Abstract
Introduction and Background:
Cosmetic procedures for hair, such as bleaching, dyeing, and straightening, are commonly used around the world. It has been suggested that excessive use of such procedures can cause damage to the hair shaft. We aimed to assess hair shaft changes using scanning electron microscopy (SEM) in female volunteers who frequently use hair treatment procedures such as bleaching, dyeing, or straightening.
Methods:
A cross-sectional, controlled study in a sample of 25 female volunteers (19 study group and 6 controls) in the age group of 18–45 years. The study group was composed of volunteers who regularly used different cosmetic hair treatment procedures such as bleaching, dyeing, and straightening (any one of these or a combination). The control group had never used any specific hair treatment procedure. The hair shaft damage as seen on SEM was assessed using a standardized scoring system and compared among the two groups statistically. The hair shafts were also examined clinically and with light microscopy.
Results:
No significant differences were seen between the test and control groups with regard to normal clinical examination and light microscopy findings. A higher degree of hair shaft damage was evident under SEM in the study group as compared to the control group. This difference was statistically significant.
Conclusions:
Regular use of procedures such as bleaching, dyeing, or straightening can lead to subtle changes in the hair shaft which can be detected early by SEM.
Keywords: Hair shaft damage, hair weathering, scanning electron microscopy
INTRODUCTION AND BACKGROUND
The use of procedures such as bleaching, dyeing, and straightening are becoming increasingly common, especially among females, the world over. Studies have suggested that repetitive stress on the hair secondary to such procedures may induce damage to the hair shaft. Electron microscopy is one of the modalities which can be used to assess and demonstrate the subtle structural damage to the hair shaft secondary to cosmetic treatments. Most of these studies are based on damage to the hair shaft induced experimentally, in vitro. We aimed to assess in vivo hair shaft damage, using scanning electron microscopy (SEM) in a sample of female patients who frequently use hair treatment procedures such as bleaching, dyeing, or straightening.[1,2]
A few studies have suggested that regular oiling of the scalp with various oils such as coconut oil may prevent hair shaft damage – either naturally occurring or secondary to hair treatments.[3,4] We also aimed to compare the SEM findings of the study group with a control group who regularly use hair oil and otherwise did not use any treatment for the hair (including machine drying of the hair).
METHODS
A cross-sectional study, in which 25 female volunteers participated after informed consent, was obtained. The participants were all staff or students at a medical school attached to a university in the Middle East. The details of the study were presented to female faculty and students at the center, and all volunteers satisfying the inclusion/exclusion criteria were included in the study of the 25 volunteers who satisfied the inclusion criteria; 19 volunteers regularly used different forms of hair treatment-bleaching, hair coloring, and straightening of the hair (in different combinations) with a frequency of at least once a month for the previous 6 months. The other inclusion criteria included: Age between 18 years and 45 years and absence of any other significant skin and/or systemic disease. Only patients with natural black hair were included. The control group included six volunteers who had not performed any procedure on the hair ever and regularly oiled their hair (all patients were using only coconut oil). The control group also had patient with natural black hair only. Both the study and control groups used over-the-counter hair shampoos with conditioners and had no other significant medical problems – systemic or related to the skin or hair.
Hair samples were collected and analyzed after cutting at a point approximately 5 cm from the hair root. Isolated gray hairs were discarded. After naked eye examination of the hair shafts, all hair samples were also examined under a handheld dermoscope (DermLite Multispectral, ×10 magnification – white light and blue light were used) and a light microscope at × 10 and × 40, to see if any discernible changes were evident.
Scanning electron microscopy procedure
Human hairs from the scalp were attached to a double coated carbon conductive tape, mounted on metal aluminum stubs, and sputter coated under high vacuum with the LEICA EM SCD 500 sputter coating machine using platinum as a metal target (10–20 nm thickness). Scanning of coated hair specimens was carried out using an SEM (Model: JSM 6390 LA, JEOL) at 15–20 kV. Multiple sections of each shaft were scanned to ensure that changes seen were uniform and not isolated.
Grading of damage under SEM: The damage to hair shaft under SEM was graded into five grades (based on the grading system developed by Kim et al.):[5]
Grade 0: Virgin intact hair with regular overlay of the cuticle
Grade 1: Irregular overlay of the cuticle without cracks or holes
Grade 2: Severe lift up of the cuticle with cracks or holes but without exposure of the cortex
Grade 3: Partial exposure of cortex
Grade 4: Complete disappearance of cortex.
