Abstract
Background:
Female pattern hair loss (FPHL) is a very common problem in women. The underlying pathophysiology remains unclear, and there are no universally agreed treatment guidelines.
Objective:
We explored the clinical features, relevant medical and family history, laboratory evaluation, and treatment and compliance of 210 patients with FPHL.
Methods:
Data analysis from case notes was performed on 210 patients with a diagnosis of FPHL seen from January 2011 to December 2011.
Results:
The youngest individual was 8 years old and the oldest was 86 years old. Nearly, 85% of the patients had a family history of androgenetic alopecia. Hypothyroidism and hypertension are the most common medical problems. Telogen effluvium (TE) is the most common concurrent hair loss condition. Only 38% of the patients were found to have normal Vitamin D level, 71% had ferritin level above 30 μg/L, and 85% had normal zinc level at the first consultation. Fifty-nine percent of the patients failed to attend any follow-up appointments.
Limitations:
One of the limitations of this study is its retrospective nature. Moreover, the severity of FPHL in terms of Ludwig score was not routinely documented in the medical charts.
Conclusion:
History of TE, hypothyroidism and hypertension, and low serum Vitamin D is common in our patients with FPHL.
Keywords: Alopecia, female pattern hair loss, hair loss
INTRODUCTION
Androgenetic alopecia (AGA) is a common form of hair loss in both men (male pattern hair loss) and women (female pattern hair loss [FPHL]). FPHL is the most common cause of alopecia in women. It was once estimated that 21 million women of all ages in the United States have some degree of FPHL.[1] The incidence increases with advancing age and it can develop at any time before or after the onset of puberty. An Australian study showed that FPHL affects 7% of women in their 20s, and more than 55% of the women aged 80 and over[2] is associated with a significant psychological morbidity. Patients with FPHL typically present with thinning over the frontal, parietal, and central scalp, with retention of the frontal hairline, and it is characterized by a widened midline part toward the front of the scalp [Figure 1]. Histopathologically, progressive hair follicle miniaturization coupled with gradual reduction of the anagen phase is seen in FPHL. There are two main ages of onset of FPHL, one in the immediate postpuberty period to the third decade and a second peak in the fifth and sixth decades.[3] The two clinical presentations are linked by phenotype and histology, but may have distinct genetic etiologies.[3] The role of androgen in FPHL is not completely understood, and most women with FPHL show no clinical or biochemical evidence of androgen excess.[4] The discovery of several AGA-susceptibility loci with genome-wide significance has certainly expanded the knowledge on the genetics of AGA; however, this has yet to explain the complex mode of inheritance in FPHL.[5] Diagnosis of FPHL is usually straightforward from the history and examination of the hair and scalp. Biopsy will only be performed if a differential diagnosis cannot be excluded. Treatment of FPHL includes 2% minoxidil solution or 5% minoxidil foam, anti-androgens, 5 alpha-reductase enzyme inhibitor, prostaglandin analogs, and hair transplantation.
Figure 1.
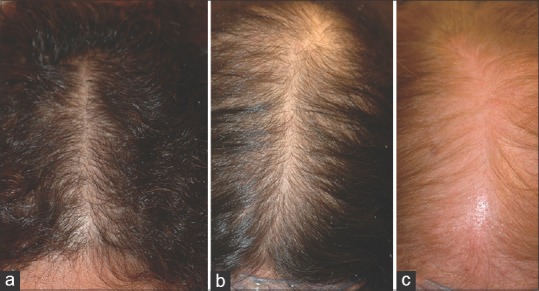
Female pattern hair loss. (a) Ludwig scale I. (b) Ludwig scale II. (c) Ludwig scale III
Here, we present the clinical features, relevant medical and family history, differential diagnoses, laboratory evaluation, treatment, and compliance of 210 patients with FPHL in a tertiary referral center.
METHODS
A retrospective chart review was performed with the Institutional Review Board approval to identify all female patients with hair loss referred to a dedicated hair clinic at the Skin Care Centre in The University of British Columbia from January 1, 2011, to December 31, 2011. The patients were identified using billing codes for alopecia. Epidemiological data for FPHL were established for the 210 patients identified, including duration of hair loss, age at evaluation, and clinical diagnosis. Additional information on disease course, concomitant hair loss problem, family and medical history, regular medications, treatment, and progress were recorded and analyzed.
The diagnosis of FPHL is made clinically based on the appearance of the scalp. Three different clinical patterns of hair loss can be observed and included, i.e. Ludwig type, frontal accentuation, and Hamilton type. British Columbia, especially Vancouver, is a multi-ethnic society with a patient population consists of Europeans, Asians, and East Indians.
