This corrigendum describes adjustments made to the ENGAGE study database following data quality review, resulting in minor changes to the published study results. The impacted parameters from Tables I, II, III, Figure 3 and Supplemental Tables II and III of the original publication are summarized in the data displays below.
Table I.
Demographics, fertility characteristics and baseline (stimulation day 1) ultrasound scan (USS) and serum hormone levels per treatment group (intent-to-treat population)
| Previously Published Results |
Corrected Results |
|||
|---|---|---|---|---|
| Corifollitropin alfa (n = 756) | rFSH (n = 750) | Corifollitropin alfa (n = 756) | rFSH (n = 750) | |
| Demographics | ||||
| Age (years) | 31.5 (3.3) | 31.5 (3.2) | 31.5 (3.3) | 31.5 (3.2) |
| Body weight (kg) | 68.8 (7.6) | 68.4 (7.3) | 68.8 (7.6) | 68.4 (7.3) |
| BMI (kg/m2) | 24.8 (2.8) | 24.8 (2.7) | 24.8 (2.8) | 24.8 (2.7) |
| Race, n (%) | ||||
| Asian | 21 (2.8) | 21 (2.8) | 22 (2.9) | 22 (2.9) |
| Black | 33 (4.4) | 28 (3.7) | 33 (4.4) | 28 (3.7) |
| Caucasian | 643 (85.1) | 650 (86.7) | 643 (85.1) | 649 (86.5) |
| Other | 59 (7.8) | 51 (6.8) | 58 (7.7) | 51 (6.8) |
| Fertility characteristics | ||||
| Primary infertility, n(%) | 403 (53.3) | 393 (52.4) | 403 (53.3) | 393 (52.4) |
| Duration of infertility (years) | 3.3 (2.4) | 3.2 (2.2) | 3.3 (2.4) | 3.2 (2.2) |
| Cause of infertilitya), n (%) | ||||
| Male factor | 388 (51.3) | 347 (46.3) | 388 (51.3) | 347 (46.3) |
| Tubal factor | 198 (26.2) | 191 (25.5) | 198 (26.2) | 191 (25.5) |
| Endometriosis | 109 (14.4) | 115 (15.3) | 109 (14.4) | 115 (15.3) |
| Other/unexplained | 246 (32.5) | 262 (34.9) | 246 (32.5) | 262 (34.9) |
| First IVF cycle, n (%) | 569 (75.3) | 552 (73.6) | 569 (75.3) | 552 (73.6) |
| Stimulation day 1 | ||||
| Total ovarian volume(mL)b) | 13.2 (8.1) | 13.2 (7.1) | 13.2 (8.1) | 13.2 (7.1) |
| Basal AFC (<11 mm) | 12.3 (4.6) | 12.4 (4.4) | 12.3 (4.5) | 12.4 (4.4) |
| FSH (IU/L) | 6.7 (2.1) | 6.6 (1.9) | 6.7 (2.1) | 6.6 (1.9) |
| LH (IU/L) | 4.8 (2.0) | 4.7 (1.8) | 4.8 (2.0) | 4.7 (1.8) |
| E2 (pmol/L) | 126.1 (39.3) | 124.8 (37.4) | 126.1 (39.2) | 124.8 (37.1) |
| Progesterone (nmol/L) | 1.8 (1.3) | 1.8 (1.4) | 1.8 (1.3) | 1.8 (1.4) |
AFC: antral follicle count, E2: estradiol, rFSH: recombinant FSH.
Numbers are mean (SD) unless otherwise indicated.
a)A patient can have multiple causes of infertility.
b)According to the formula for a polate ellipsoid: 0.523 x longitudinal x antero-posterior x transverse diameters as measured per transvaginal ultrasound.
Table II.
Primary end-points of the ENGAGE trial: ongoing pregnancy rate (assessed at least 10 weeks after embryo transfer) and the mean (SD) number of cumulus-oocyte-complexes retrieved (intent-to-treat population)
| Previously Published Results |
Corrected Results |
|||||||
|---|---|---|---|---|---|---|---|---|
| Corifollitropin alfa (n = 756) | rFSH (n = 750) | Estimated differencea) [95% CI] | P-value | Corifollitropin alfa (n = 756) | rFSH (n = 750) | Estimated differencea) [95% CI] | P-value | |
| Ongoing pregnancies (n) | 294 | 286 | 295 | 286 | ||||
| Per started cycle | 38.9% | 38.1% | 0.9 [−3.9; 5.7] | 0.71 | 39.0% | 38.1% | 1.1 [−3.8; 5.9] | 0.67 |
| Per embryo transfer | 43.8% | 40.6% | 3.1 [−2.0; 8.2] | 0.24 | 43.9% | 40.6% | 3.3 [−1.9; 8.4] | 0.21 |
| Cumulus-oocyte-complexes retreived | ||||||||
| Per started cycle | 13.7 (8.2) | 12.5 (6.7) | 1.2 [0.5; 1.9] | 0.001 | 13.8 (8.3) | 12.6 (6.8) | 1.2 [0.5; 1.9] | 0.001 |
| Per oocyte retrieval | 14.1 (7.9) | 12.7 (6.7) | 1.6 [0.8; 2.3] | <0.001 | 14.2 (8.0) | 12.7 (6.2) | 1.6 [0.8; 2.3] | <0.001 |
P-value corresponds to the test whether the treatment difference equals zero.
