher on H. splendens in September compared with June (Figures 3a and b). Further, the community composition changed considerably between June and September for both H. splendens and P. schreberi (Figures 3a and b). In June, the ‘Nostoc cluster I' was the dominant cluster on both H. splendens and P. schreberi, and represented 70% and 84% of the total cyanobacterial community, respectively. However, in September, the ‘Stigonema cluster' became the most abundant cluster on H. splendens, representing 65% of the total cyanobacterial community, while the ‘nifH2 cluster' (which includes the cyanobacterial genera Nostoc, Calothrix and Fischerella) became the most abundant cluster on P. schreberi, constituting 48% of the total community. Repeated measure ANOVA showed that nifH gene abundance was influenced by sampling date, moss species and cyanobacterial cluster, as well as all but one of the possible interactions among these factors (Supplementary Table 3).
Figure 3.
Average (±s.e.) nifH gene abundance and expression relative to the 16S rRNA gene of the cyanobacterial community associated with H. splendens and P. schreberi in June versus September. (a) Relative nifH gene abundance in the cyanobacterial community. (b) nifH gene abundance expressed as a percentage of each nifH cluster in the cyanobacterial community. (c) Relative nifH gene expression in the cyanobacterial community. (d) nifH gene expression as a percentage of each nifH cluster in the cyanobacterial community. For (a) and (c), note the difference in scale between H. splendens and P. schreberi. Significant differences between moss species × month combinations are indicated by different capital letters on the top of groups of three histogram bars, following Tukey's HSD test (α<0.05). Different lower case letters represent significant differences between the nifH clusters, following Tukey's HSD test (α<0.05). ANOVA results are given in Supplementary Table 3.
nifH expression of individual cyanobacterial clusters
Our result shows that the nifH expression of the different cyanobacterial clusters increased between June and September for H. splendens and remained unchanged for P. schreberi (Figures 3c and d). The ‘Stigonema cluster' had the highest nifH expression on both H. splendens and P. schreberi for both sampling dates, constituting 82–94% of the total nifH expression in the community, while ‘Nostoc cluster I' and ‘nifH2 cluster' had the second and third highest nifH gene expression, respectively (Figures 3c and d). When comparing nifH gene abundance (Figures 3a and b) and nifH expression (Figures 3c and d), it is apparent that although the ‘Stigonema cluster' was not the most abundant (with exception of H. splendens in September), it accounts for the majority of the nifH gene expression, irrespective of moss species and sampling date. Conversely, ‘Nostoc cluster I' was dominating on both moss species in June but its nifH expression only accounted for 4–5% of the total nifH expression in the community. Our results from repeated measure ANOVA revealed that community nifH expression was influenced by sampling date, moss species and cyanobacterial cluster, as well as all possible interactions among these factors (Supplementary Table 3).
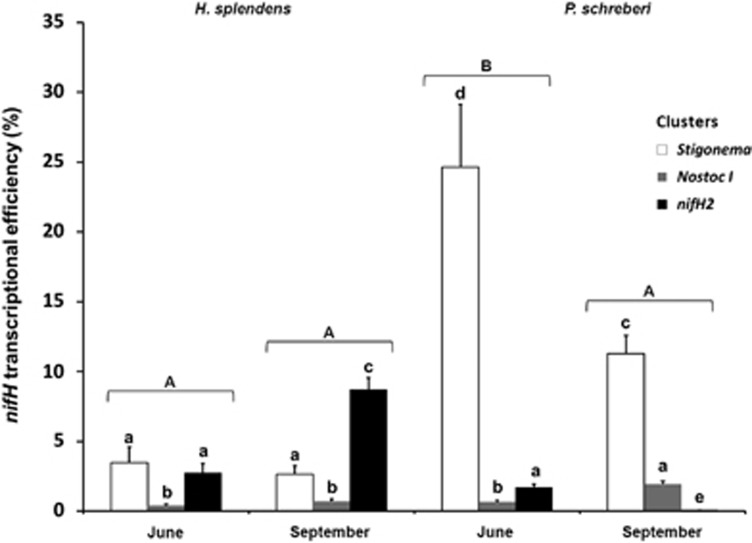
The ‘Stigonema cluster' was the most transcriptionally efficient cyanobacterial cluster on P. schreberi in both sampling dates. For H. splendens, both ‘Stigonema cluster' and ‘nifH2 cluster' are equally efficient in June, while ‘nifH2 cluster' was the most efficient cyanobacterial cluster in September (Figure 4). Repeated measure ANOVA revealed that nifH transcriptional efficiency was influenced by moss species and nifH cluster, and all except one of the possible interactions involving these two factors and sampling date (Supplementary Table 3).
