Abstract
Elastographic techniques have recently become available as advanced diagnostic tools for tissue characterization. Strain elastography is a real-time technique used with transcutaneous ultrasound (US) and endoscopic US. Convincing evidence is available demonstrating a significant value of strain elastography for the discrimination of benign and malignant lymph nodes (LNs). This paper reviews preliminary data demonstrating the feasibility of performing real-time elastography during endobronchial US (EBUS) and a potential application of this technique for selection of LNs for EBUS-guided transbronchial needle aspiration in patients with lung cancer and extrathoracic malignancies.
Keywords: Elastography, endobronchial ultrasound, guidelines, lung cancer, recommendations, staging, training
INTRODUCTION
Conventional endoscopic ultrasound (EUS) and endobronchial US (EBUS) have gained importance in recent years, especially for the clinical important mediastinal lymph node (LN) staging of lung cancer.[1,2] For the evaluation of mediastinal lesions, EBUS and EUS are complimentary methods as in combination virtually all mediastinal and hilar nodal stations can be visualized and sampled.[3] The added value of EUS to EBUS can be summarized by the complementary diagnostic reach of the lower mediastinum and aortopulmonary window in selected cases and the evaluation of the left adrenal gland and other infradiaphragmal metastatic sites.[4,5] Both EUS and EBUS have also been successfully used for the assessment of mediastinal tumor spread of patients with extrathoracic neoplastic diseases and for the evaluation of mediastinal lymphadenopathy of unknown origin and especially for the diagnosis and differentiation of mediastinal granulomatous disease and malignant lymphoma.[4,5]
Computed tomography (CT) provides detailed anatomical information of the mediastinum, hilum, lung parenchyma, and chest wall. Fludeoxyglucose positron emission tomography scanning, preferable in combination with CT, can provide important physiological information regarding mediastinal nodes and lesions. Recently published lung cancer staging guidelines recommend that endosonography (EBUS and EUS) should be the initial tissue sampling test over surgical staging. Mediastinal LN biopsies can be taken using EBUS combined with transbronchial needle aspiration (EBUS-TBNA) or using the esophageal approach (EUS-fine needle aspiration). EBUS and EUS are safe techniques.[4,5] The value of all available US technologies for mediastinal LN staging[4,5,6] and lung US[7] have been recently published.
In addition to the published reviews, the aim of this report is to discuss the possible role of endobronchial elastography in identifying LN features for a better selection of biopsies. The preliminary data are illustrated by examples.
INTRODUCTION INTO ELASTOGRAPHY
Elastography is a noninvasive method in which the relative stiffness of tissues can be imaged as a color map or measured as shear wave velocity. Two main techniques are used currently: Strain elastography and shear wave elastography. Strain elastography is a real-time technique and can be applied with EUS.[8] The European Federation of Societies for US in Medicine and Biology (EFSUMB) has published the guidelines and recommendations describing the technology and assessing the clinical use of real-time elastography (RTE).[9,10] In addition, the World Federation for US in Medicine and Biology has updated this knowledge.[11,12,13] Comments and reviews about elastography have been published as well.[14,15,16,17,18,19,20,21]
Endobronchial ultrasound elastography
Initial reports on EBUS-elastography (EBUS-E) have been published in textbooks.[22,23] More recently a few other publications have been published.[24,25,26,27,28,29,30,31,32]
Longitudinal EBUS-Scopes allow US-guided biopsy and have been introduced in 2004.[33] More recently, US technology including strain imaging techniques (RTE) has been introduced[9,10] which allows LN characterization transcutaneously[15,20,34,35,36,37] and via EUS.[8,14,15,20,38] Since the introduction of the new generation of EBUS processors those features are now also available for the EBUS procedure. EBUS-E can only be applied with the linear EBUS scope. It is worthwhile to recall that real-time EBUS-TBNA has been shown to have a higher diagnostic yield in mediastinal staging than blind TBNA and has similar sensitivity to mediastinoscopy.[4] Contrast-enhanced US does not influence elastographic imaging which is true at least for shear wave elastography of the liver.[39]
The role of EBUS-E has not been discussed in detail so far.
