Abstract
Endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) is widely used to obtain a definitive diagnosis of pancreatic tumors. Good results have been reported for its diagnostic accuracy, with high sensitivity and specificity of around 90%; however, technological developments and adaptations to improve it still further are currently underway. The endosonographic technique can be improved when several tips and tricks useful to overcome challenges of EUS-FNA are known. This review provides various techniques and equipment for improvement in the diagnostic accuracy in EUS-FNA.
Keywords: Diagnostic accuracy, endoscopic ultrasound-guided fine-needle aspiration, pancreatic tumors
INTRODUCTION
Since its initial development in the early 1980s, endoscopic ultrasonography (EUS) has become an essential therapeutic tool for gastroenterology and many other nongastrointestinal applications. However, EUS-based diagnosis solely on the basis of ultrasound patterns has limitations.[1] EUS-guided fine-needle aspiration (EUS-FNA) has therefore been developed as a method of obtaining a pathological diagnosis as a qualitative diagnosis. Vilmann et al.[2] were the first to introduce EUS-FNA into clinical use in the 1990s, and since then it has been adopted as a technique that provides a safe approach of tissue diagnosis under ultrasound guidance in real time. Its greatest advantage is that it enables specimens to be obtained safely and accurately from lesions that were formerly not readily accessible for pathological diagnosis. The reported diagnostic yield of EUS-FNA for pancreatic tumorous lesions to date is good, with a diagnostic accuracy of 78%–95%, sensitivity 78%–95%, and specificity 75%–100%.[3,4,5] A number of studies have reported adaptations and variations in the technique of EUS-FNA to further improve its specimen acquisition rate and diagnostic yield. In this paper, we describe and review efforts to improve the diagnostic accuracy of EUS-FNA for pancreatic masses.
ADAPTATIONS TO NEEDLES
Kind of puncture needle
A number of attempts have been made to improve the puncture device [Table 1]. Several studies have examined EUS-guided trucut biopsy using a core-cut needle (Quick-core™, Cook Medical Co.). A consensus on its value has yet to be reached, with one study finding no difference in the specimen acquisition rate or diagnostic yield with those of EUS-FNA,[3] but another reporting an exceptionally high true-positive rate of 97%.[4] This device was cumbersome to use and was recently withdrawn from sale. It would be desirable if other puncture needles based on the same concept were to be developed that were simpler to use. Puncture needles with a side hole at the tip have recently been developed as core biopsy needles, and a number of reports have addressed their utility. The Echo Tip ProCore™ has enabled diagnosis with fewer needle passes than conventional puncture needles without side holes, with no significant difference in diagnostic adequacy, diagnostic accuracy, and rate of histological core specimen acquisition.[6,7] Ishiwatari et al.[8] compared puncture needles of the same type with and without a side hole and found that although there was no significant difference in the true positive rate, needles with side holes had a higher rate of acquisition of specimens usable for histological diagnosis. Vanbiervliet et al.,[9] however, reported that conventional needles acquired higher-quality specimens compared with those obtained with core biopsy needles. Other studies have found that the type and size of puncture needle had no effect on diagnostic yield,[10,11,12] and it is to be hoped that puncture needles based on new concepts will be developed in future.
Table 1.
Adaptations to endoscopic ultrasound-fine-needle aspiration needles
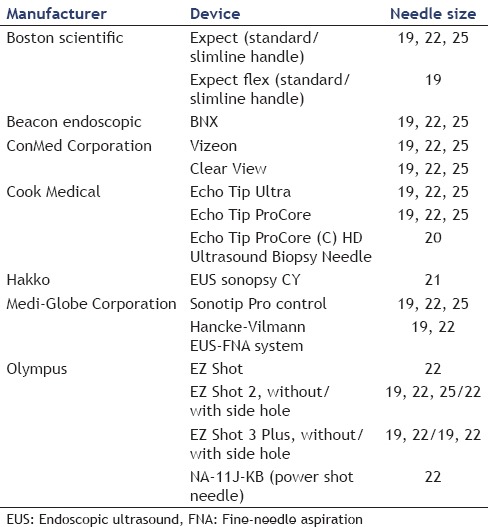
Needle size
Available needle sizes are 19-G, 22-G, and 25-G. Although the 22-G or 25-G needle seems to be the most commonly used for sampling of the pancreas, the optimal needle size for FNA depends on the nature and location of the suspected lesion. There are many reports that have compared the diagnostic yield based on needle size. For the cytopathological yield of FNA for 22-G and 25-G needles, some investigators[13,14,15,16,17,18,19,20,21,22,23,24] showed that the overall diagnostic accuracy of these needles was approximately the same, and there is no clear-cut superiority of either size. In addition, Affolter et al.[11] summarized data from the past studies and estimated the effect of needle size (19-G, 22-G, and 25-G) on reported outcomes such as accuracy, adequacy, and complications. In this report, they suggested that while the 25-G needles might confer an advantage in adequacy relative to 22-G needles, there were no advantages with respect to accuracy, number of passes, or complications between them. On the other hand, a meta-analysis involving six studies and 1064 patients suggested that the 25-G needle was more sensitive than the 22-G needle (93% vs. 84%) for diagnosing pancreatic malignancy.[25] Furthermore, in a prospective randomized trial comparing 25-G and 22-G needles by Carrara et al.,[26] sampling was more adequate in the 25-G compared to the 22-G group (81% vs. 68%; P = 0.09). Moreover, Ramesh et al.[12] compared the flexible 19-G and 25-G needle and reported there was no significant difference in the performance of flexible 19-G and 25-G needles. As a whole, the 25-G needle might be both more flexible and technically easier to use in the pancreatic head and uncinate process although it is still controversial which needle size is better.