Figure 4.
Average (±s.e.) nifH gene transcriptional efficiency (expressed as the ratio between the nifH expression and abundance) of the cyanobacterial clusters associated with H. splendens and P. schreberi in June and September. Significant differences between moss species × month combinations are indicated by different capital letters on top of groups of three histogram bars, following Tukey's HSD test (α<0.05). Different lower case letters represent significant differences between the nifH clusters, following Tukey's HSD test (α<0.05). ANOVA results are given in Supplementary Table 3.
Parameters influencing the temporal dynamics of N2-fixation rates
The correlation analysis showed that N2-fixation rates were positively correlated with sampling date (June versus September), nifH gene abundance of ‘Stigonema cluster' and ‘nifH2 cluster', nifH gene expression of ‘Stigonema cluster' and ‘Nostoc cluster I', and light intensity (Table 2). Stepwise multiple regression analysis using data from the diurnal study showed that N2-fixation rates could be best predicted by a model incorporating three variables, that is, nifH expression of ‘Stigonema cluster', sampling date and nifH abundance of ‘nifH2 cluster (R2=0.969, P<0.001). ‘Stigonema cluster' nifH expression and sampling date explained most of the variation observed for the dependent variable (51% and 33%, respectively).
Table 2. Results from Spearman correlation analysis between N2-fixation measured at the highest peak of the day and sampling date, moss species, nifH expression and abundance for each individual cluster, light intensity and temperature.
| Factors | N2-fixation (μg N g−1 fresh weight moss d−1) |
|---|---|
| Sampling date | 0.871 (0.000) |
| Moss species | −0.375 (0.071) |
| nifH abundance of ‘Stigonema cluster' | 0.667 (0.000) |
| nifH abundance of ‘Nostoc cluster I' | 0.282 (0.182) |
| nifH abundance of ‘nifH2 cluster' | 0.449 (0.028) |
| nifH expression of ‘Stigonema cluster' | 0.514 (0.010) |
| nifH expression of ‘Nostoc cluster I' | 0.472 (0.020) |
| nifH expression of ‘nifH2 cluster' | 0.284 (0.178) |
| Light intensity | 0.826 (0.000) |
| Temperature | −0.176 (0.411) |
Values show the correlation coefficient and the P-value in parentheses; bold values show statistically significant correlations at P<0.05.
Discussion
Diazotrophs associated with pleurocarpous feather mosses have a key role for the N cycle of boreal forests (DeLuca et al., 2002; Zackrisson et al., 2004; Turetsky et al., 2012; Lindo et al., 2013; Jonsson et al., 2014). The majority of studies on this topic report N2-fixation rate variations between growing seasons (Deluca et al., 2002) and forest stands (Zackrisson et al., 2004; Stuiver et al., 2015), and most of these observed differences have been attributed to local environmental conditions (Smith, 1984; Zackrisson et al., 2004; Gentili et al., 2005; Gundale et al., 2009, 2012a, 2012b; Jackson et al., 2011; Jean et al., 2012; Sorensen et al., 2012; Rousk et al., 2013). N2-fixation rates also differ between feather moss species (Lagerström et al., 2007) and these fluctuations are in part related to differing composition and diversity of cyanobacteria on these mosses (Ininbergs et al., 2011). With the use of specific nifH primers targeting four major cyanobacterial clusters in the cyanobacterial community and qPCR, we showed that the temporal variation of N2-fixation rates is mostly determined by the nifH gene expression of cyanobacteria belonging to the ‘Stigonema cluster'.