Elastographic lymph node evaluation
The differentiation of malignant from benign LNs by US, CT, and magnetic resonance imaging traditionally relies mainly on the size measurements and topographic distribution and size. However, sensitivity and specificity in the differentiation of benign and malignant LNs are disappointing using only size parameters. Reasons for the low accuracy include that malignant LN infiltration occurs in up to 30% in LNs of <5 mm which has been shown for lung, esophageal, gastric, pancreatic, and rectal carcinoma.[15]
Many papers have been published on the use of elastography for LN evaluation[14,15,17,20,35,38,40,41,42,43] with the EUS scope. It could be shown that normal LNs reveal a significantly harder cortex than the medulla and the hilum[14,20,40] which is also true for mediastinal LNs [Figure 1]. The same is true for inflammatory lymphadenopathy [Figures 2 and 3]. The very early metastatic infiltration of an LN is circumscribed with localized stiffer neoplastic infiltration. This stage is rarely seen using EBUS-E [Figure 4]. At a later stage, the diffuse stiff infiltration of an LN is typical [Figures 5 and 6]. A meta-analysis describes a pooled sensitivity of 88% and a pooled specificity of 85% with EUS-elastography for the discrimination of malignant versus benign superficial LNs.[44]
Figure 1.
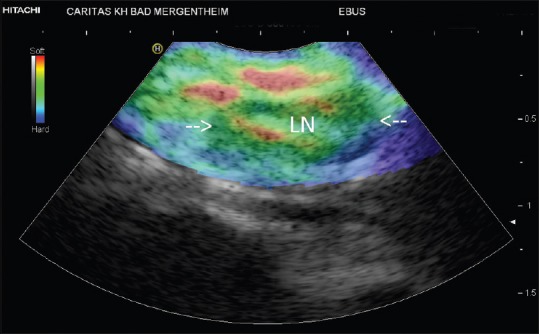
Normal lymph node (in between arrows)
Figure 2.
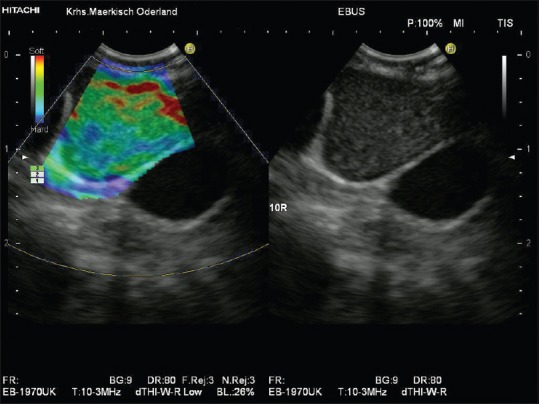
Inflammatory lymph node. Unspecific inflammatory mediastinal lymphadenopathy showed by endobronchial ultrasound-elastography (homogeneously green lymph node parenchyma with softer hilum [shown in red])
Figure 3.
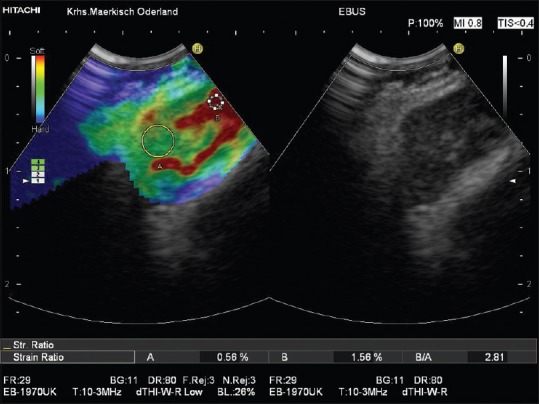
Inflammatory lymph node in a patient with sarcoidosis (homogeneously green lymph node parenchyma with softer hilum [shown in red]). The low strain ratio (2.81) is indicated as well
Figure 4.