Statistical analysis
Mann–Whitney U-test was used to compare the SEM scores between the study and control group. Spearman's rho was used to evaluate correlation between SEM scores within the study group with primary hair type/ethnicity and the specific procedure done.
RESULTS
Scanning electron microscopy
Of the study population, 8 (42.1%) had changes corresponding to Grade 2, 7 (36.8%) had changes corresponding to Grade 1, and 3 (15.7%) had changes corresponding to Grade 3. Only 1 (5.1%) had intact hair (Grade 0) [Figures 1–4].
Figure 1.
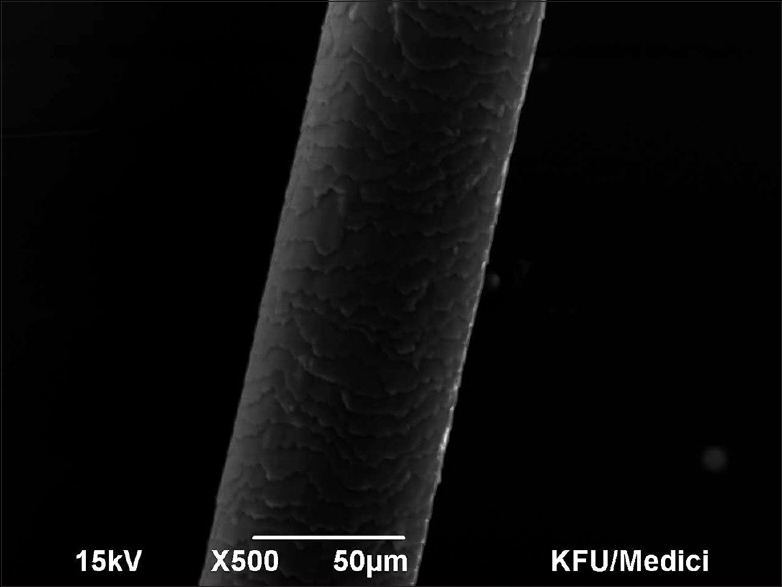
Scanning electron microscopy Grade 0 – Virgin intact hair with regular overlay of the cuticle. 50 micron, ×500, 15 kV
Figure 4.
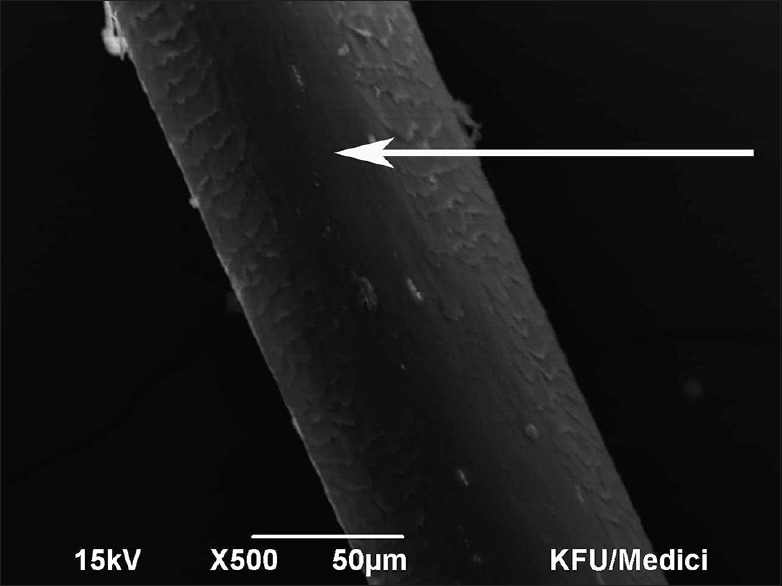
Scanning electron microscopy Grade 3 – Partial exposure of cortex. 50 micron, ×500, 15 kV
Figure 2.