In support of our first hypothesis, our data showed that the abundance and community composition of the cyanobacteria inhabiting H. splendens and P. schreberi differed, and that the cyanobacterial composition changed over the growing season for both species. The total relative cyanobacterial nifH gene abundance was higher in September than in June for H. splendens as well as higher on H. splendens compared with that of P. schreberi. Therefore, these results not only provide evidence that composition is host-specific (Ininbergs et al., 2011), but also suggest a promiscuous symbiosis with a mixed community changing over time. As the changes in the community composition are different for the two moss species, the drivers of cyanobacterial community composition might be directed by the host species and/or by different abiotic conditions provided by the host. Under dry conditions, the cyanobacterial community associated with P. schreberi was shown to fix more N than the community on H. splendens, suggesting that different hosts provide different abiotic conditions (Gundale et al., 2012a). In addition, it was recently shown that the host controls the degree of colonization by secretion of chemo-attractants when depleted in N (Bay et al., 2013). A host control mechanism by P. schreberi could explain why we found no variation in cyanobacterial abundance over the growing season, contrary to the abundance increase observed for H. splendens. However, we cannot exclude the possibility that our observations could be explained in part by potential antagonistic or mutually beneficial interactions between the members of the epiphytic microbial communities in the manner that has been shown for microbial communities in the phyllosphere (Delmotte et al., 2009; Dulla and Lindow, 2009; Vorholt 2012).
In support of our second hypothesis, we found that the nifH expressions of individual cyanobacterial clusters changed temporally for both moss species and were higher on H. splendens than on P. schreberi. Although ‘Stigonema cluster' showed the highest nifH expression on both moss species in both June and September, it only dominated the cyanobacterial community on H. splendens in September. Although Stigonema has been observed in association with both H. splendens and P. schreberi in previous studies (Gentili et al., 2005; Houle et al., 2006; Ininbergs et al., 2011), this is the first study to show that the ‘Stigonema cluster', despite its overall relatively low occurrence in the community, is the main contributor to N input into the boreal forest. These findings are indicative that Stigonema is a far more important genus for N input than previously thought. The dominance of ‘Stigonema cluster' on H. splendens as seen in this study has also been observed in a previous study from the boreal forest of Quebec, Canada; Stigonema sp. was the most abundant genus on H. splendens compared with P. schreberi during the late autumn season, that is, September—October (Houle et al., 2006). In addition, a higher number of phylotypes affiliated to the ‘Stigonema cluster' was also observed on H. splendens compared with P. schreberi in the work of Ininbergs et al. (2011). It should be noted that the 16S rRNA primers used for normalizing the nifH abundance and expression could potentially cross-react with the non-cyanobacteria community, for example, co-occurring bacteria and archaea. Therefore, one should be cautious in interpreting the differences between samples because the co-occurring non-target community likely differs in abundance. However, in sillico study of the 16S rRNA primers showed a high coverage and specificity for the common cyanobacterial genera found on P. schreberi and H. splendens and a low coverage with non-cyanobacteria sequences and therefore amplification of non-target bacteria was negligible.
Both feather mosses, and especially H. splendens, show distinct seasonal growth patterns (Busby et al., 1978; Callaghan et al., 1978; Campioli et al., 2009). These studies have inferred that abiotic environmental factors, such as high rainfall frequency or low temperature, are merely responsible for their biomass accumulation over time. However, none of these earlier works have recognized that these mosses host cyanobacteria that are capable of transferring N to the host (Bay et al., 2013). Rainfall frequency is a strong determinant of annual growth increment, carbon fixation (Campioli et al., 2009) and N2-fixation rates (Jackson et al., 2011) of feather mosses, meaning that seasonal rainfall patterns also may have indirect effects on moss growth. Interestingly, this is also the period when the relative mass increment of the youngest (current year+1 year) segments of H. splendens has been reported to be the highest (Campioli et al., 2009). The relative growth rate and shoot biomass of P. schreberi also increase from early to late season, but this increase is less marked than that for H. splendens (Campioli et al., 2009). This pattern is also reflected in our findings where the relative increase in N2-fixation rate from June to September is lower than that of H. splendens and that nifH gene expression of cyanobacteria associated with P. schreberi is lower than that of H. splendens.