ADAPTATIONS OF PUNCTURE AND SPECIMEN ACQUISITION METHODS
Puncture method (needle movement inside the tumor)
To date, various techniques of needling for improvement in diagnostic accuracy of EUS-FNA were reported [Table 2]. Recent studies have reported the value of the fanning technique, in which the puncture needle is moved in a fan-like motion. Bang et al.[27] compared EUS-FNA performed with the fanning technique and the normal technique and found that the true positive rate was 96.4% when the fanning technique was used and 76.9% with the normal technique (P = 0.05), and although this difference was not significant, there was a clearly significant difference in the number of needles passes required to reach a diagnosis. Recently, the door-knocking method, which is maximally quick needle advancement technique within the target lesion, has developed. Mukai et al.[28] compared the conventional puncture method with the door-knocking method and found that although there was no difference in the true positive rate, the door-knocking method did enable the acquisition of larger specimens. Attam et al.[29] found that when EUS-FNA was performed using the “wet suction” technique, in which the puncture needle is filled with saline solution, good quantities of better-quality specimens could be acquired compared with the conventional method, whereas Villa et al.[30] reported that not only were specimens of better quality but also diagnostic yield for the wet suction technique was significantly better at 85.5% compared with 75.2% for the conventional method (P < 0.035). Nakai et al.[31] passed 0.75 mm biopsy forceps through a 19-G FNA needle to perform direct biopsy and found that the tissue acquisition rate for a single puncture was 67% with 0.75 mm biopsy forceps alone but rose to 88% when this technique was used in combination with regular EUS-FNA, indicating that this is a useful technique for acquiring tissue with a small number of needle passes.
Table 2.
Various techniques for improvement in diagnostic accuracy of endoscopic ultrasound-fine-needle aspiration
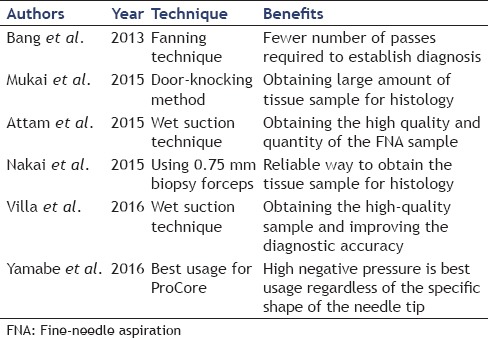
Because of the unique characteristics of the Echo Tip® HD ProCore™ needle, we theorized that moving it with a whipping back technique might yield more tissue than moving it at natural speed because the needle has a “reverse side-bevel” that cuts the specimen. Therefore, we carried out bench-top experiments to investigate the hypothesis that the whipping back technique would be more useful than the conventional technique.[32] However, we found no significant difference in the volume of specimen acquired, and even when using the ProCore™, with its special shape, the best results were produced by the regular puncture action.
Number of fine-needle aspiration passes
In the absence of rapid on-site evaluation (ROSE), earlier reports recommended 5–7 passes for cases with a pancreatic mass.[33,34] Some other studies, however, have found that a smaller number of passes are sufficient,[35,36,37] and Itoi et al.[36] reported that even without ROSE, a mean 2.88 needle passes were adequate for diagnosis, yielding 93.3% accuracy, 91.8% sensitivity, 100% specificity, 100% positive predictive value, and 77.6% negative predictive value. Most studies have shown that maximal diagnostic yield can be obtained after 2–5 passes for pancreatic masses if ROSE is available.[37,38,39,40] Suzuki et al.[41] compared the results when the number of passes was fixed at 4 and when ROSE was used for 25-G EUS-FNA and found that there was no significant difference in either sensitivity or specificity for passes (93% and 100%) and when ROSE was used (94% and 100%). In another study of the number of passes and diagnostic yield, Erickson and Garza[42] found that if ROSE was also used the average number of needle passes was 3.4 ± 2.2 (range 1–10), but that the average number of passes was affected by the differentiation level of cancer (well-differentiated cancer: 5.5 ± 2.7; moderately differentiated: 2.7 ± 1.2; moderately to poorly differentiated: 3.4 ± 2.1; poorly differentiated: 2.3 ± 1.1) (P < 0.001). Wani et al.[43] carried out a similar study and found that well-differentiated cancer was a predictive factor for a larger number of needle passes.
Suction techniques
To date, various suction techniques of EUS-FNA were reported [Table 3]. The standard EUS-FNA is done under negative pressure, usually applied with a 10–20 mL syringe.[44] Recently, however, a number of studies of suction pressure in EUS-FNA for pancreatic tumors have reported other techniques such as the nonsuction technique,[45,46,47] the slow pull technique,[48,49] and the high negative pressure technique.[33,44]
Table 3.