a)Estimated treatment difference (corifollitropin alfa – rFSH) adjusted for covariates.
Table III.
Clinical parameters from stimulation phase up to embryo transfer (intent-to-treat population)
| Previously Published Results |
Corrected Results |
|||
|---|---|---|---|---|
| Corifollitropin alfa (n = 756) | rFSH (n = 750) | Corifollitropin alfa (n = 756) | rFSH (n = 750) | |
| Stimulation characteristicsa) | ||||
| Total dose of rFSH (IU) | 400 (0–2000) | 1800 (400–2800) | 400 (0–2000) | 1800 (400–2800) |
| Total dose of rFSH from day 8 onwards (IU) | 400 (0–2000) | 400 (0–1400) | 400 (0–2000) | 400 (0–1400) |
| Total duration of stimulation (days)b) | 9 (6–18) | 9 (6–15) | 9 (6–18) | 9 (6–15) |
| Follicles, day of hCGa) | ||||
| ≥11 mm | 16.0 (7.0) | 13.9 (6.1) | 16.0 (7.0) | 13.9 (6.1) |
| ≥15 mm | 9.6 (4.8) | 8.7 (4.0) | 9.6 (4.8) | 8.7 (4.0) |
| ≥17 mm | 5.7 (3.2) | 5.6 (2.9) | 5.7 (3.2) | 5.6 (2.9) |
| Serum parameters, day of hCGa) | ||||
| FSH (IU/L) | 12.5 (3.3) | 11.6 (2.8) | 12.5 (3.3) | 11.6 (2.8) |
| LH (IU/L) | 1.4 (1.8) | 1.9 (1.6) | 1.4 (1.8) | 1.9 (1.6) |
| E2 (pmol/L) | 5508.8 (3469.8) | 5165.3 (435.6) | 5486.7 (3461.9) | 5154.9 (2999.5) |
| Inhibin-B (pg/mL) | 610.3 (492.3) | 614.8 (435.6) | 610.3 (492.3) | 614.8 (435.6) |
| Progesterone (nmol/L) | 3.0 (2.1) | 3.2 (1.5) | 3.2 (4.9) | 3.2 (1.5) |
| Clinical outcome per started cycle | ||||
| Oocytes retrieved, ICSI only | 13.8 (7.6) | 12.1 (6.3) | 13.8 (7.7) | 12.2 (6.4) |
| Metaphase II oocytes (ICSI only) | 10.8 (6.5) | 9.2 (5.1) | 10.8 (6.5) | 9.2 (5.1) |
| % of total | 78.9 (18.9) | 77.4 (18.1) | 78.9 (18.7) | 77.2 (18.1) |
| Fertilization rate (%)c,d) | 66.0 (23.4) | 67.6 (22.9) | 66.0 (23.5) | 67.6 (22.9) |
| Total number of embryos obtained (day 3)c) | 8.3 (5.6) | 7.4 (4.8) | 8.4 (5.5) | 7.5 (4.8) |
| Excellent (top) quality embryos (grade 1)c) | 2.6 (3.4) | 2.5 (3.4) | 2.6 (3.4) | 2.5 (3.4) |
| Good quality embryos (grade 1 + 2)c) | 4.6 (4.3) | 4.4 (3.9) | 4.7 (4.3) | 4.5 (3.9) |
| Single embryo transfer (%)d) | 25.7 | 27.0 | 25.7 | 27.0 |
| Embryos transferrede) | 1.7 (0.4) | 1.7 (0.4) | 1.7 (0.4) | 1.7 (0.4) |
| Embryos cryopreservedf) | 4.3 (3.6) | 3.9 (2.7) | 4.3 (3.5) | 3.9 (2.7) |
Numbers are mean (SD) unless otherwise indicated.
a)Restricted to patients with hCG injection.
b)Number of days up to and including the day of hCG administration.
c)Restricted to patients with IVF and/or ICSI.
d)Defined as 100 times the number of mature oocytes (with two pronuclei) obtained divided by the number of oocytes used for fertilization.
e)Restricted to patients with embryo transfer.
f)Restricted to patients with cryopreserved embryos.