Consistent with our third hypothesis, we found that only a few clusters are responsible for the majority of N being fixed by the cyanobacterial community, in particular, the ‘Stigonema cluster', which explained most of the temporal variation for N2-fixation. ‘Stigonema cluster' is the most transcriptionally efficient cyanobacterial cluster (relative to its abundance), especially on P. schreberi in June when it only represents 11% of the total nifH gene abundance but contributes to more than 90% of the total nifH gene expression. We can potentially explain this observation by the fact that P. schreberi might provide a more optimal habitat to its cyanobacteria (Gundale et al., 2012a). However, the nifH gene expressions of individual cyanobacterial clusters retrieved on P. schreberi in September are surprisingly low considering the difference in N2-fixation rates observed between June and September. One explanation might be the existence in our samples of an untargeted cyanobacterial cluster or other diazotrophs highly capable of N2-fixation. In addition, N2-fixation activity was observed in the dark period in September, and this suggests that there may also be other diazotrophs present. As such, it was recently reported that Alphaproteobacteria are more abundant than cyanobacteria in the microbial communities associated with Sphagnum mosses (Bragina et al., 2011). However, as Nostocaceae are capable of N2-fixation in darkness both when free-living (Whitton et al., 1979; Huber, 1986) and in symbiosis with plants (Rai et al., 2002), the existence of an untargeted cyanobacterial cluster cannot be excluded.
We found a positive correlation of the N2-fixation rates with the nifH expression of ‘Stigonema cluster' and ‘Nostoc cluster I'. Nevertheless, nifH expression of ‘Nostoc cluster I' poorly explains the N2-fixation rate variations compared with the nifH expression of ‘Stigonema cluster'. In addition, the N2-fixation rates were correlated with the nifH gene abundance of the ‘Stigonema cluster' and the ‘nifH2 cluster'. The dominance of Nostoc species (represented in the ‘Nostoc cluster I' and ‘nifH2 cluster') in the cyanobacterial community is evident. However, their contribution to the nifH gene expression pool is minor. As a result, Nostoc species could most likely be considered as a ‘cheater' with regards to N input into boreal forests. It is known from other symbiotic systems that hosts can select specific members of the N2-fixing community. For instance, soybean (Glycine max) selects the ‘cooperative strains' of Bradyrhizobium japonicum from a mixed community using mechanisms of sanctions/impositions and directed reciprocation (Trivers, 1971; Crespi, 2001; Kiers et al., 2003). A bryophytic mechanism of sanction to control the cyanobacterial community could result from the secretion of antimicrobial cationic peptides or oxylipin molecules; these compounds have been found to be synthesized by mosses (Matsui, 2006; Skripnikov et al., 2011). Finally, light intensity was the only abiotic factor found correlated with N2-fixation, and in spite of having an important role in this metabolically costly process (Staal et al., 2002; Rabouille et al., 2006), it was shown to be of secondary importance in predicting N2-fixation rate variations.
In conclusion our results demonstrate a temporal and host-dependent dynamic of the cyanobacterial communities (that is, composition, nifH abundance and nifH expression) in symbiosis with H. splendens and P. schreberi. Variation in N2-fixation rates are greatly explained by temporal changes in cyanobacterial nifH expression, especially of the genus Stigonema, which implies that this cyanobacterial genus might—although not dominating—be the most influential N2-fixer in this ecosystem. On the contrary, Nostoc proves to be the most abundant cyanobacterial genus but shows poor nifH transcriptional activity and could then be considered as a ‘cheater' in the cyanobacterial community. Consequently, our findings not only provide new and valuable insights into N2-fixation rate variations but also highlight the lack of knowledge about factors controlling the maintenance, composition and activity of the moss–cyanobacteria symbiosis and its eventual consequences for N input into boreal forests. Studying the role of the host and potential mechanisms regulating the cyanobacterial community appears an essential step towards a deeper understanding of the spatial and temporal dynamics of N2-fixation in boreal forest ecosystems.