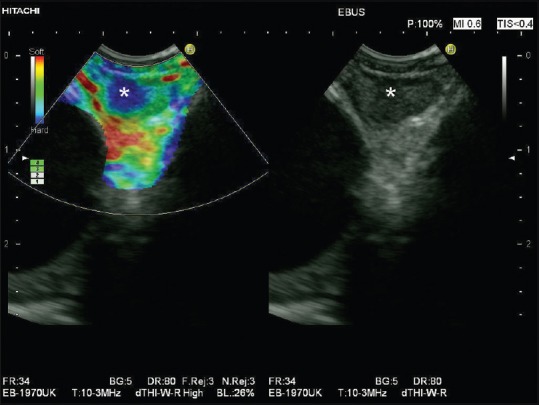
Circumscribed metastatic infiltration of a mediastinal lymph node shown by endobronchial ultrasound-elastography (circumscribed blue area within the lymph node marked by asterisk)
Figure 5.
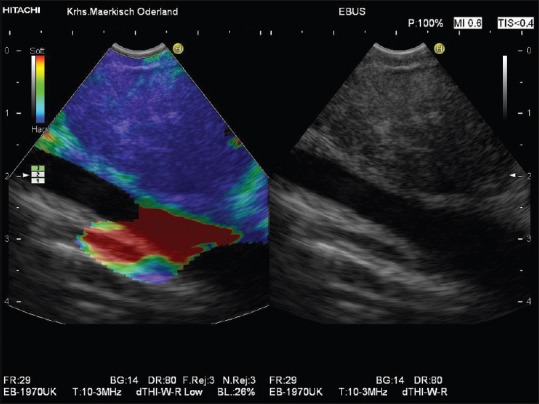
Diffuse malignant infiltration by lung cancer of a mediastinal lymph node shown by endobronchial ultrasound-elastography (homogeneously blue lymph node tissue)
Figure 6.
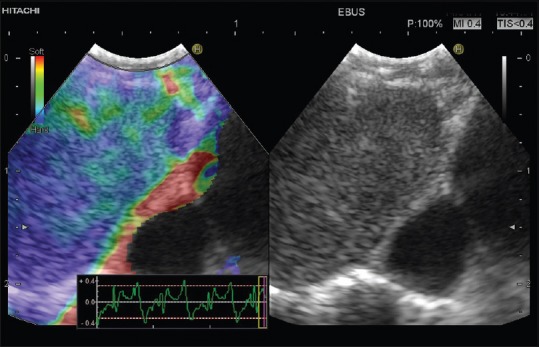
Diffuse metastatic infiltration of a mediastinal lymph node shown by endobronchial ultrasound-elastography (homogeneously blue lymph node tissue)
Elastography-guided lymph node aspiration
A systematic endosonographic sampling of all clinically relevant mediastinal LN stations is necessary in lung cancer staging. LN features as hypoechoicity, distinct margins, roundness, and diameter >10 mm traditionally are used to identify the most suspicious LNs.[5] However, a definitive correct classification as either malignant or benign is possible only in approximately 25% of mediastinal LNs using those criteria.[45] For esophageal cancer, two studies have demonstrated that the use of EUS-elastography significantly improves the accuracy of mediastinal LN staging.[42] Therefore, EFSUMB guidelines recommend the use of endosonographic real-time elastography in patients admitted for tumor staging in order to identify the most suspicious LNs of a particular LN station and/or circumscribed stiff areas within a particular LN as targets of EUS-guided biopsy.[10,46] Thus, endosonographic elastography (EUS-elastography and EBUS-E) may save time in mediastinal nodal staging, reduce the risk of false-negative cytopathological results, and prevent repeat endosonographic sampling procedures and surgical staging. However, comparative studies of EUS-elastography-guided versus EUS-B-mode-guided biopsy of LNs are lacking so far.
Elastography with the endobronchial ultrasound scope
Recently, there have been several reports suggesting that EUS elastography had a high sensitivity and specificity for detecting malignant involvement of pancreatic lesions and LNs. Subsequently, elastography has become available for use during EBUS. As of yet, evidence is needed to assess whether elastography can be a valuable tool in the noninvasive discrimination between benign and malignant thoracic LNs during EBUS-TBNA. At present, only a few articles focusing on this topic are available.