Comparative studies of each suction technique
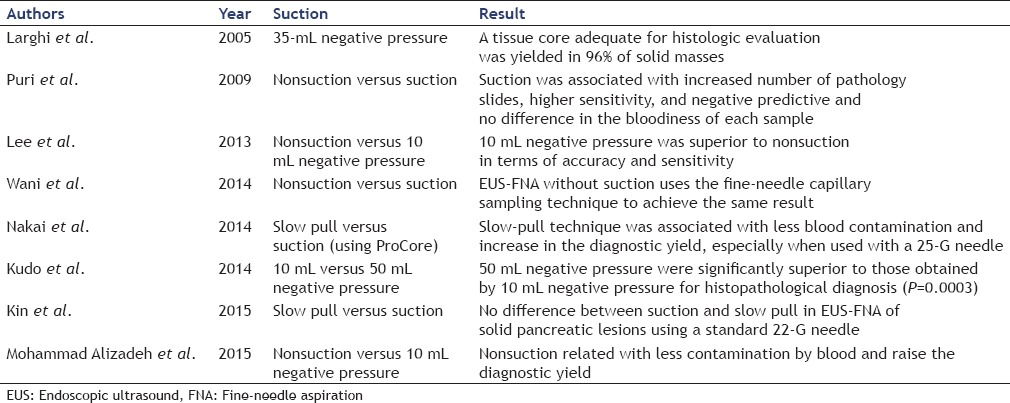
In terms of the nonsuction method, Mohammad Alizadeh et al.[47] compared nonsuction and 10 mL negative pressure and found that not only was there significantly less blood contamination when nonsuction was used compared with 10 mL negative pressure (20% vs. 50%; P < 0.002) but that the diagnostic yield also increased. Puri et al.,[45] however, carried out a similar study and found that sensitivity and negative predictive values were higher when suction was applied, as compared to the nonsuction group (85.7% vs. 66.7%; P = 0.05), and no difference in the bloodiness of each sample. Lee et al.[46] also reported that 10 mL negative pressure was superior to nonsuction in terms of accuracy (85.2% vs. 75.9%; P = 0.004) and sensitivity (82.4% vs. 72.1%; P = 0.005) although specificity was similar (95.8% vs. 100%; P = 0.999). At this point, therefore, the utility of the nonsuction technique may best be described as controversial. The value of the slow pull technique was described by Nakai et al.,[48] who stated that the slow pull technique provides less bloody specimens without reducing cellularity in EUS-FNA for pancreatic malignant lesions, and the sensitivity of the slow pull technique is similar in terms of cytology but higher than that of histology. In contrast, Kin et al.[49] found no difference between suction and slow pull in EUS-FNA of solid pancreatic lesions using a standard 22-G needle. With respect to the high negative pressure technique, Kudo et al.[44] compared the rate of specimen acquisition when 10 mL negative pressure and 50 mL negative pressure were used and found that this was significantly higher with 50 mL negative pressure (72.2% vs. 90%; P = 0.0003). Although blood contamination was significantly greater when the high negative pressure technique was used (P = 0.004), this was not considered to affect the histological diagnosis. A few recent studies, however, have stated that although high negative pressure enables the acquisition of larger specimens, blood contamination does actually affect the diagnostic yield.[47,48] Further studies of the value of the slow pull technique are required, but at this point, it may be better to use the conventional 10–20 mL negative pressure as the suction pressure for EUS-FNA for pancreatic tumors.
USE OF IMAGE PROCESSING (ENDOSCOPIC ULTRASOUND-GUIDED FINE-NEEDLE ASPIRATION UNDER CONTRAST-ENHANCED HARMONIC IMAGING)
EUS-FNA with the use of ultrasound contrast agent is also used in the attempt to improve its diagnostic accuracy. The use of ultrasound contrast agent may enable the recognition of better puncture sites on the basis of differences in blood flow patterns at the lesion site. Kitano et al.[50] have reported on the usefulness of contrast-enhanced harmonic EUS (CE-EUS) and EUS-FNA using CE-EUS. They demonstrated that CE-EUS could indentify pancreatic adenocarcinomas as solid lesions exhibiting hypoenhancement, with a sensitivity and specificity of 88%–96% and 88%–94%, respectively. In particular, 80%–100% of false-negative cases in EUS-FNA are correctly classified by CE-EUS, suggesting that CE-EUS complements EUS-FNA. They also reported that CE-EUS-depicted hypoenhancement diagnosed ductal carcinomas with a sensitivity and specificity of 95.1% (95% confidence interval [CI]: 92.7%–96.7%) and 89.0% (95% CI: 83.0%–93.1%), respectively, whereas hypervascular enhancement diagnosed neuroendocrine tumors with a sensitivity and specificity of 78.9% (95% CI: 61.4%–89.7%) and 98.7% (95% CI: 96.7%–98.8%), respectively.[51] Therefore, CE-EUS will be useful for detecting masses that are difficult to identify on conventional images using B-mode, particularly in patients with severe chronic pancreatitis.
Hocke et al.[52] stated that the use of CE-EUS improved the sensitivity with which chronic pancreatitis could be differentiated from inflammatory pseudotumors and pancreatic carcinoma from 73% to 91% and the specificity from 83% to 93%. According to a study by Seicean et al.,[53] EUS-FNA under CE-EUS imaging improved the diagnostic accuracy for pancreatic masses to 86.5% from the 78.4% achieved with regular EUS-FNA (P = 0.35), and they also reported that the accuracy increased to 94% when the two methods’ results were combined. Sugimoto et al.[54] reported that EUS-FNA under CE-EUS imaging enables a diagnosis to be obtained with fewer needle passes compared with conventional EUS-FNA, whereas Hou et al.[55] stated that the percentage of adequate biopsy specimens in the CE-EUS group (96.6%) was greater than that in the EUS group (86.7%). These studies suggest that the use of CE-EUS in combination with EUS-FNA may both enable the acquisition of a good volume of specimen and improve the true positive rate.
