Abstract
Background:
The efficacy and safety of telmisartan combined with clopidogrel, leflunomide, or both drugs for immunoglobulin A nephropathy (IgAN) are unclear. This study was designed to evaluate the efficacy and safety of telmisartan combined with clopidogrel, leflunomide, or both drugs for IgAN.
Methods:
It is a multicenter, prospective, double-dummy randomized controlled trial. Primary IgAN patients were recruited in 13 renal units across Beijing, China, from July 2010 to June 2012. After a 4-week telmisartan (80 mg/d) wash-in, 400 patients continuing on 80 mg/d telmisartan were randomly assigned to additionally receive placebo (Group A), 50 mg/d clopidogrel (Group B), 20 mg/d leflunomide (Group C), or 50 mg/d clopidogrel and 20 mg/d leflunomide (Group D). The 24-week intervention was completed by 360 patients. The primary endpoint was change in 24-h proteinuria at 24 weeks. A linear mixed-effect model was used to analyze the changes at 4, 12, and 24 weeks. Generalized estimating equations were used to evaluate changes in hematuria grade. This trial was registered at the Chinese Clinical Trial Registry.
Results:
The effects of telmisartan combined with leflunomide on changes in proteinuria (0.36 [95% confidence interval (CI) 0.18–0.55] g/d, P < 0.001), in serum uric acid (76.96 [95% CI 57.44–96.49] μmol/L, P < 0.001), in serum creatinine (9.49 [95% CI 6.54–12.44] μmol/L, P < 0.001), and in estimated glomerular filtration rate (−6.72 [95% CI −9.46 to −3.98] ml∙min−1∙1.73 m−2, P < 0.001) were statistically significant, whereas they were not statistically significant on changes in systolic and diastolic blood pressure and weight (P > 0.05). Telmisartan combined with clopidogrel had no statistical effect on any outcome, and there was no interaction between the interventions. No obvious adverse reactions were observed.
Conclusions:
Telmisartan combined with leflunomide, not clopidogrel, is safe and effective for decreasing proteinuria in certain IgAN patients.
Trial Registration:
chictr.org.cn, ChiCTR-TRC-10000776; http://www.chictr.org.cn/showproj.aspx?proj=8760.
Keywords: Clopidogrel, Immunoglobulin A Nephropathy, Leflunomide, Randomized Controlled Trial
INTRODUCTION
Immunoglobulin A nephropathy (IgAN) is the most prevalent primary chronic glomerular disease worldwide.[1] In China, IgAN accounts for 33.19% of total renal biopsy diagnoses and 45.26% of primary glomerular diseases.[2] Approximately 10% of IgAN patients demonstrate an accelerated loss of renal function and 30–40% slowly progress to renal failure;[3] however, proteinuria control can improve the prognoses for IgAN.[4] Pathogenesis and progression of IgAN are related to many factors, including heredity, inflammation, renin-angiotensin system activity, extracellular matrix metabolism, and abnormal blood coagulation.[5,6] An effective delay or blockade of IgAN progression may require multiple treatments. Despite a better understanding of the pathogenic mechanisms of IgAN, no targeted treatment is available.
Several classes of drugs have been shown to be efficacious in managing IgAN. Brown et al.[7] reported that prednisolone, azathioprine or cyclophosphamide, dipyridamole, and heparin followed by warfarin improve renal function in patients with rapidly progressive glomerulonephritis. Other studies have found that immunosuppressants combined with antiplatelet/anticoagulant drugs improve renal function in patients with IgAN and other renal diseases.[8,9,10] These are single-center studies with a small sample size, and results of these studies qualified for a lower level of evidence. Therefore, in a multicenter, prospective, double-dummy randomized clinical trial (RCT), we evaluated the efficacy and safety of the angiotensin receptor blocker (ARB) telmisartan in combination with the antiplatelet drug clopidogrel, the immunosuppressant leflunomide, or both drugs for managing IgAN. The study was implemented before the Kidney Disease: Improving Global Outcomes (KDIGO) 2012 guidelines[11] were issued.