Acknowledgments
This work was financially supported by Carl Tryggers Foundation for Scientific Research and the Foundation in Memory of Oscar and Lili Lamm.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on The ISME Journal website (http://www.nature.com/ismej)
Supplementary Material
References
- Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W et al. (1997). Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25: 3389–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bay G, Nahar N, Oubre M, Whitehouse MJ, Wardle DA, Zackrisson O et al. (2013). Boreal feather mosses secrete chemical signals to gain nitrogen. New Phytol 200: 54–60. [DOI] [PubMed] [Google Scholar]
- Bragina A, Maier S, Berg C, Müller H, Chobot V, Hadacek F et al. (2011). Similar diversity of Alphaproteobacteria and nitrogenase gene amplicons on two related Sphagnum mosses. Front Microbiol 2: 275–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busby JR, Bliss LC, Hamilton CD. (1978). Microclimate control of growth rates and habitats of the boreal forest mosses, Tomenthypnum nitens and Hylocomium splendens. Ecol Monograph 48: 95–110. [Google Scholar]
- Callaghan TV, Collins NJ, Callaghan CH. (1978). Photosynthesis, growth and reproduction of Hylocomium splendens and Polytrichum commune in Swedish Lapland. Strategies of growth and population dynamics of Tundra plants 4. Oikos 31: 73–88. [Google Scholar]
- Campioli M, Samson R, Michelsen A, Jonasson S, Baxter R, Lemeur R. (2009). Nonvascular contribution to ecosystem NPP in a subarctic heath during early and late growing season. Plant Ecol 202: 41–53. [Google Scholar]
- Crespi BJ. (2001). The evolution of social behavior in microorganisms. Trends Ecol Evolut 16: 178–183. [DOI] [PubMed] [Google Scholar]
- Cronberg N, Molau U, Sonesson M. (1997). Genetic variation in the clonal bryophyte Hylocomium splendens at hierarchical geographical scales in Scandinavia. Heredity 78: 293–301. [Google Scholar]
- Delmotte N, Knief C, Chaffron S, Innerebner G, Roschitzki B, Schlapbach R et al. (2009). Community proteogenomics reveals insights into the physiology of phyllosphere bacteria. Proc Natl Acad Sci USA 106: 16428–16433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLuca TH, Zackrisson O, Gentili F, Sellstedt A, Nilsson M-C. (2007). Ecosystem controls on nitrogen fixation in boreal feather moss communities. Oecologia 152: 121–130. [DOI] [PubMed] [Google Scholar]
- DeLuca TH, Zackrisson O, Nilsson M-C, Sellstedt A. (2002). Quantifying nitrogen-fixation in feather moss carpets of boreal forests. Nature 419: 917–920. [DOI] [PubMed] [Google Scholar]
- Dulla GFJ, Lindow SE. (2009). Acyl-homoserine lactone-mediated cross talk among epiphytic bacteria modulates behavior of Pseudomonas syringae on leaves. ISME J 3: 825–834. [DOI] [PubMed] [Google Scholar]
- Gentili F, Nilsson M-C, Zackrisson O, DeLuca TH, Sellstedt A. (2005). Physiological and molecular diversity of feather moss associative N2-fixing cyanobacteria. J Exp Bot 56: 3121–3127. [DOI] [PubMed] [Google Scholar]
- Gundale MJ, Gustafsson H, Nilsson M-C. (2009). The sensitivity of nitrogen fixation by a feathermoss–cyanobacteria association to litter and moisture variability in young and old boreal forests. Can J Forest Res 39: 2542–2549. [Google Scholar]
- Gundale MJ, Nilsson M, Bansal S, Jäderlund A. (2012. a). The interactive effects of temperature and light on biological nitrogen fixation in boreal forests. New Phytol 194: 453–463. [DOI] [PubMed] [Google Scholar]