RAPID ON-SITE EVALUATION
Although the use of ROSE has been shown to improve the true positive rate of EUS-FNA.[10,38,40,56,57,58,59,60] The number of institutions capable of having a pathologist on staff when EUS-FNA is performed is limited,[36,48,61] and this is a factor that at least hinders the widespread use of ROSE. Hikichi et al.[62] compared the sensitivity, specificity, and true positive rate of ROSE performed by the pathologist and the endoscopist and found no significant difference between the two. Hayashi et al.[63] reported that the performance of ROSE by the endoscopist who had received a certain level of cytology training improved the true positive rate from 62.9% to 91.8%. This suggested that even in the absence of a pathologist, the performance of ROSE by an endoscopist may be sufficient to improve diagnostic yield.
Recent studies have shown that macroscopic on-site quality evaluation (MOSE) also improves diagnostic accuracy to a degree comparable with ROSE. On this point, Iwashita et al.[64] investigated the size of the macroscopically visible core and the diagnostic yield of EUS-FNA and found that MVC of ≥4 mm on MOSE can be an indicator of specimen adequacy and can improve diagnostic yield. MOSE offers a procedure that can be reliably carried out by an endoscopist even if ROSE is infeasible, and can be regarded as obligatory for endoscopists who intend to improve their diagnostic accuracy.
CYTOLOGIC AND HISTOLOGIC SAMPLE PROCESSING
The obtained material is expressed onto glass slides or into a container for pathologic examination by expelling air from a syringe and/or reinsertion of the stylet. Inserting the stylet into the needle should be done for expression of the material if the operator feels a strong pressure when expressing the material with the syringe.[65] Specimens collected by fine needle would contain the cyto-histopathological sample and blood/clot. Therefore, it is often difficult to confirm whether the obtained sample contains an adequate specimen within. Matsumoto et al.[66] developed a useful target sample check illuminator (TSCI) and reported that using the TSCI in EUS-FNA made it possible to both collect the minimum necessary target samples by EUS-FNA and to end further procedures, even without performing ROSE.
The aspirated material can be processed as direct smears, collected in a preservative solution or media for subsequent processing, or both. In performing ROSE, the preparation of good smears is the final important step because smearing error may lead to tissue loss, artifacts, and interpretation difficulties. At dry fixation, the air-dried preparation requires immediate drying of the cytologic smears. Usually, these slides are stained utilizing Romanowsky-type stains (i.e., Diff-Quik staining, Siemens, USA) to provide morphologic assessment on-site. Although Diff-Quik staining usually provides adequate findings for a preliminary diagnosis, ethanol-fixed and Papanicolaou-stained material provides the best nuclear detail. The smears must be placed immediately into alcohol to minimize air drying artifacts.[67] However, in wet fixation, processing must be carried out while the slide is still damp. This can be accomplished by directly immersing the slides in 95% ethanol, or by spraying the smeared slides using alcohol-based spray fixatives. Typically, wet fixed smear preparations are subsequently stained using Papanicolaou stain in the laboratory. The Papanicolaou stain is preferred by many pathologists because of its near transparency, allowing for the nuclear features, chromatin pattern, and thicker tissue fragments to be visualized.
Material also may be placed in a liquid medium or fixative for cell block, which can then be formalin-fixed, paraffin-embedded, and sectioned for standard hematoxylin and eosin staining or other ancillary testing. Although cell blocks are used as a complement in many cases,[68] Noda et al.[69] reported that the cell block method with immunostaining showed a higher diagnostic accuracy than smear cytology in patients who had undergone EUS-FNA without ROSE (93.9% and 60.6%, P = 0.003). Haba et al.[58] also reported that the diagnostic performance was significantly higher when both cytological and cell-block examinations were carried out than with only cytological examination. These types of processing will be performed in cases without ROSE.
Although the success rate of EUS-FNA increases with the endoscopist's experience, the diagnostic success of EUS-FNA also depends on the cytopathologist's experience. Unlike percutaneous biopsies, EUS-FNA material is contaminated by gastrointestinal epithelium[70,71] that can lead to errors in diagnosis. It is important for the cytopathologist to be aware of the route traversed by the needle for proper evaluation of the smears. Training courses are required for cytopathologists with no previous experience with EUS-FNA.[72,73]
CONCLUSION