METHODS
Patients
Patients were recruited across 13 renal centers in Beijing, China. Inclusion criteria were age 18–55 years, biopsy confirmed (within the past year) IgAN of Lee's grade II–IV, proteinuria of 0.5–3.5 g/d, serum creatinine <265 μmol/L, and blood pressure between 90/60 and 130/80 mmHg with or without antihypertensive treatments. Exclusion criteria were IgAN secondary to other diseases; previous adverse reaction to telmisartan, clopidogrel, or leflunomide; diabetes mellitus; pregnancy or unreliable contraception; and use of corticosteroids or other immunosuppressive agents (including leflunomide) in the preceding 3 months. Patients were recruited through workplace flyers and posters. Individuals who met all eligibility criteria were asked to complete a written informed consent document.
Study design
Participants were recruited from July 2010 to September 2011, and the trial follow-up was completed in June 2012. Patients, providers, and researchers were blinded to treatment allocation. The trial was registered at the Chinese Clinical Trial Registry (No. ChiCTR-TRC-10000776). Ethical approval was obtained from the People's Liberation Army General Hospital Clinical Research Ethics Committee (No. 20100127-002). Each participating center also obtained the local ethical approval. All patients gave written informed consent. There were no changes to the methods after trial commencement. The study was conducted in conformity with the Declaration of Helsinki.
Randomization and interventions
Before recruitment, a computer-generated randomization list was produced by a staff member at the Peking University Clinical Research Institute (Beijing, China) who was not otherwise involved in the trial. The randomization sequence was created using SAS statistical software 13.0 (SAS Institute, Cary, NC, USA) with a 1:1:1:1 allocation using a block size of eight, with two participants assigned to each of the four arms. Corresponding supervision measures were taken, and a detailed blind coding was recorded and covertly preserved in the coordinating center. Each study center was randomly stratified according to the enrollment order. Patients who met the eligibility criteria underwent a 4-week wash-in period with 80 mg/d of telmisartan. Baseline data were recorded after the wash-in period. Morphological indices were assessed by light microscopy according to the scoring system of Katafuchi et al.[12] Patients were randomly assigned to Groups A (telmisartan [80 mg/d] + clopidogrel placebo + leflunomide placebo), B (telmisartan [80 mg/d] + clopidogrel [50 mg/d] + leflunomide placebo), and C (telmisartan [80 mg/d] + clopidogrel placebo + leflunomide [20 mg/d]), and 99 were assigned to Group D (telmisartan [80 mg/d] + clopidogrel [50 mg/d] + leflunomide [20 mg/d]). The treatment period was 24 weeks. Leflunomide, clopidogrel, and corresponding placebos were produced by their respective manufacturers in prepacked bottles that were consecutively numbered for each patient according to the randomization schedule. The shape, color, and smell of the placebos were the same as the “true” medicine. The use of conventional antihypertensive drugs, including calcium channel blockers (amlodipine or felodipine), α- and β-receptor antagonists, and thiazide or loop diuretics, was allowed with the aim of achieving a target blood pressure of 130/80 mmHg. The use of steroids, other renin-angiotensin system inhibitors, immunosuppressants, antiplatelet agents, and anticoagulants was excluded during the study period. Patients were followed up to the end of 24-week treatment period.
Outcome measures
The primary outcome was a change in the 24-h urinary protein excretion at 24 weeks. The secondary outcomes included changes in the serum creatinine and estimated glomerular filtration rate (eGFR) as calculated by the chronic kidney disease (CKD) epidemiology collaboration equation which is adjusted for Asian populations,[13] serum uric acid, hematuria, and blood pressure at 24 weeks. These indices were measured at 4, 12, and 24 weeks. To avoid possible drift among different centers, critical laboratory examinations, such as urine protein and serum creatinine, were all conducted at Chinese People's Liberation Army General Hospital. Urinary protein concentration was measured using the biuret method (Siemens; ADVIA 2400 Biochemical Analyzer, Germany), and 24-h urinary protein excretion was calculated based on concentration and 24-h urine volume. Sarcosine oxidase was used to assay serum creatinine (Roche Cobas 8000 Biochemical Analyzer, Switzerland).