Izumo et al. reported on 75 LNs.[26] They analyzed three different patterns that were classified based on color distribution: Type 1, predominantly nonblue (green, yellow, and red); Type 2, partially blue, partially nonblue (green, yellow, and red); and Type 3, predominantly blue. The elastographic patterns were compared with the final pathologic results from EBUS-TBNA. On pathological evaluation of the LNs, 33 were benign and 42 were malignant. The LNs that were classified as Type 1 on EBUS-E were benign in 24/24 (100%); for Type 2 LNs, 6/14 (46.9%) were benign and 8/14 (57.1%) were malignant; and Type 3 LNs were benign in 2/37 (5.4%) and malignant in 35/37 (94.6%). In classifying Type 1 as “benign” and Type 3 as “malignant,” the sensitivity, specificity, positive predictive value, negative predictive value (NPV), and diagnostic accuracy rates were 100%, 92.3%, 94.6%, 100%, and 96.7%.
Nakajima et al. evaluated 49 LNs (16 malignant) in 21 patients by EBUS-E.[28] Mean stiff area ratios were significantly greater for malignant LNs (0.478) than for benign nodes (0.216; P = 0.0002). Using a cutoff value of 0.311 for stiff area ratios, the group was able to show a sensitivity and specificity for predicting metastatic disease of 0.81 and 0.85. The stiff area was histologically compatible with metastatic distribution in surgically resected LNs. The group did not used any color distribution for an additional analysis.
Rozman et al. underwent a prospective, single-center trial, enrolling patients with an indication for mediastinal staging of a lung tumor.[29] EBUS with standard B-mode evaluation and elastography with strain ratio measurement were performed before EBUS-TBNA. Thirteen patients with eighty suspicious mediastinal LNs were evaluated. Malignancy was confirmed in 34 LNs. The area under the curve for the strain ratio was 0.87 (P < 0.0001). At a strain ratio ≥8, the accuracy for prediction of malignancy was 86.3% (sensitivity 88%, specificity 85%, positive predictive value 81%, and NPV 91%). The strain ratio was more accurate than conventional B-mode EBUS features for differentiating between malignant and benign LNs.
In a group of forty lung cancer patients, a Chinese group evaluated the accuracy of B-Mode features, a qualitative elastographic score, and the elastographic strain ratio to predict metastatic LN involvement. The elastographic score proved to be more sensitive and specific in determining the malignant LN than all B-mode EBUS criteria. Moreover, the combination of B-Mode criteria and elastography further improved the diagnostic accuracy of EBUS to discriminate between benign and metastatic mediastinal LNs (area under the curve [AUC] of combined B-mode features and elastographic score: 0.911).[24] The best accuracy was reported for strain ratio (AUC 0.933 using a cutoff value of 32.07).[25]
In none of the studies, a complication was reported.
Summary
Preliminary data suggest that elastography is a promising diagnostic tool for the differentiation between benign and malignant LNs also using EBUS. Most inflammatory processes do not change the elastographic architecture of LNs, whereas metastatic infiltration causes initially circumscribed and a later stage diffuse hard infiltration of the LN parenchyma. Actually, the evidence is limited to retrospective and single-institution data. EBUS-E may be used to facilitate endosonographic staging by identifying the most suspicious LNs and/or LN area of a particular mediastinal LN station for EBUS-TBNA. Prospective, randomized, multi-center trials are needed in the future to confirm the utility and clinical impact of qualitative and quantitative elastography during EBUS-TBNA. There is a clear need to evaluate the different color distributions, as well as other features like the strain ratio.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Vansteenkiste J, De Ruysscher D, Eberhardt WE, et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(Suppl 6):vi89–98. doi: 10.1093/annonc/mdt241. [DOI] [PubMed] [Google Scholar]