A range of adaptations to improve the accuracy of EUS-FNA is currently under development. Although the diagnostic yield of regular EUS-FNA is around 90% and as such is already satisfactory, we must constantly develop and implement new procedures in the quest to attain the 100% level. It is our hope that this article will be of value to as many endoscopists as possible.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Rösch T, Classen M. Endosonography – what are the limits in gastroenterological diagnostics? Endoscopy. 1991;23:144–6. doi: 10.1055/s-2007-1010642. [DOI] [PubMed] [Google Scholar]
- 2.Vilmann P, Jacobsen GK, Henriksen FW, et al. Endoscopic ultrasonography with guided fine needle aspiration biopsy in pancreatic disease. Gastrointest Endosc. 1992;38:172–3. doi: 10.1016/s0016-5107(92)70385-x. [DOI] [PubMed] [Google Scholar]
- 3.Wiersema MJ, Vilmann P, Giovannini M, et al. Endosonography-guided fine-needle aspiration biopsy: Diagnostic accuracy and complication assessment. Gastroenterology. 1997;112:1087–95. doi: 10.1016/s0016-5085(97)70164-1. [DOI] [PubMed] [Google Scholar]
- 4.Williams DB, Sahai AV, Aabakken L, et al. Endoscopic ultrasound guided fine needle aspiration biopsy: A large single centre experience. Gut. 1999;44:720–6. doi: 10.1136/gut.44.5.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoshinaga S, Suzuki H, Oda I, et al. Role of endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) for diagnosis of solid pancreatic masses. Dig Endosc. 2011;23(Suppl 1):29–33. doi: 10.1111/j.1443-1661.2011.01112.x. [DOI] [PubMed] [Google Scholar]
- 6.Witt BL, Adler DG, Hilden K, et al. A comparative needle study: EUS-FNA procedures using the HD ProCore(™) and EchoTip(®) 22-gauge needle types. Diagn Cytopathol. 2013;41:1069–74. doi: 10.1002/dc.22971. [DOI] [PubMed] [Google Scholar]
- 7.Bang JY, Hawes R, Varadarajulu S. A meta-analysis comparing ProCore and standard fine-needle aspiration needles for endoscopic ultrasound-guided tissue acquisition. Endoscopy. 2016;48:339–49. doi: 10.1055/s-0034-1393354. [DOI] [PubMed] [Google Scholar]
- 8.Ishiwatari H, Hayashi T, Kawakami H, et al. Randomized trial comparing a side-port needle and standard needle for EUS-guided histology of pancreatic lesions. Gastrointest Endosc. 2016:S0016–510701591-1. doi: 10.1016/j.gie.2016.03.1329. [DOI] [PubMed] [Google Scholar]
- 9.Vanbiervliet G, Napoléon B, Saint Paul MC, et al. Core needle versus standard needle for endoscopic ultrasound-guided biopsy of solid pancreatic masses: A randomized crossover study. Endoscopy. 2014;46:1063–70. doi: 10.1055/s-0034-1377559. [DOI] [PubMed] [Google Scholar]
- 10.Alper E, Onur I, Arabul M, et al. Endoscopic ultrasound-guided tissue sampling: How can we improve the results? Turk J Gastroenterol. 2016;27:1–3. doi: 10.5152/tjg.2015.150497. [DOI] [PubMed] [Google Scholar]
- 11.Affolter KE, Schmidt RL, Matynia AP, et al. Needle size has only a limited effect on outcomes in EUS-guided fine needle aspiration: A systematic review and meta-analysis. Dig Dis Sci. 2013;58:1026–34. doi: 10.1007/s10620-012-2439-2. [DOI] [PubMed] [Google Scholar]
- 12.Ramesh J, Bang JY, Hebert-Magee S, et al. Randomized trial comparing the flexible 19G and 25G needles for endoscopic ultrasound-guided fine needle aspiration of solid pancreatic mass lesions. Pancreas. 2015;44:128–33. doi: 10.1097/MPA.0000000000000217. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Gerke H. EUS-guided FNA: Better samples with smaller needles? Gastrointest Endosc. 2009;70:1098–100. doi: 10.1016/j.gie.2009.06.033. [DOI] [PubMed] [Google Scholar]
- 14.Yusuf TE, Ho S, Pavey DA, et al. Retrospective analysis of the utility of endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) in pancreatic masses, using a 22-gauge or 25-gauge needle system: A multicenter experience. Endoscopy. 2009;41:445–8. doi: 10.1055/s-0029-1214643. [DOI] [PubMed] [Google Scholar]
- 15.Sakamoto H, Kitano M, Komaki T, et al. Prospective comparative study of the EUS guided 25-gauge FNA needle with the 19-gauge trucut needle and 22-gauge FNA needle in patients with solid pancreatic masses. J Gastroenterol Hepatol. 2009;24:384–90. doi: 10.1111/j.1440-1746.2008.05636.x. [DOI] [PubMed] [Google Scholar]
- 16.Siddiqui UD, Rossi F, Rosenthal LS, et al. EUS-guided FNA of solid pancreatic masses: A prospective, randomized trial comparing 22-gauge and 25-gauge needles. Gastrointest Endosc. 2009;70:1093–7. doi: 10.1016/j.gie.2009.05.037. [DOI] [PubMed] [Google Scholar]
- 17.Fabbri C, Polifemo AM, Luigiano C, et al. Comparative study of EUS-guided 25 gauge needle versus EUS-guided 22 gauge needle for FNA in patients with solid pancreatic masses: Preliminary results. Dig Liver Dis. 2009;41:S60–1. [Google Scholar]
- 18.Lee JH, Stewart J, Ross WA, et al. Blinded prospective comparison of the performance of 22-gauge and 25-gauge needles in endoscopic ultrasound-guided fine needle aspiration of the pancreas and peri-pancreatic lesions. Dig Dis Sci. 2009;54:2274–81. doi: 10.1007/s10620-009-0906-1. [DOI] [PubMed] [Google Scholar]
- 19.Song TJ, Kim JH, Lee SS, et al. The prospective randomized, controlled trial of endoscopic ultrasound-guided fine-needle aspiration using 22G and 19G aspiration needles for solid pancreatic or peripancreatic masses. Am J Gastroenterol. 2010;105:1739–45. doi: 10.1038/ajg.2010.108. [DOI] [PubMed] [Google Scholar]