Safety evaluation
Patients whose proteinuria increased to >3.5 g/d, serum creatinine increased to >50% of the previous value or >265 μmol/L, serum potassium exceeded 5.5 mmol/L, or blood pressure dropped below 90/60 mmHg were dropped from the trial. Adverse events such as leukopenia, abnormal liver function, and high potassium were also assessed. Clinicians collected adverse events and assessed severity and potential causality at each visit. The chief investigator categorized the adverse events rated the severity and potential causality of adverse events and decided whether to classify adverse events as open-ended according to the checklist of possible adverse events in the protocol.
Sample size
SAS version 9.2 (GLMPOWER, SAS Institute, Cary, NC, USA) was used to determine the study sample size required to detect changes in urinary protein excretion at 24 weeks with a power of 90% at a significance level of 5%. Based on our previous study, we assumed a mean urinary protein excretion of 1 g/24 h and mean (standard deviation [SD]) decreases in urinary protein excretion of 0.20 ± 0.70, 0.40 ± 0.70, 0.40 ± 0.70, and 0.60 ± 0.70 g/24 h for Group A (telmisartan alone), Group B (telmisartan + clopidogrel), Group C (telmisartan + leflunomide), and Group D (telmisartan + clopidogrel + leflunomide), respectively. Analysis of variance showed that 89 patients per group (total 356 patients) would be required. Allowing for an estimated dropout rate of 10%, we concluded that 400 patients should be recruited.
Statistical analysis
Data were checked manually and double-entered; any obvious error was corrected in the primary records. Categorical variables are presented as number (%) and normally and nonnormally distributed continuous variables as mean (SD) and median (interquartile range), respectively. All patients who completed any of the outcome measures at 4, 12, or 24 weeks were included in the analysis. A linear mixed-effect model with the missing data was used to analyze the changes in proteinuria, serum creatinine, eGFR, serum uric acid, systolic and diastolic blood pressure, and weight at 4,12, and 24 weeks. Generalized estimating equations were used to evaluate changes in hematuria grade. Categorical variables are compared by Chi-square test. Over the 24-week period, compliance, defined as the ratio of the actual amount of medication taken by the patient to the amount provided by the investigator, was assessed at each visit. Good compliance was defined as a ratio ranging from 0.8 to 1.2. Bad compliance was defined as the ratio <0.8 or >1.2. Corresponding 95% confidence interval (CI) was calculated. All statistical analyses were performed using SPSS 16.0 software (SPSS, Chicago, IL, USA) and SAS version 9.1 (SAS Institute, Cary, NC, USA).
RESULTS
Patient characteristics
We screened 472 patients between July 2010 and September 2011. After a 4-week wash-in period, 72 patients were excluded from the study and 400 patients were randomly assigned (1:1:1:1) to groups using a random number table. After randomization, one patient was deemed ineligible based on a serum creatinine level of 407 µmol/L after the wash-in period. This patient did not receive the allocated intervention and was not included in the primary analysis. Of the remaining 399 patients, 100 each were assigned to Groups A (telmisartan 80 mg/d + clopidogrel placebo + leflunomide placebo), B (telmisartan 80 mg/d + clopidogrel 50 mg/d + leflunomide placebo), and C (telmisartan80 mg/d + clopidogrel placebo + leflunomide 20 mg/d), and 99 were assigned to Group D (telmisartan 80 mg/d + clopidogrel 50 mg/d + leflunomide 20 mg/d). Ultimately, 360 patients completed the 24-week intervention [Figure 1]. Baseline measurements were similar among the treatment groups [Table 1]. The number of patients receiving concomitant therapy with the calcium channel blockers, α- and β-receptor antagonists, and diuretics was similar among the four groups.
Figure 1.