- 2.Rivera MP, Mehta AC, Wahidi MM. Establishing the diagnosis of lung cancer: Diagnosis and management of lung cancer, 3 rd ed: American College of chest physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e142S–65S. doi: 10.1378/chest.12-2353. [DOI] [PubMed] [Google Scholar]
- 3.Vilmann P, Clementsen PF, Colella S, et al. Combined endobronchial and esophageal endosonography for the diagnosis and staging of lung cancer: European Society of Gastrointestinal Endoscopy (ESGE) guideline, in cooperation with the European Respiratory Society (ERS) and the European Society of Thoracic Surgeons (ESTS) Endoscopy. 2015;47:c1. doi: 10.1055/s-0034-1392453. [DOI] [PubMed] [Google Scholar]
- 4.Dietrich CF, Annema JT, Clementsen P, et al. Ultrasound techniques in the evaluation of the mediastinum, part I: Endoscopic ultrasound (EUS), endobronchial ultrasound (EBUS) and transcutaneous mediastinal ultrasound (TMUS), introduction into ultrasound techniques. J Thorac Dis. 2015;7:E311–25. doi: 10.3978/j.issn.2072-1439.2015.09.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jenssen C, Annema JT, Clementsen P, et al. Ultrasound techniques in the evaluation of the mediastinum, part 2: Mediastinal lymph node anatomy and diagnostic reach of ultrasound techniques, clinical work up of neoplastic and inflammatory mediastinal lymphadenopathy using ultrasound techniques and how to learn mediastinal endosonography. J Thorac Dis. 2015;7:E439–58. doi: 10.3978/j.issn.2072-1439.2015.10.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Leyn P. Clinical value of ESTS guidelines on preoperative lymph node staging for NSCLC. Eur J Cardiothorac Surg. 2011;40:280–1. doi: 10.1016/j.ejcts.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 7.Dietrich CF, Mathis G, Cui XW, et al. Ultrasound of the pleurae and lungs. Ultrasound Med Biol. 2015;41:351–65. doi: 10.1016/j.ultrasmedbio.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 8.Dietrich CF, Saftoiu A, Jenssen C. Real time elastography endoscopic ultrasound (RTE-EUS), a comprehensive review. Eur J Radiol. 2014;83:405–14. doi: 10.1016/j.ejrad.2013.03.023. [DOI] [PubMed] [Google Scholar]
- 9.Bamber J, Cosgrove D, Dietrich CF, et al. EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography. Part 1: Basic principles and technology. Ultraschall Med. 2013;34:169–84. doi: 10.1055/s-0033-1335205. [DOI] [PubMed] [Google Scholar]
- 10.Cosgrove D, Piscaglia F, Bamber J, et al. EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography. Part 2: Clinical applications. Ultraschall Med. 2013;34:238–53. doi: 10.1055/s-0033-1335375. [DOI] [PubMed] [Google Scholar]
- 11.Barr RG, Nakashima K, Amy D, et al. WFUMB guidelines and recommendations for clinical use of ultrasound elastography: Part 2: breast. Ultrasound Med Biol. 2015;41:1148–60. doi: 10.1016/j.ultrasmedbio.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 12.Ferraioli G, Filice C, Castera L, et al. WFUMB guidelines and recommendations for clinical use of ultrasound elastography: Part 3: liver. Ultrasound Med Biol. 2015;41:1161–79. doi: 10.1016/j.ultrasmedbio.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 13.Shiina T, Nightingale KR, Palmeri ML, et al. WFUMB guidelines and recommendations for clinical use of ultrasound elastography: Part 1: Basic principles and terminology. Ultrasound Med Biol. 2015;41:1126–47. doi: 10.1016/j.ultrasmedbio.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 14.Cui XW, Chang JM, Kan QC, et al. Endoscopic ultrasound elastography: Current status and future perspectives. World J Gastroenterol. 2015;21:13212–24. doi: 10.3748/wjg.v21.i47.13212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cui XW, Jenssen C, Saftoiu A, et al. New ultrasound techniques for lymph node evaluation. World J Gastroenterol. 2013;19:4850–60. doi: 10.3748/wjg.v19.i30.4850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dietrich CF. Elastography, the new dimension in ultrasonography. Praxis (Bern 1994) 2011;100:1533–42. doi: 10.1024/1661-8157/a000735. [DOI] [PubMed] [Google Scholar]