- 20.Camellini L, Carlinfante G, Azzolini F, et al. A randomized clinical trial comparing 22G and 25G needles in endoscopic ultrasound-guided fine-needle aspiration of solid lesions. Endoscopy. 2011;43:709–15. doi: 10.1055/s-0030-1256482. [DOI] [PubMed] [Google Scholar]
- 21.Fabbri C, Polifemo AM, Luigiano C, et al. Endoscopic ultrasound-guided fine needle aspiration with 22- and 25-gauge needles in solid pancreatic masses: A prospective comparative study with randomisation of needle sequence. Dig Liver Dis. 2011;43:647–52. doi: 10.1016/j.dld.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 22.Gimeno-García AZ, Elwassief A, Paquin SC, et al. Randomized controlled trial comparing stylet-free endoscopic ultrasound-guided fine-needle aspiration with 22-G and 25-G needles. Dig Endosc. 2014;26:467–73. doi: 10.1111/den.12204. [DOI] [PubMed] [Google Scholar]
- 23.Mavrogenis G, Weynand B, Sibille A, et al. 25-gauge histology needle versus 22-gauge cytology needle in endoscopic ultrasonography-guided sampling of pancreatic lesions and lymphadenopathy. Endosc Int Open. 2015;3:E63–8. doi: 10.1055/s-0034-1390889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berzosa M, Villa N, El-Serag HB, et al. Comparison of endoscopic ultrasound guided 22-gauge core needle with standard 25-gauge fine-needle aspiration for diagnosing solid pancreatic lesions. Endosc Ultrasound. 2015;4:28–33. doi: 10.4103/2303-9027.151320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Madhoun M, Wani SB, Early DS, et al. The diagnostic accuracy of 22- and 25-gauge needles in EUS-FNA of solid pancreatic lesions: A meta-analysis. Gastrointest Endosc. 2011;73:AB154. doi: 10.1055/s-0032-1325992. [DOI] [PubMed] [Google Scholar]
- 26.Carrara S, Anderloni A, Jovani M, et al. A prospective randomized study comparing 25-G and 22-G needles of a new platform for endoscopic ultrasound-guided fine needle aspiration of solid masses. Dig Liver Dis. 2016;48:49–54. doi: 10.1016/j.dld.2015.09.017. [DOI] [PubMed] [Google Scholar]
- 27.Bang JY, Magee SH, Ramesh J, et al. Randomized trial comparing fanning with standard technique for endoscopic ultrasound-guided fine-needle aspiration of solid pancreatic mass lesions. Endoscopy. 2013;45:445–50. doi: 10.1055/s-0032-1326268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mukai S, Itoi T, Ashida R, et al. Multicenter, prospective, crossover trial comparing the door-knocking method with the conventional method for EUS-FNA of solid pancreatic masses (with videos) Gastrointest Endosc. 2016;83:1210–7. doi: 10.1016/j.gie.2015.10.025. [DOI] [PubMed] [Google Scholar]
- 29.Attam R, Arain MA, Bloechl SJ, et al. “Wet suction technique (WEST)”: A novel way to enhance the quality of EUS-FNA aspirate. Results of a prospective, single-blind, randomized, controlled trial using a 22-gauge needle for EUS-FNA of solid lesions. Gastrointest Endosc. 2015;81:1401–7. doi: 10.1016/j.gie.2014.11.023. [DOI] [PubMed] [Google Scholar]
- 30.Villa NA, Berzosa M, Wallace MB, et al. Endoscopic ultrasound-guided fine needle aspiration: The wet suction technique. Endosc Ultrasound. 2016;5:17–20. doi: 10.4103/2303-9027.175877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakai Y, Isayama H, Chang KJ, et al. A pilot study of EUS-guided through-the-needle forceps biopsy (with video) Gastrointest Endosc. 2016:S0016–510700002-X. doi: 10.1016/j.gie.2015.12.033. [DOI] [PubMed] [Google Scholar]
- 32.Yamabe A, Irisawa A, Shibukawa G, et al. An experimental study to assess the best maneuver when using a reverse side-bevel histology needle for EUS-guided fine-needle biopsy. Endosc Int Open. 2016;4:E56–61. doi: 10.1055/s-0041-107801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Erickson RA, Sayage-Rabie L, Beissner RS. Factors predicting the number of EUS-guided fine-needle passes for diagnosis of pancreatic malignancies. Gastrointest Endosc. 2000;51:184–90. doi: 10.1016/s0016-5107(00)70416-0. [DOI] [PubMed] [Google Scholar]
- 34.LeBlanc JK, Ciaccia D, Al-Assi MT, et al. Optimal number of EUS-guided fine needle passes needed to obtain a correct diagnosis. Gastrointest Endosc. 2004;59:475–81. doi: 10.1016/s0016-5107(03)02863-3. [DOI] [PubMed] [Google Scholar]
- 35.Turner BG, Cizginer S, Agarwal D, et al. Diagnosis of pancreatic neoplasia with EUS and FNA: A report of accuracy. Gastrointest Endosc. 2010;71:91–8. doi: 10.1016/j.gie.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 36.Itoi T, Tsuchiya T, Itokawa F, et al. Histological diagnosis by EUS-guided fine-needle aspiration biopsy in pancreatic solid masses without on-site cytopathologist: A single-center experience. Dig Endosc. 2011;23(Suppl 1):34–8. doi: 10.1111/j.1443-1661.2011.01142.x. [DOI] [PubMed] [Google Scholar]
- 37.Ecka RS, Sharma M. Rapid on-site evaluation of EUS-FNA by cytopathologist: An experience of a tertiary hospital. Diagn Cytopathol. 2013;41:1075–80. doi: 10.1002/dc.23047. [DOI] [PubMed] [Google Scholar]