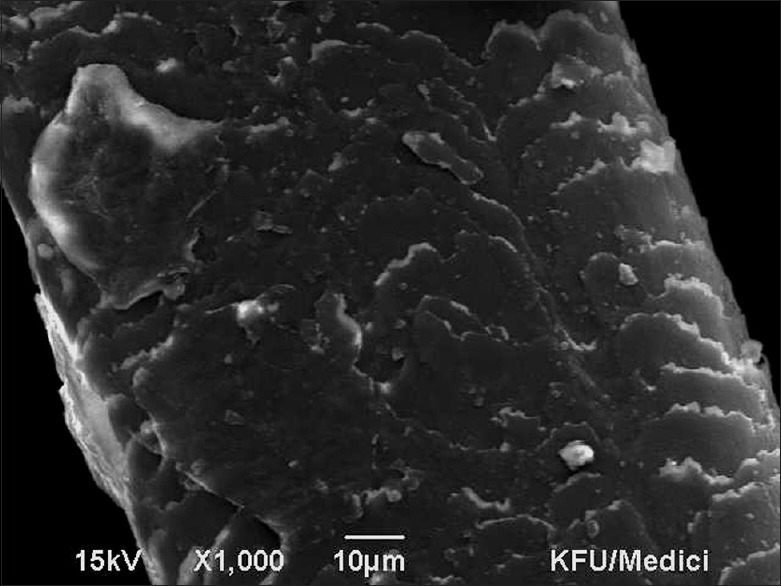
Scanning electron microscopy Grade 1 – Irregular overlay of the cuticle without cracks or holes. 10 micron, ×1000, 15 kV
Figure 3.
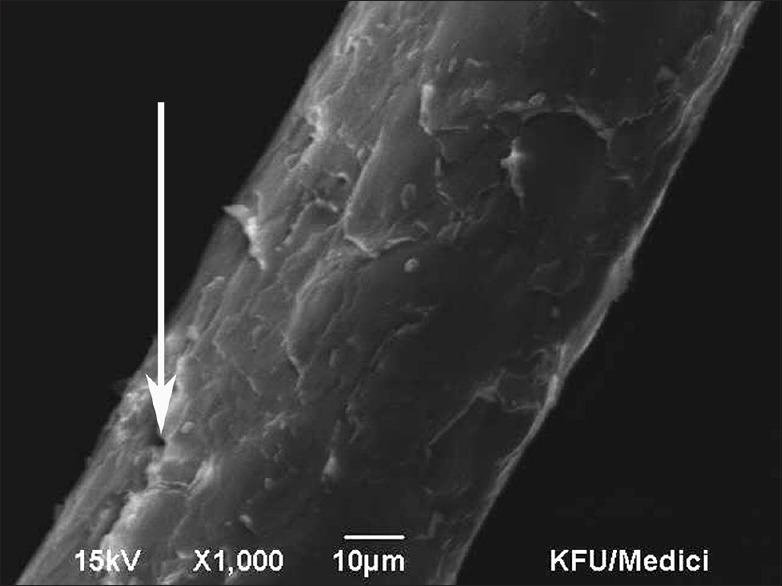
Scanning electron microscopy Grade 2 – Severe lift up of the cuticle with cracks or holes but without exposure of the cortex. 10 micron, ×1000, 15 kV
Of the control samples - 2 out 6 (33.3%) had Grade 1 changes, whereas the remaining 4 (66.6%) showed intact hair on SEM (Grade 0) [Tables 1 and 2].
Table 1.
Study group
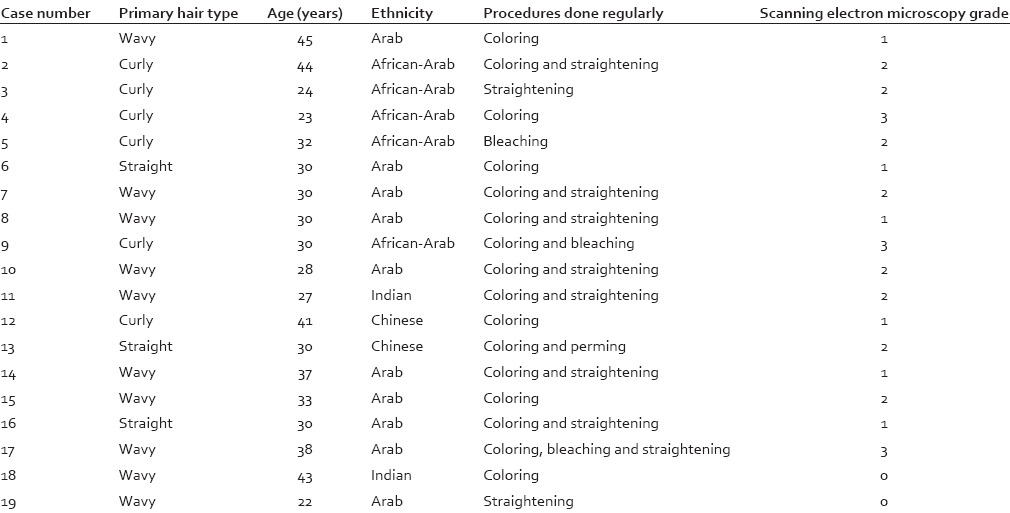
Table 2.