- 17.Dietrich CF, Cantisani V. Current status and perspectives of elastography. Eur J Radiol. 2014;83:403–4. doi: 10.1016/j.ejrad.2013.02.028. [DOI] [PubMed] [Google Scholar]
- 18.Dietrich CF, Hirche TO, Ott M, et al. Real-time tissue elastography in the diagnosis of autoimmune pancreatitis. Endoscopy. 2009;41:718–20. doi: 10.1055/s-0029-1214866. [DOI] [PubMed] [Google Scholar]
- 19.Dietrich CF, Jenssen C. Endoscopic ultrasound-guided sampling in gastroenterology: European society of gastrointestinal endoscopy technical guidelines. Endosc Ultrasound. 2013;2:117–22. doi: 10.7178/eus.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dietrich CF, Jenssen C, Arcidiacono PG, et al. Endoscopic ultrasound: Elastographic lymph node evaluation. Endosc Ultrasound. 2015;4:176–90. doi: 10.4103/2303-9027.162995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dietrich CF, Jenssen C, Hocke M, et al. Imaging of gastrointestinal stromal tumours with modern ultrasound techniques – A pictorial essay. Z Gastroenterol. 2012;50:457–67. doi: 10.1055/s-0031-1282076. [DOI] [PubMed] [Google Scholar]
- 22.Dietrich CF. In: Endobronchialer ultraschall (EBUS). Interventionelle Sonographie. Dietrich CF, Nuernberg D, editors. Stuttgart: Thieme; 2011. pp. 387–9. [Google Scholar]
- 23.Dietrich CF. In: Endobronchialer Ultraschall (EBUS). Interventionelle Sonographie. Dietrich CF, Nurnberger D, editors. Stuttgart, New York: Thieme Verlag; 2011. [Google Scholar]
- 24.He H, Lu X, Ma H, et al. Value of endobronchial ultrasound elastography in the diagnosis of mediastinal and hilar lymph node metastasis in lung cancer. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2016;41:30–6. doi: 10.11817/j.issn.1672-7347.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 25.He HY, Huang M, Zhu J, et al. Endobronchial ultrasound elastography for diagnosing mediastinal and hilar lymph nodes. Chin Med J (Engl) 2015;128:2720–5. doi: 10.4103/0366-6999.167296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Izumo T, Sasada S, Chavez C, et al. Endobronchial ultrasound elastography in the diagnosis of mediastinal and hilar lymph nodes. Jpn J Clin Oncol. 2014;44:956–62. doi: 10.1093/jjco/hyu105. [DOI] [PubMed] [Google Scholar]
- 27.Jiang JH, Turner JF, Jr, Huang JA. Endobronchial ultrasound elastography: A new method in endobronchial ultrasound-guided transbronchial needle aspiration. J Thorac Dis. 2015;7(Suppl 4):S272–8. doi: 10.3978/j.issn.2072-1439.2015.12.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakajima T, Inage T, Sata Y, et al. Elastography for predicting and localizing nodal metastases during endobronchial ultrasound. Respiration. 2015;90:499–506. doi: 10.1159/000441798. [DOI] [PubMed] [Google Scholar]
- 29.Rozman A, Malovrh MM, Adamic K, et al. Endobronchial ultrasound elastography strain ratio for mediastinal lymph node diagnosis. Radiol Oncol. 2015;49:334–40. doi: 10.1515/raon-2015-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sivokozov IV, Silina TL, Korolev VN, et al. The first experience in using elastography in combination with endobronchial ultrasonography for mediastinal pathology: Preliminary assessment of feasibility and comparison of characteristics via different approaches. Vestn Rentgenol Radiol. 2014;96:13–9. [PubMed] [Google Scholar]