- Gundale MJ, Wardle DA, Nilsson M-C. (2012. b). The effect of altered macroclimate on N-fixation by boreal feather mosses. Biol Lett 8: 805–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundale MJ, Wardle DA, Nilsson M-C. (2010). Vascular plant removal effects on biological N fixation vary across a boreal forest island gradient. Ecology 91: 1704–1714. [DOI] [PubMed] [Google Scholar]
- Hardy RW, Holsten RD, Jackson EK, Burns RC. (1968). The acetylene-ethylene assay for N2 fixation: Laboratory and field evaluation 1. Plant Physiol 43: 1185–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houle D, Bilodeau Gauthier S, Paquet S, Planas D, Warren A. (2006). Identification of two genera of N2-fixing cyanobacteria growing on three feather moss species in boreal forests of Quebec, Canada. Can J Bot 84: 1025–1029. [Google Scholar]
- Huber A. (1986). Nitrogen fixation by Nodularia spumigena Martens (Cyanobacteria). I. Field studies on the contribution of blooms to the nitrogen budget of the Peel-Harvey Estuary, Western Australia. Hydrobiologia 131: 193–203. [Google Scholar]
- Ininbergs K, Bay G, Rasmussen U, Wardle DA, Nilsson M-C. (2011). Composition and diversity of nifH genes of nitrogen-fixing cyanobacteria associated with boreal forest feather mosses. New Phytol 192: 507–517. [DOI] [PubMed] [Google Scholar]
- Jackson BG, Martin P, Nilsson M-C, Wardle DA. (2011). Response of feather moss associated N2 fixation and litter decomposition to variations in simulated rainfall intensity and frequency. Oikos 120: 570–581. [Google Scholar]
- Jean M-E, Cassar N, Setzer C, Bellenger J-P. (2012). Short-term N2 fixation kinetics in a moss-associated cyanobacteria. Environ Sci Technol 46: 8667–8671. [DOI] [PubMed] [Google Scholar]
- Jonsson M, Kardol P, Gundale MJ, Bansal S, Nilsson M-C, Metcalfe DB et al. (2014). Direct and indirect drivers of moss community structure, function, and associated microfauna across a successional gradient. Ecosys 18: 154–169. [Google Scholar]
- Kiers ET, Rousseau RA, West SA, Denison RF. (2003). Host sanctions and the legume–rhizobium mutualism. Nature 425: 78–81. [DOI] [PubMed] [Google Scholar]
- Lagerström A, Nilsson M-C, Zackrisson O, Wardle DA. (2007). Ecosystem input of nitrogen through biological fixation in feather mosses during ecosystem retrogression. Funct Ecol 21: 1027–1033. [Google Scholar]
- Leppänen SM, Salemaa M, Smolander A, Mäkipää R, Tiirola M. (2013). Nitrogen fixation and methanotrophy in forest mosses along a N deposition gradient. Environ Exper Bot 90: 62–69. [Google Scholar]
- Lindo Z, Nilsson M-C, Gundale MJ. (2013). Bryophyte-cyanobacteria associations as regulators of the northern latitude carbon balance in response to global change. Glob Change Biol 19: 2022–2035. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 25: 402–408. [DOI] [PubMed] [Google Scholar]
- Ludwig W, Strunk O, Westram R, Richter L, Meier H, Yadhukumar et al. (2004). ARB: a software environment for sequence data. Nucl Acids Res 32: 1363–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui K. (2006). Green leaf volatiles: hydroperoxide lyase pathway of oxylipin metabolism. Curr Opin Plant Biol 9: 274–280. [DOI] [PubMed] [Google Scholar]
- Nübel U, Garcia-Pichel F, Muyzer G. (1997). PCR primers to amplify 16S rRNA genes from cyanobacteria. Appl Environ Microbiol 63: 3327–3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okonechnikov K, Golosova O, Fursov M, UGENE team. (2012). Unipro UGENE: a unified bioinformatics toolkit. Bioinformatics 28: 1166–1167. [DOI] [PubMed] [Google Scholar]