RESULTS
Four hundred and sixty-three new patients with alopecia were seen in the hair clinic in 2011. Of the 463 patients, 210 (45%) were diagnosed with FPHL. The mean age of the patients was 45.5 years. The youngest individual was 8 years old and the oldest was 86 years old. About 90 patients were above the age of 50, 76 were between the age of 30 and 40, and 44 were under the age of 30. The average time from the onset of hair loss to seeing a hair specialist was 4.1 years.
Previous treatments
Twenty-eight (13%) of 210 patients had previously received treatments for FPHL. Topical minoxidil was the most common medication used by the majority of patients (89%) before presenting to the hair clinic.
Family history
A family history was recorded in 178 of 210 patients with FPHL (85%). Overall, 91 of the 178 (51%) patients reported a family history of AGA in paternal relatives, 35 (20%) in maternal relatives, and 42 (24%) in both sides of the family. Thirty (14%) patients denied any family history of AGA, ten patients reported AGA only in their siblings, and two patients were uncertain of the history of AGA in the family.
Past medical history
Twenty-six (12%) patients (age ranged from 24 to 73 years) had a long-standing history of hypothyroidism, four patients were known to have polycystic ovarian syndrome (PCOS), and one patient had a history of iron deficiency anemia. Eighteen (9%) patients had a history of depression and anxiety disorders. Other common medical problems among patients included hypertension (17.6%), cancers (8%), hyperlipidemia (6%), and diabetes mellitus (5%).
Dermatologic history
Telogen effluvium (TE) is the most concurrent hair loss condition which was found in 81 of 210 (38.6%) patients. Four patients had seborrheic dermatitis, two patients had chemotherapy-induced hair loss, and one patient was found to have alopecia areata.
Biopsy
Scalp biopsies were performed in three patients to confirm the diagnosis. Two biopsies were done by the referring physicians. Both biopsies showed typical features of AGA with a reduction in the terminal-to-vellus hair ratio, presence of fibrous streamer in the subcutaneous tissue, and miniaturized follicles. There was no evidence of peribulbar inflammation, granulomas, or scarring.
Laboratory evaluation
One hundred and fifty-two patients who had laboratory evaluation of the mean Vitamin D (normal range: 40–190 nmol/L), ferritin (normal range: 15–180 µg/L), and zinc level (normal range: 9.2–26.0 µmol/L) were 70.17 ± 35.84 nmol/L, 76.94 ± 105.83 µg/L, and 10.63 ± 1.70 µmol/L, respectively. In addition, only 38% of the patients were found to have normal Vitamin D level, 71% had ferritin level above 30 µg/L, and 85% had normal zinc level at the first consultation. New abnormal thyroid-stimulating hormone level was found in one patient. Androgen levels including free and total testosterone, dehydroepiandrosterone, and 17-hydroxyprogesterone were performed in 13 (age ranged from 19 to 35 years) patients with a history of irregular periods, acne, or hirsutism, and all were found to be within normal range.
Initial treatment
Minoxidil was recommended in 174 patients on their first visit.
Follow-up
One hundred and twenty-three of 210 (59%) failed to attend any follow-up appointments after the initial consultation, 23 patients attended one follow-up, and 25 patients attended two follow-up visits within 16-month period. Only 39 patients attended all the three follow-ups at 4th, 10th, and 16th months.
Response to treatment
Forty-four patients felt that their conditions had become stable by their first follow-up visit at 4th month, 12 patients at 8th month review, and eight patients at 16th month visit. Six patients felt that their condition failed to become stabilized at the 16th month visit.
Side effects
Twenty-three patients reported side effects from treatments. Twenty-one patients developed irritation of the scalp, facial hair, and increased hair shedding after started using minoxidil solution, one patient complained of weight gain while taking finasteride, and one patient developed redness and swollen face after adjusting spironolactone to a higher dose.
DISCUSSION
Our results demonstrated that AGA is the most common hair loss condition for patients to be referred by the primary care physicians and other dermatologists to our dedicated hair specialist clinic. Specifically, FPHL was found to contribute 45% of newly referred cases within the 1-year study period. AGA has been reported in prepubertal children as young as 6 years of age, all patients shared a common feature of having a strong genetic predisposition to the disease.[6] The youngest patient observed in our study was aged 8 years whose father and paternal grandfather were both known to have AGA. Our data have also demonstrated the classical trend of increased incidence of FPHL with advancing age, as 42% of our patients were above the age of 50. Previously, a popular myth assumed that baldness in a person is inherited from the maternal grandfather, and this misconception has certainly gone in vogue. At present, the mode of inheritance of AGA remains a gray area. Our data demonstrated that majority of our patients have a positive family history of AGA and 51% of the patients reported a family history of AGA only in paternal relatives.