Control group
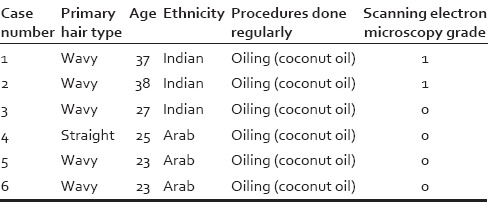
A statistically significant difference was found between the study and control group with respect to the SEM scores with the extent of damage being more in the study group as compared to the control group (Mann–Whitney U-test – 14.000, Z – 02.852, P < 0.05). However, no statistically significant correlation was seen between SEM score and primary hair type/ethnicity or specific hair treatment procedure, within the study group (Spearman's rho, P > 0.05 for both sets of variables).
Normal clinical examination, hair microscopy, and dermoscopy did not show any significant changes in any of the samples, including the ones which had a Grade 3 on SEM.
DISCUSSION
Twenty-five female subjects voluntarily participated in the study which was done to assess hair shaft damage in those who frequently use bleaching, dyeing, and straightening treatments. None of the patients in the study group were using coconut oil over the scalp. SEM was used to assess the hair shaft and visualize the extent of damage in the study group (those frequently using hair treatments) and control group (those regularly using hair oil and no other treatment). Of the total, 42.1% of subjects in the study group had Grade 2 changes, and 15.7% had Grade 3 changes, whereas 33.3% of the control group had Grade 1 changes. No subject in the control group had Grade 2 or higher changes. The results from this small study indicate that treatments such as bleaching, dyeing, and straightening can have an adverse effect on the hair shaft. Ali et al. also documented changes in the hair such as breaking off or lifting up of cuticular scales, roughness in hair surface, tearing, and fragmentation of cuticle in hair samples that have undergone chemical (bleaching, coloring, and waving) and physical treatments (hot straightening).[2] Another experimental in vitro study has demonstrated that hair that was dried using a hair dryer showed more damage of hair surfaces and hair surfaces tend to become more damaged as temperatures increase.[1] Frequent use of chemical agents is considered to be a major cause of damage to the hair shaft which can produce characteristic changes visible on SEM.[6] It has been conclusively proven that for bleached and heat damaged hair, the water retention index (WRI) is much higher than that of untreated hair due to chemical degradation of protein, generating hydrophilic groups.[3] The change in the WRI might be the first step, which in turn leads to the other characteristic changes seen on SEM in hair, which has been frequently exposed to chemical or physical trauma.
Subjects in the control group were regularly applying hair oils. It is postulated that regular oiling with coconut/olive oil might be protective against damage by straightening/bleaching. Faria et al. demonstrated that oiling the scalp with coconut/olive oil might be protective against damage by straightening/bleaching.[4] It is also observed that coconut oil, when used as a prewash conditioner, has a protective effect not only on undamaged hair but also on heat exposed, chemically and ultraviolet treated hair.[3] All the subjects who participated in the study were regularly using head scarves when they went out in the sun. There could be a protective effect of covering the head against environmental damage to the hair. This feature requires further investigation.