- Olson JB, Steppe TF, Litaker RW, Paerl HW. (1998). N2-fixing microbial consortia associated with the ice cover of Lake Bonney, Antarctica. Microb Ecol 36: 231–238. [DOI] [PubMed] [Google Scholar]
- Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P et al. (2013). The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucl Acids Res 41(D1): D590–D596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabouille S, Staal M, Stal LJ, Soetaert K. (2006). Modeling the dynamic regulation of nitrogen fixation in the cyanobacterium Trichodesmium sp. Appl Environ Microbiol 72: 3217–3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai AN, Bergman B, Rasmussen U. (2002) Cyanobacteria in Symbiosis. Kluwer Academic Publishers: Dordrecht, The Netherlands. [Google Scholar]
- Rousk K, Jones DL, DeLuca TH. (2013). Moss-cyanobacteria associations as biogenic sources of nitrogen in boreal forest ecosystems. Front Microbiol 4: 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skripnikov AY, Anikanov NA, Kazakov VS, Dolgov SV, Ziganshin RK, Govorun VM et al. (2011). The search for and identification of peptides from the moss Physcomitrella patens. Russ J Bioorg Chem 37: 95–104. [DOI] [PubMed] [Google Scholar]
- Smith VR. (1984). Effects of abiotic factors on acetylene reduction by cyanobacteria epiphytic on moss at a subantarctic island. Appl Environ Microbiol 48: 594–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solheim B, Wiggen H, Røberg S, Spaink HP. (2004). Associations between arctic cyanobacteria and mosses. Symbiosis 37: 169–187. [Google Scholar]
- Sorensen PL, Lett S, Michelsen A. (2012). Moss-specific changes in nitrogen fixation following two decades of warming, shading, and fertilizer addition. Plant Ecol 213: 695–706. [Google Scholar]
- Staal M, te Lintel Hekkert S, Herman P, Stal LJ. (2002). Comparison of models describing light dependence of N2 Fixation in heterocystous cyanobacteria. Appl Environ Microbiol 68: 4679–4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuiver BM, Gundale MJ, Wardle DA, Nilsson M-C. (2015). Nitrogen fixation rates associated with the feather mosses Pleurozium schreberi and Hylocomium splendens during forest stand development following clear-cutting. Forest Ecol Manag 347: 130–139. [Google Scholar]
- Trivers RL. (1971). The evolution of reciprocal altruism. Q Rev Biol 46: 35–57. [Google Scholar]
- Turetsky MR, Bond-Lamberty B, Euskirchen E, Talbot J, Frolking S, McGuire AD et al. (2012). The resilience and functional role of moss in boreal and arctic ecosystems. New Phytol 196: 49–67. [DOI] [PubMed] [Google Scholar]
- Vorholt JA. (2012). Microbial life in the phyllosphere. Nat Rev Micro 10: 828–840. [DOI] [PubMed] [Google Scholar]
- Whitton BA, Donaldson A, Potts M. (1979). Nitrogen fixation by Nostoc colonies in terrestrial environments of Aldabra Atoll, Indian Ocean. Phycologia 18: 278–287. [Google Scholar]
- Zackrisson O, DeLuca TH, Gentili F, Sellstedt A, Jäderlund A. (2009). Nitrogen fixation in mixed Hylocomium splendens moss communities. Oecologia 160: 309–319. [DOI] [PubMed] [Google Scholar]
- Zackrisson O, DeLuca TH, Nilsson M-C, Sellstedt A, Berglund LM. (2004). Nitrogen fixation increases with successional age in boreal forests. Ecology 85: 3327–3334. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.