Hair loss can potentially result in low self-esteem and poor body image, especially in women. It is not uncommon that patients tried different shampoos, supplements, and medical treatment promising hair regrowth but in vain before seeking a hair specialist. This could explain the average gap of 4.1 years between the onset of hair loss and the first consultation visit in the hair clinic.
Consequently, their compliance could be poor, particularly if there are unrealistic expectations in treatment outcomes.
PCOS is the most common endocrinologic abnormality associated with FPHL. Previous studies have looked at the association between androgenic alopecia and PCOS. Cela et al. at the reproductive endocrinology service in London reported the prevalence of PCOS in patients with AGA as high as 67%[7] while Quinn et al. recently reported a much lower prevalence at 22%.[8] However, our data showed that only four patients were diagnosed with PCOS. This could be explained by different population group included in the study as well as different screening methods used in the diagnosis of PCOS.
Hypertension and hyperlipidemia are the common medical problems in our study population. Several studies have associated AGA with the risk of cardiovascular disease[9,10] in both men and women. Moreover, one recent report documents AGA as an independent predictor of mortality from diabetes mellitus and heart disease in both sexes.[11] However, a proper comparative study will be needed to affirm the association of hypertension and hyperlipidemia to FPHL. Our data showed that a very high percentage (38.6%) of our patients had histories of concomitant TE, and this trend has not been reported in the literature. On the other hand, 15 patients (7%) came in for TE and were incidentally diagnosed with FPHL. AGA can be unmasked and made worse by the episodes of TE, which is often triggered by physiological, physical, and extreme emotional stress as well as medications. It is not impossible that patients with AGA could develop TE through the extreme emotional stress of losing hair, or perhaps, there is a genetically determined increased tendency for the hair follicles to shift from anagen to telogen phase via various stimuli in patients with AGA. Further studies will be required to clarify this observation.
A scalp biopsy is rarely required to diagnose AGA. We only performed a scalp biopsy on one patient to exclude other causes of hair loss. Assessment of serum ferritin, Vitamin D, and zinc levels is routinely performed as part of investigation for patients with FPHL. Low serum ferritin, Vitamin D, and zinc levels have been shown to be the possible contributory factors in hair loss.[12] Iron deficiency has been demonstrated to be more common in patients with hair loss conditions such as FPHL, TE, and alopecia areata;[12,13,14,15,16] however, other studies found no significant association between iron deficiency and hair loss.[17,18] Mean serum ferritin level (76.2 µg/L) in our patient group was much higher compared to the previous studies (37.3 µg/L, 23.9 µg/L).[12,13] This difference could be explained by diet patterns, genetic and ethnic variations compared to other study populations. While the association and definition of iron deficiency in hair loss remain to be clarified, we routinely prescribe iron supplement to patients with a serum ferritin level below 50 µg/L.
Vitamin D is a hormone that plays an important role in calcium homeostasis and skeletal health. The main source of Vitamin D is an endogenous synthesis in the skin. It is converted to 25 hydroxyvitamin D2 (25(OH)D2) in the liver, then to its active form, 1α,25-dihydroxyvitamin D3 in the kidney. 25(OH)D2 has been regarded as a stable indicator of Vitamin D status, and deficiency is defined as a serum 25(OH)D2 concentration of <25 nmol/L and insufficiency is defined as a serum 25(OH)D2 concentration of between 25 and 75 nmol/L. A trend between low serum Vitamin D level and FPHL has also been described.[13] Our results agree with a recent report[13] (29.1 nmol/L) demonstrating low serum Vitamin D level in patients with FPHL, although the mean serum 25(OH)D2 in our patient group (70.17 nmol/L) was considerably higher. The discrepancy could be due to different patterns of Sun exposure as well as genetic and dietary variations.
Our data did not support the association of low serum zinc and FPHL as demonstrated in recent studies,[19,20] as the mean serum zinc level of our patient group was within the normal range and only 15% (23/149) of our patients were found to have low zinc level on their first visit.