- 31.Trosini-Désert V, Jeny F, Taillade L, et al. Bronchial endoscopic ultrasound elastography: Preliminary feasibility data. Eur Respir J. 2013;41:477–9. doi: 10.1183/09031936.00124812. [DOI] [PubMed] [Google Scholar]
- 32.Andreo García F, Centeno Clemente CÁ, Sanz Santos J, et al. Initial experience with real-time elastography using an ultrasound bronchoscope for the evaluation of mediastinal lymph nodes. Arch Bronconeumol. 2015;51:e8–11. doi: 10.1016/j.arbres.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 33.Yasufuku K, Chhajed PN, Sekine Y, et al. Endobronchial ultrasound using a new convex probe: A preliminary study on surgically resected specimens. Oncol Rep. 2004;11:293–6. [PubMed] [Google Scholar]
- 34.Cui XW, Hocke M, Jenssen C, et al. Conventional ultrasound for lymph node evaluation, update 2013. Z Gastroenterol. 2014;52:212–21. doi: 10.1055/s-0033-1356153. [DOI] [PubMed] [Google Scholar]
- 35.Dietrich CF, Hocke M, Jenssen C. Ultrasound for abdominal lymphadenopathy. Dtsch Med Wochenschr. 2013;138:1001–18. doi: 10.1055/s-0032-1333027. [DOI] [PubMed] [Google Scholar]
- 36.Dietrich CF, Ponnudurai R, Bachmann Nielsen M. Is there a need for new imaging methods for lymph node evaluation? Ultraschall Med. 2012;33:411–4. doi: 10.1055/s-0032-1325384. [DOI] [PubMed] [Google Scholar]
- 37.Hirche TO, Hirche H, Cui XW, et al. Ultrasound evaluation of mediastinal lymphadenopathy in patients with sarcoidosis. Med Ultrason. 2014;16:194–200. doi: 10.11152/mu.2013.2066.163.2hh. [DOI] [PubMed] [Google Scholar]
- 38.Janssen J, Dietrich CF, Will U, et al. Endosonographic elastography in the diagnosis of mediastinal lymph nodes. Endoscopy. 2007;39:952–7. doi: 10.1055/s-2007-966946. [DOI] [PubMed] [Google Scholar]
- 39.Cui XW, Pirri C, Ignee A, et al. Measurement of shear wave velocity using acoustic radiation force impulse imaging is not hampered by previous use of ultrasound contrast agents. Z Gastroenterol. 2014;52:649–53. doi: 10.1055/s-0034-1366036. [DOI] [PubMed] [Google Scholar]
- 40.Chiorean L, Barr RG, Braden B, et al. Transcutaneous ultrasound: Elastographic lymph node evaluation. Current clinical applications and literature review. Ultrasound Med Biol. 2016;42:16–30. doi: 10.1016/j.ultrasmedbio.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 41.Saftoiu A, Vilmann P, Ciurea T, et al. Dynamic analysis of EUS used for the differentiation of benign and malignant lymph nodes. Gastrointest Endosc. 2007;66:291–300. doi: 10.1016/j.gie.2006.12.039. [DOI] [PubMed] [Google Scholar]
- 42.Knabe M, Günter E, Ell C, et al. Can EUS elastography improve lymph node staging in esophageal cancer? Surg Endosc. 2013;27:1196–202. doi: 10.1007/s00464-012-2575-y. [DOI] [PubMed] [Google Scholar]
- 43.Giovannini M, Thomas B, Erwan B, et al. Endoscopic ultrasound elastography for evaluation of lymph nodes and pancreatic masses: A multicenter study. World J Gastroenterol. 2009;15:1587–93. doi: 10.3748/wjg.15.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ying L, Hou Y, Zheng HM, et al. Real-time elastography for the differentiation of benign and malignant superficial lymph nodes: A meta-analysis. Eur J Radiol. 2012;81:2576–84. doi: 10.1016/j.ejrad.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 45.Faige DO. EUS in patients with benign and malignant lymphadenopathy. Gastrointest Endosc. 2001;53:593–8. doi: 10.1067/mge.2001.114060. [DOI] [PubMed] [Google Scholar]
- 46.Jenssen C, Hocke M, Fusaroli P, et al. EFSUMB guidelines on interventional ultrasound (INVUS), part IV – EUS-guided Interventions: General aspects and EUS-guided sampling (long version) Ultraschall Med. 2016;37:E33–76. doi: 10.1055/s-0035-1553785. [DOI] [PubMed] [Google Scholar]