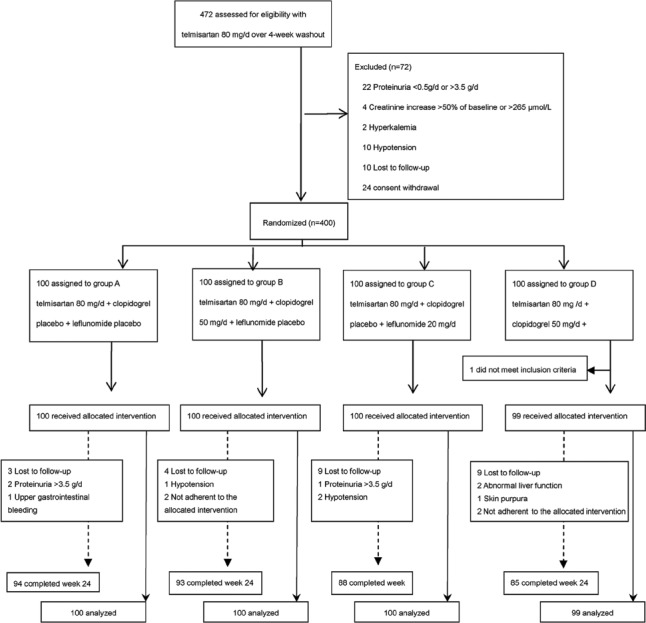
Study flow diagram.
Table 1.
Baseline characteristics of the study population*
| Characteristics | Group A† (n = 100) | Group B† (n = 100) | Group C† (n = 100) | Group D†,‡ (n = 99) | P |
|---|---|---|---|---|---|
| Sex (male/female) | 54/46 | 61/39 | 54/46 | 62/37 | 0.466 |
| Age (years)§ | 39.01 (9.78) | 36.52 (9.59) | 38.12 (10.62) | 37.06 (10.46) | 0.309 |
| BMI (kg/m2)§ | 24.48 (3.87) | 24.06 (3.04) | 24.99 (3.48) | 24.58 (3.71) | 0.331 |
| Weight (kg)§ | 69.27 (13.74) | 68.15 (11.27) | 70.77 (14.44) | 70.16 (13.77) | 0.536 |
| Systolic blood pressure (mmHg)|| | 120.00 (110.00–123.50) | 120.00 (110.00–120.00) | 120.00 (110.00–124.00) | 120.00 (110.00–122.00) | 0.637 |
| Diastolic blood pressure (mmHg)|| | 75.00 (70.00–80.00) | 75.50 (70.00–80.00) | 78.00 (70.00–80.00) | 80.00 (70.00–80.00) | 0.615 |
| Proteinuria (g/d)|| | 1.01 (0.66–1.48) | 0.99 (0.68–1.26) | 1.04 (0.63–1.50) | 1.05 (0.69–1.52) | 0.974 |
| Hematuria | |||||
| 0/1+/2+/3+ | 13/48/26/13 | 19/38/32/11 | 18/36/25/21 | 15/49/29/6 | 0.097 |
| Serum creatinine (µmol/L)§ | 88.18 (34.34) | 89.03 (29.32) | 90.37 (28.44) | 96.61 (37.95) | 0.258 |
| Serum uric acid (µmol/L)§ | 361.49 (92.09) | 367.64 (92.00) | 387.46 (100.09) | 385.53 (99.23) | 0.151 |
| Albumin (g/L)§ | 43.56 (3.77) | 44.17 (3.20) | 44.61 (4.01) | 44.22 (3.64) | 0.253 |
| Blood potassium (mmol/L)§ | 4.30 (0.40) | 4.38 (0.40) | 4.31 (0.38) | 4.28 (0.42) | 0.325 |
| eGFR-EPI (ml∙min−1∙1.73 m−2)§ | 95.16 (29.02) | 96.03 (28.00) | 92.06 (26.39) | 91.87 (31.33) | 0.650 |
| Morphologic indices (n)¶ | |||||
| Glomerular lesions§ | 4.27 (1.71) | 4.54 (1.78) | 4.74 (1.75) | 4.78 (1.57) | 0.155 |
| Tubulointerstitial lesions§ | 3.57 (1.91) | 3.49 (1.74) | 3.65 (1.76) | 3.56 (1.77) | 0.957 |
| Arterial lesions§ | 1.32 (1.64) | 1.17 (1.77) | 0.94 (1.12) | 1.06 (1.56) | 0.433 |
| Sum§ | 9.06 (4.10) | 9.39 (3.96) | 9.61 (3.59) | 9.61 (3.90) | 0.827 |
*Baseline characteristics were measured at the start of the study treatment in each group after a 4-week wash-in period; †Group A: Telmisartan 80 mg/d + placebo, Group B: Telmisartan 80 mg/d + 50 mg/d clopidogrel, Group C: Telmisartan 80 mg/d + 20 mg/d leflunomide, Group D: Telmisartan 80 mg/d + 50 mg/d clopidogrel + 20 mg/d leflunomide; ‡One patient did not receive the allocated intervention and was not included in the primary analysis; §Normally distributed continuous variables are presented as mean (SD); ||Nonnormally distributed continuous variables are presented as median (IQR); ¶Morphologic indices of patients in the present study were assessed by light microscopy according to the scoring system of Katafuchi et al. BMI: Body mass index; eGFR-EPI: Estimated glomerular filtration rate as calculated by the Chronic Kidney Disease Epidemiology Collaboration equation adjusted for Asian populations; IQR: Interquartile range; SD: Standard deviation.