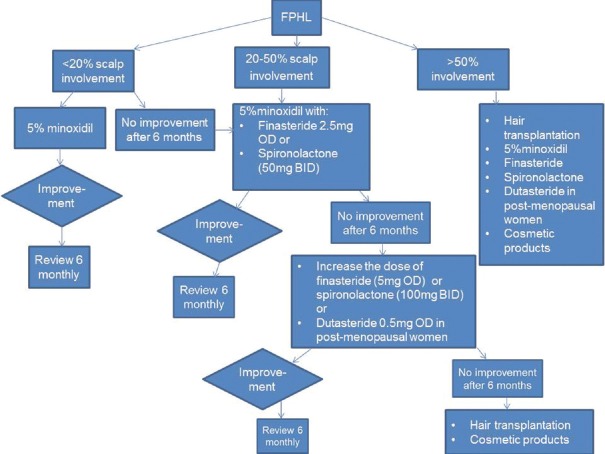
Different treatment modalities for FPHL were comprehensively discussed in the previous studies.[21,22] We recommend 5% minoxidil solution/foam as the first-line treatment for many of our patients with FPHL. The superior benefit of using combination treatment which involves instituting two agents with a different mode of action has been reported in literature.[23,24] Therefore, the authors often recommend 5% minoxidil solution/foam in a combination of spironolactone or finasteride, especially if patients failed to demonstrate improvement while on monotherapy. We also successfully treated two patients with a combination of 5% minoxidil, finasteride, and spironolactone. Other treatment options which the authors sometimes consider include prostaglandin analogs, hair pieces, and hair transplantation. Our treatment algorithm for patients with FPHL is shown in Figure 2.
Figure 2.
Treatment algorithm for female pattern hair loss
Patients with FPHL appear to have a poor attendance at follow-up appointments. As high as 59% of the patients failed to attend any follow-up and only 19% of the patients managed to attend three follow-up visits at 4–6-month interval. Unrealistic expectations of treatment outcomes, dissatisfaction with the current treatment options, and potential treatment side effects are considered contributing to the poor attendance rate at follow-up visits.
Side effects from the treatments are uncommon, and most are due to scalp irritation from using minoxidil solution. The irritation is often resolved following discontinuation or switching to foam preparation.
FPHL is the most common hair loss condition seen in our dedicated specialist hair clinic. History of TE, hypothyroidism and hypertension, and low serum Vitamin D are common in our patients with FPHL. Treatment options and efficacy for FPHL are limited resulting in dissatisfaction and subsequent low attendance rate for follow-up.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Leavitt M. Understanding and management of female pattern alopecia. Facial Plast Surg. 2008;24:414–27. doi: 10.1055/s-0028-1102905. [DOI] [PubMed] [Google Scholar]
- 2.Gan DC, Sinclair RD. Prevalence of male and female pattern hair loss in Maryborough. J Investig Dermatol Symp Proc. 2005;10:184–9. doi: 10.1111/j.1087-0024.2005.10102.x. [DOI] [PubMed] [Google Scholar]
- 3.Olsen EA. Female pattern hair loss. J Am Acad Dermatol. 2001;45(3 Suppl):S70–80. doi: 10.1067/mjd.2001.117426. [DOI] [PubMed] [Google Scholar]
- 4.Futterweit W, Dunaif A, Yeh HC, Kingsley P. The prevalence of hyperandrogenism in 109 consecutive female patients with diffuse alopecia. J Am Acad Dermatol. 1988;19(5 Pt 1):831–6. doi: 10.1016/s0190-9622(88)70241-8. [DOI] [PubMed] [Google Scholar]
- 5.Nuwaihyd R, Redler S, Heilmann S, Drichel D, Wolf S, Birch P, et al. Investigation of four novel male androgenetic alopecia susceptibility loci: No association with female pattern hair loss. Arch Dermatol Res. 2014;306:413–8. doi: 10.1007/s00403-013-1436-4. [DOI] [PubMed] [Google Scholar]
- 6.Tosti A, Iorizzo M, Piraccini BM. Androgenetic alopecia in children: Report of 20 cases. Br J Dermatol. 2005;152:556–9. doi: 10.1111/j.1365-2133.2004.06279.x. [DOI] [PubMed] [Google Scholar]
- 7.Cela E, Robertson C, Rush K, Kousta E, White DM, Wilson H, et al. Prevalence of polycystic ovaries in women with androgenic alopecia. Eur J Endocrinol. 2003;149:439–42. doi: 10.1530/eje.0.1490439. [DOI] [PubMed] [Google Scholar]
- 8.Quinn M, Shinkai K, Pasch L, Kuzmich L, Cedars M, Huddleston H. Prevalence of androgenic alopecia in patients with polycystic ovary syndrome and characterization of associated clinical and biochemical features. Fertil Steril. 2014;101:1129–34. doi: 10.1016/j.fertnstert.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 9.Youssef SS, Abdel-Khalek YI, Mostafa AE, Abdel-Fattah A. Female androgenetic alopecia: A risk factor for cardiovascular disease. J Egypt Womens Dermatol Soc. 2013;10:69–74. [Google Scholar]