- 38.Lee LS, Nieto J, Watson RR, et al. Randomized noninferiority trial comparing diagnostic yield of cytopathologist-guided versus 7 passes for EUS-FNA of Pancreatic Masses. Dig Endosc. 2015 doi: 10.1111/den.12594. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 39.Ganc RL, Carbonari AP, Colaiacovo R, et al. Rapid on-site cytopathological examination (ROSE) performed by endosonagraphers and its improvement in the diagnosis of pancreatic solid lesions. Acta Cir Bras. 2015;30:503–8. doi: 10.1590/S0102-865020150070000009. [DOI] [PubMed] [Google Scholar]
- 40.Mehmood S, Jahan A, Loya A, et al. Onsite cytopathology evaluation and ancillary studies beneficial in EUS-FNA of pancreatic, mediastinal, intra-abdominal, and submucosal lesions. Diagn Cytopathol. 2015;43:278–86. doi: 10.1002/dc.23207. [DOI] [PubMed] [Google Scholar]
- 41.Suzuki R, Irisawa A, Bhutani MS, et al. Prospective evaluation of the optimal number of 25-gauge needle passes for endoscopic ultrasound-guided fine-needle aspiration biopsy of solid pancreatic lesions in the absence of an onsite cytopathologist. Dig Endosc. 2012;24:452–6. doi: 10.1111/j.1443-1661.2012.01311.x. [DOI] [PubMed] [Google Scholar]
- 42.Erickson RA, Garza AA. Impact of endoscopic ultrasound on the management and outcome of pancreatic carcinoma. Am J Gastroenterol. 2000;95:2248–54. doi: 10.1111/j.1572-0241.2000.02310.x. [DOI] [PubMed] [Google Scholar]
- 43.Wani S, Early D, Kunkel J, et al. Diagnostic yield of malignancy during EUS-guided FNA of solid lesions with and without a stylet: A prospective, single blind, randomized, controlled trial. Gastrointest Endosc. 2012;76:328–35. doi: 10.1016/j.gie.2012.03.1395. [DOI] [PubMed] [Google Scholar]
- 44.Kudo T, Kawakami H, Hayashi T, et al. High and low negative pressure suction techniques in EUS-guided fine-needle tissue acquisition by using 25-gauge needles: A multicenter, prospective, randomized, controlled trial. Gastrointest Endosc. 2014;80:1030–7-e1. doi: 10.1016/j.gie.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 45.Puri R, Vilmann P, Saftoiu A, et al. Randomized controlled trial of endoscopic ultrasound-guided fine-needle sampling with or without suction for better cytological diagnosis. Scand J Gastroenterol. 2009;44:499–504. doi: 10.1080/00365520802647392. [DOI] [PubMed] [Google Scholar]
- 46.Lee JK, Choi JH, Lee KH, et al. A prospective, comparative trial to optimize sampling techniques in EUS-guided FNA of solid pancreatic masses. Gastrointest Endosc. 2013;77:745–51. doi: 10.1016/j.gie.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 47.Mohammad Alizadeh AH, Hadizadeh M, Padashi M, et al. Comparison of two techniques for endoscopic ultrasonography fine-needle aspiration in solid pancreatic mass. Endosc Ultrasound. 2014;3:174–8. doi: 10.4103/2303-9027.138790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nakai Y, Isayama H, Chang KJ, et al. Slow pull versus suction in endoscopic ultrasound-guided fine-needle aspiration of pancreatic solid masses. Dig Dis Sci. 2014;59:1578–85. doi: 10.1007/s10620-013-3019-9. [DOI] [PubMed] [Google Scholar]
- 49.Kin T, Katanuma A, Yane K, et al. Diagnostic ability of EUS-FNA for pancreatic solid lesions with conventional 22-gauge needle using the slow pull technique: A prospective study. Scand J Gastroenterol. 2015;50:900–7. doi: 10.3109/00365521.2014.983155. [DOI] [PubMed] [Google Scholar]
- 50.Kitano M, Kamata K, Imai H, et al. Contrast-enhanced harmonic endoscopic ultrasonography for pancreatobiliary diseases. Dig Endosc. 2015;27(Suppl 1):60–7. doi: 10.1111/den.12454. [DOI] [PubMed] [Google Scholar]
- 51.Kitano M, Kudo M, Yamao K, et al. Characterization of small solid tumors in the pancreas: The value of contrast-enhanced harmonic endoscopic ultrasonography. Am J Gastroenterol. 2012;107:303–10. doi: 10.1038/ajg.2011.354. [DOI] [PubMed] [Google Scholar]
- 52.Hocke M, Schulze E, Gottschalk P, et al. Contrast-enhanced endoscopic ultrasound in discrimination between focal pancreatitis and pancreatic cancer. World J Gastroenterol. 2006;12:246–50. doi: 10.3748/wjg.v12.i2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Seicean A, Badea R, Moldovan-Pop A, et al. Harmonic contrast-enhanced endoscopic ultrasonography for the guidance of fine-needle aspiration in solid pancreatic masses. Ultraschall Med. 2015 doi: 10.1055/s-0035-1553496. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 54.Sugimoto M, Takagi T, Hikichi T, et al. Conventional versus contrast-enhanced harmonic endoscopic ultrasonography-guided fine-needle aspiration for diagnosis of solid pancreatic lesions: A prospective randomized trial. Pancreatology. 2015;15:538–41. doi: 10.1016/j.pan.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 55.Hou X, Jin Z, Xu C, et al. Contrast-enhanced harmonic endoscopic ultrasound-guided fine-needle aspiration in the diagnosis of solid pancreatic lesions: A retrospective study. PLoS One. 2015;10:e0121236. doi: 10.1371/journal.pone.0121236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen J, Yang R, Lu Y, et al. Diagnostic accuracy of endoscopic ultrasound-guided fine-needle aspiration for solid pancreatic lesion: A systematic review. J Cancer Res Clin Oncol. 2012;138:1433–41. doi: 10.1007/s00432-012-1268-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Krishnan K, Dalal S, Nayar R, et al. Rapid on-site evaluation of endoscopic ultrasound core biopsy specimens has excellent specificity and positive predictive value for gastrointestinal lesions. Dig Dis Sci. 2013;58:2007–12. doi: 10.1007/s10620-013-2613-1. [DOI] [PubMed] [Google Scholar]