The authors also noted that dermoscopy or light microscopy does not recognize hair damage due to chemical changes. SEM is a better modality to demonstrate hair shaft damage due to different cosmetic treatments. According to Miteva and Tosti, although dermoscopy is a useful tool in the diagnosis of hair shaft abnormalities in general, it may not be useful in all conditions associated with hair shaft changes.[7] We feel that practically the hair shaft damage in our study group is too subtle to produce significant changes, which are visible on light microscopy or dermoscopy. This highlights the importance of SEM as a tool to pick up early and subtle hair shaft damage. In general, SEM is considered to be a powerful complementary tool in the study of hair shaft abnormalities.[8]
Although this study was to the best of our knowledge the first if its kind to be conducted to be conducted to assess actual in vivo damage to hair shaft secondary to cosmetic treatments, it had certain limitations. The sample size was relatively small. Only SEM and not transmission electron microscopy were used in the present study. Another limitation of this study was that hair root was not examined in this study. We also did not study other relevant parameters such as the WRI. However, we meant this study to only be a pilot study, and we hope to follow-up the same with a more detailed study with a larger sample size.
We were expecting to obtain a clinical correlation in terms of quality of hair, with the extent of damage visible on SEM. However, we did not find any significant clinical correlation. We assume that this might be partly because none of the patients had Grade 4 changes and studies have shown that minor hair shaft damage tends to recover completely within a relatively short period.[9] We postulate that the percentage of scalp hairs involved and the severity of involvement are the two factors which might correlate with clinically visible changes.
The study, we feel, opens up newer research questions like whether or not the regular use of hair oil can have a protective effect against hair damage due to different chemicals or environmental factors. Studies with a larger sample size possibly incorporating transmission electron microscopy also are warranted in pursuing these research questions.
CONCLUSION
Regular use of cosmetic procedures for the hair such as bleaching, dyeing, or straightening can lead to subtle changes in the hair shaft which can be detected early by SEM.
Financial support and sponsorship
Deanship of Scientific Research, King Faisal University, annual research grants.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Lee Y, Kim YD, Hyun HJ, Pi LQ, Jin X, Lee WS. Hair shaft damage from heat and drying time of hair dryer. Ann Dermatol. 2011;23:455–62. doi: 10.5021/ad.2011.23.4.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ali N, Zohra RR, Qader SA, Mumtaz M. Scanning electron microscopy analysis of hair index on Karachi's population for social and professional appearance enhancement. Int J Cosmet Sci. 2015;37:312–20. doi: 10.1111/ics.12201. [DOI] [PubMed] [Google Scholar]
- 3.Rele AS, Mohile RB. Effect of mineral oil, sunflower oil, and coconut oil on prevention of hair damage. J Cosmet Sci. 2003;54:175–92. [PubMed] [Google Scholar]
- 4.Faria PM, Camargo LN, Carvalho RS, Paludetti LA, Velasco MVR, da Gama RM. Hair protective effect of Argan oil (Arganiaspinosa Kernel Oil) and Cupuassu butter (Theobroma grandifl orum seed butter) post treatment with hair dye. J Cosmet Dermatol Sci Appl. 2013;3:40–4. [Google Scholar]
- 5.Kim YD, Jeon SY, Ji JH, Lee WS. Development of a classification system for extrinsic hair damage: Standard grading of electron microscopic findings of damaged hairs. Am J Dermatopathol. 2010;32:432–8. doi: 10.1097/DAD.0b013e3181c38549. [DOI] [PubMed] [Google Scholar]
- 6.Lee Y, Kim YD, Pi LQ, Lee SY, Hong H, Lee WS. Comparison of hair shaft damage after chemical treatment in Asian, White European, and African hair. Int J Dermatol. 2014;53:1103–10. doi: 10.1111/ijd.12247. [DOI] [PubMed] [Google Scholar]
- 7.Miteva M, Tosti A. Dermatoscopy of hair shaft disorders. J Am Acad Dermatol. 2013;68:473–81. doi: 10.1016/j.jaad.2012.06.041. [DOI] [PubMed] [Google Scholar]
- 8.Echeverría XP, Romero WA, Carreño NR, Zegpi MS, González SJ. Hair shaft abnormalities. Pili bifurcati: A scanning electron microscopy analysis. Pediatr Dermatol. 2009;26:169–70. doi: 10.1111/j.1525-1470.2009.00877.x. [DOI] [PubMed] [Google Scholar]
- 9.Ahn HJ, Lee WS. An ultrastuctural study of hair fiber damage and restoration following treatment with permanent hair dye. Int J Dermatol. 2002;41:88–92. doi: 10.1046/j.1365-4362.2002.01375.x. [DOI] [PubMed] [Google Scholar]