- 10.Arias-Santiago S, Gutiérrez-Salmerón MT, Castellote-Caballero L, Buendía-Eisman A, Naranjo-Sintes R. Androgenetic alopecia and cardiovascular risk factors in men and women: A comparative study. J Am Acad Dermatol. 2010;63:420–9. doi: 10.1016/j.jaad.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 11.Su LH, Chen LS, Lin SC, Chen HH. Association of androgenetic alopecia with mortality from diabetes mellitus and heart disease. JAMA Dermatol. 2013;149:601–6. doi: 10.1001/jamadermatol.2013.130. [DOI] [PubMed] [Google Scholar]
- 12.Kantor J, Kessler LJ, Brooks DG, Cotsarelis G. Decreased serum ferritin is associated with alopecia in women. J Invest Dermatol. 2003;121:985–8. doi: 10.1046/j.1523-1747.2003.12540.x. [DOI] [PubMed] [Google Scholar]
- 13.Rasheed H, Mahgoub D, Hegazy R, El-Komy M, Abdel Hay R, Hamid MA, et al. Serum ferritin and vitamin d in female hair loss: Do they play a role? Skin Pharmacol Physiol. 2013;26:101–7. doi: 10.1159/000346698. [DOI] [PubMed] [Google Scholar]
- 14.Moeinvaziri M, Mansoori P, Holakooee K, Safaee Naraghi Z, Abbasi A. Iron status in diffuse telogen hair loss among women. Acta Dermatovenerol Croat. 2009;17:279–84. [PubMed] [Google Scholar]
- 15.Deloche C, Bastien P, Chadoutaud S, Galan P, Bertrais S, Hercberg S, et al. Low iron stores: A risk factor for excessive hair loss in non-menopausal women. Eur J Dermatol. 2007;17:507–12. doi: 10.1684/ejd.2007.0265. [DOI] [PubMed] [Google Scholar]
- 16.Rushton DH, Ramsay ID. The importance of adequate serum ferritin levels during oral cyproterone acetate and ethinyl oestradiol treatment of diffuse androgen-dependent alopecia in women. Clin Endocrinol (Oxf) 1992;36:421–7. doi: 10.1111/j.1365-2265.1992.tb01470.x. [DOI] [PubMed] [Google Scholar]
- 17.Olsen EA, Reed KB, Cacchio PB, Caudill L. Iron deficiency in female pattern hair loss, chronic telogen effluvium, and control groups. J Am Acad Dermatol. 2010;63:991–9. doi: 10.1016/j.jaad.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 18.Sinclair R. There is no clear association between low serum ferritin and chronic diffuse telogen hair loss. Br J Dermatol. 2002;147:982–4. doi: 10.1046/j.1365-2133.2002.04997.x. [DOI] [PubMed] [Google Scholar]
- 19.Ozturk P, Kurutas E, Ataseven A, Dokur N, Gumusalan Y, Gorur A, et al. BMI and levels of zinc, copper in hair, serum and urine of Turkish male patients with androgenetic alopecia. J Trace Elem Med Biol. 2014;28:266–70. doi: 10.1016/j.jtemb.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 20.Kil MS, Kim CW, Kim SS. Analysis of serum zinc and copper concentrations in hair loss. Ann Dermatol. 2013;25:405–9. doi: 10.5021/ad.2013.25.4.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sinclair R, Patel M, Dawson TL, Jr, Yazdabadi A, Yip L, Perez A, et al. Hair loss in women: Medical and cosmetic approaches to increase scalp hair fullness. Br J Dermatol. 2011;165(Suppl 3):12–8. doi: 10.1111/j.1365-2133.2011.10630.x. [DOI] [PubMed] [Google Scholar]
- 22.Blumeyer A, Tosti A, Messenger A, Reygagne P, Del Marmol V, Spuls PI, et al. Evidence-based (S3) guideline for the treatment of androgenetic alopecia in women and in men. J Dtsch Dermatol Ges. 2011;9(Suppl 6):S1–57. doi: 10.1111/j.1610-0379.2011.07802.x. [DOI] [PubMed] [Google Scholar]
- 23.Khandpur S, Suman M, Reddy BS. Comparative efficacy of various treatment regimens for androgenetic alopecia in men. J Dermatol. 2002;29:489–98. doi: 10.1111/j.1346-8138.2002.tb00314.x. [DOI] [PubMed] [Google Scholar]
- 24.Hoedemaker C, van Egmond S, Sinclair R. Treatment of female pattern hair loss with a combination of spironolactone and minoxidil. Australas J Dermatol. 2007;48:43–5. doi: 10.1111/j.1440-0960.2007.00332.x. [DOI] [PubMed] [Google Scholar]