- 58.Haba S, Yamao K, Bhatia V, et al. Diagnostic ability and factors affecting accuracy of endoscopic ultrasound-guided fine needle aspiration for pancreatic solid lesions: Japanese large single center experience. J Gastroenterol. 2013;48:973–81. doi: 10.1007/s00535-012-0695-8. [DOI] [PubMed] [Google Scholar]
- 59.Schmidt RL, Walker BS, Howard K, et al. Rapid on-site evaluation reduces needle passes in endoscopic ultrasound-guided fine-needle aspiration for solid pancreatic lesions: A risk-benefit analysis. Dig Dis Sci. 2013;58:3280–6. doi: 10.1007/s10620-013-2750-6. [DOI] [PubMed] [Google Scholar]
- 60.Hébert-Magee S, Bae S, Varadarajulu S, et al. The presence of a cytopathologist increases the diagnostic accuracy of endoscopic ultrasound-guided fine needle aspiration cytology for pancreatic adenocarcinoma: A meta-analysis. Cytopathology. 2013;24:159–71. doi: 10.1111/cyt.12071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yasuda I, Goto N, Tsurumi H, et al. Endoscopic ultrasound-guided fine needle aspiration biopsy for diagnosis of lymphoproliferative disorders: Feasibility of immunohistological, flow cytometric, and cytogenetic assessments. Am J Gastroenterol. 2012;107:397–404. doi: 10.1038/ajg.2011.350. [DOI] [PubMed] [Google Scholar]
- 62.Hikichi T, Irisawa A, Bhutani MS, et al. Endoscopic ultrasound-guided fine-needle aspiration of solid pancreatic masses with rapid on-site cytological evaluation by endosonographers without attendance of cytopathologists. J Gastroenterol. 2009;44:322–8. doi: 10.1007/s00535-009-0001-6. [DOI] [PubMed] [Google Scholar]
- 63.Hayashi T, Ishiwatari H, Yoshida M, et al. Rapid on-site evaluation by endosonographer during endoscopic ultrasound-guided fine needle aspiration for pancreatic solid masses. J Gastroenterol Hepatol. 2013;28:656–63. doi: 10.1111/jgh.12122. [DOI] [PubMed] [Google Scholar]
- 64.Iwashita T, Yasuda I, Mukai T, et al. Macroscopic on-site quality evaluation of biopsy specimens to improve the diagnostic accuracy during EUS-guided FNA using a 19-gauge needle for solid lesions: A single-center prospective pilot study (MOSE study) Gastrointest Endosc. 2015;81:177–85. doi: 10.1016/j.gie.2014.08.040. [DOI] [PubMed] [Google Scholar]
- 65.Irisawa A, Hikichi T, Bhutani MS, et al. Basic technique of FNA. Gastrointest Endosc. 2009;69(2 Suppl):S125–9. doi: 10.1016/j.gie.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 66.Matsumoto K, Ueki M, Takeda Y, et al. Development of a device for detecting target specimens from EUS-guided FNA samples. Endosc Int Open. 2015;3:E662–4. doi: 10.1055/s-0034-1393076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kulesza P, Eltoum IA. Endoscopic ultrasound-guided fine-needle aspiration: Sampling, pitfalls, and quality management. Clin Gastroenterol Hepatol. 2007;5:1248–54. doi: 10.1016/j.cgh.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 68.Polkowski M, Larghi A, Weynand B, et al. Learning, techniques, and complications of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Technical Guideline. Endoscopy. 2012;44:190–206. doi: 10.1055/s-0031-1291543. [DOI] [PubMed] [Google Scholar]
- 69.Noda Y, Fujita N, Kobayashi G, et al. Diagnostic efficacy of the cell block method in comparison with smear cytology of tissue samples obtained by endoscopic ultrasound-guided fine-needle aspiration. J Gastroenterol. 2010;45:868–75. doi: 10.1007/s00535-010-0217-5. [DOI] [PubMed] [Google Scholar]
- 70.Stelow EB, Bardales RH, Stanley MW. Pitfalls in endoscopic ultrasound-guided fine-needle aspiration and how to avoid them. Adv Anat Pathol. 2005;12:62–73. doi: 10.1097/01.pap.0000155053.68496.ad. [DOI] [PubMed] [Google Scholar]
- 71.Jenssen C, Möller K, Wagner S, et al. Endoscopic ultrasound-guided biopsy: Diagnostic yield, pitfalls, quality management part 1: Optimizing specimen collection and diagnostic efficiency. Z Gastroenterol. 2008;46:590–600. doi: 10.1055/s-2008-1027413. [DOI] [PubMed] [Google Scholar]
- 72.Skov BG, Baandrup U, Jakobsen GK, et al. Cytopathologic diagnoses of fine-needle aspirations from endoscopic ultrasound of the mediastinum: Reproducibility of the diagnoses and representativeness of aspirates from lymph nodes. Cancer. 2007;111:234–41. doi: 10.1002/cncr.22866. [DOI] [PubMed] [Google Scholar]
- 73.Eltoum IA, Chhieng DC, Jhala D, et al. Cumulative sum procedure in evaluation of EUS-guided FNA cytology: The learning curve and diagnostic performance beyond sensitivity and specificity. Cytopathology. 2007;18:143–50. doi: 10.1111/j.1365-2303.2007.00433.x. [DOI] [PubMed] [Google Scholar]