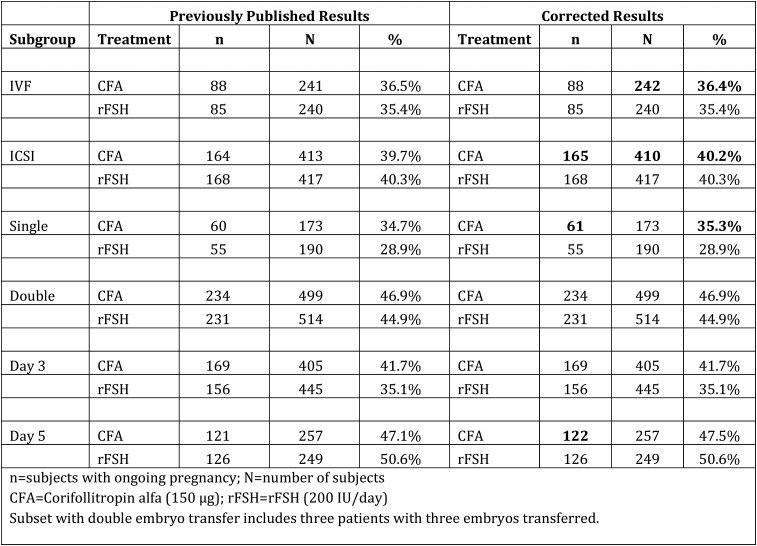
Figure 3.
Data are displayed here in tabular format rather than as a figure per the original publication. Ongoing pregnancy rates stratified for fertilization procedure, number of embryos transferred and day of transfer.
In 2011, an audit of one of the ENGAGE study sites revealed inconsistencies in the way oocytes were counted in this study. Specifically, it was discovered that some sites included atretic oocytes and cumulus oocyte complexes without an oocyte present (“empty shells”) in the count for number of oocytes retrieved, while others excluded them from the count. In response to these findings, a uniform criterion was developed so that atretic oocytes and “empty shells” were always included in the total oocyte counts. The records from all of the sites were then reexamined and this new criterion was applied. This resulted in corrections to the number of oocytes retrieved in 55 out of 1506 subjects and, in most of these cases, the use of the new criterion increased the total number of oocytes by 1 or 2. At the same time, a separate review of pregnancy and infant follow-up data revealed a single, previously unreported, pregnancy in the corifollitropin alfa treatment group (ultrasound data were not available 10 weeks after embryo transfer and follow-up information could not initially be obtained, so by protocol the patient was considered to be ”not pregnant”). This information was corrected after receiving confirmation of a live birth. The corrected results for ongoing pregnancies and number of oocytes retrieved are noted in bold font in Table II.
In Table IV of the original publication, clinical efficacy outcomes per started cycle (intent-to-treat population), the numbers (%) of subjects with an ongoing pregnancy and a multiple pregnancy in the corifollitropin alfa group should be 295 (39.0%) and 84 (28.5%), respectively, rather than the originally reported numbers of 294 (38.9%) and 83 (28.2%), respectively. The resulting p-values for the between-treatment group differences in the ongoing pregnancy and multiple pregnancy rates are p = 0.67 and 0.16, respectively, rather than the originally reported values of p = 0.71 and 0.18, respectively. A corrected version of Figure 3, ‘Ongoing pregnancy rates stratified for fertilization procedure, number of embryos transferred and the day of transfer’, is available upon request.
In addition, four previously unreported serious adverse events (SAEs) were identified. One patient had a SAE of pleurisy prior to receiving study drug. Three other SAEs, which occurred in the intervention portion of the study, were reported during the pregnancy and infant follow-up studies. Of these 3 SAEs, two occurred in the recombinant FSH (rFSH) group (i.e., one subject with a possibly drug-related report of ovarian cyst and one subject with an unlikely drug-related report of hyperemesis gravidarum), and one occurred in the corifollitropin alfa group (i.e., one subject with a non-drug-related report of antepartum haemorrhage). None of the SAEs in this study resulted in discontinuations. The number of subjects with SAEs in the corifollitropin alfa and rFSH groups shown in the safety section of the published paper should be 38 and 39, respectively, rather than the originally reported numbers of 37 for both treatment groups.
In summary, the re-analysis of the oocyte data, the additional pregnancy and SAE reports resulted in relatively minor changes to the previously published data. The overall conclusions of the ENGAGE study remain unchanged.
Other changes in the data relative to the original paper are noted by bold font in the tables adapted from the original publication.
Supplementary Material
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.