Efficacy analysis
Treatment crossovers did not occur during the trial. Two patients in Groups B and D had bad compliance. Compliance was not statistically different among the four treatment groups. Estimated differences in the changes in proteinuria, serum creatinine, eGFR, serum uric acid, and systolic and diastolic blood pressure between no leflunomide and leflunomide groups (linear mixed-effects model) during the 24-week treatment period are presented in Table 2 and Figure 2. Changes in proteinuria (0.36 [95% CI 0.18–0.55] g/d, F = 8.44, P = 0.004), serum uric acid (76.96 [95% CI 57.44–96.49] µmol/L, F = 61.07, P < 0.001), serum creatinine (9.49 [95% CI 6.54–12.44] µmol/L, F = 32.89, P < 0.001), and eGFR (−6.72 [95% CI −9.46 to −3.98] ml∙min−1∙1.73 m−2, F = 23.35, P < 0.001) were statistically different in telmisartan combined with leflunomide, whereas changes in systolic and diastolic blood pressure and weight were not statistically different.
Table 2.
Estimated differences in the changes in proteinuria, serum creatinine, estimated glomerular filtration rate, serum uric acid, and systolic and diastolic blood pressure between no leflunomide and leflunomide groups during the 24-week treatment period (linear mixed-effects model)
| Characteristic | No leflunomide | Leflunomide | Differences | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | 95% CI | n | Mean | 95% CI | Mean | 95% CI | F | P | |
| Changes in proteinuria from baseline (g/24 h) (weeks) | ||||||||||
| 4 | 200 | −0.14 | −0.23 to −0.05 | 199 | −0.18 | −0.27 to −0.09 | 0.36 | 0.18 to 0.55 | 8.44 | 0.004 |
| 12 | 197 | −0.06 | −0.17 to 0.05 | 193 | −0.18 | −0.29 to −0.07 | ||||
| 24 | 189 | −0.17 | −0.27 to −0.08 | 176 | −0.59 | −0.69 to −0.49 | ||||
| Changes in serum creatinine from baseline (μmol/L) (weeks) | ||||||||||
| 4 | 200 | 2.22 | 0.90 to 3.54 | 199 | −0.25 | −1.57 to 1.07 | 9.49 | 6.54 to 12.44 | 32.89 | <0.001 |
| 12 | 197 | 4.19 | 2.52 to 5.86 | 193 | −1.95 | −3.63 to −0.26 | ||||
| 24 | 189 | 4.30 | 2.62 to 5.98 | 176 | −2.90 | −4.62 to −1.17 | ||||
| Changes in eGFR from baseline (ml·min−1·1.73 m−2) (weeks) | ||||||||||
| 4 | 200 | −1.83 | −3.07 to −0.60 | 199 | −0.34 | −1.58 to 0.90 | −6.72 | −9.46 to −3.98 | 23.35 | <0.001 |
| 12 | 197 | −3.64 | −5.18 to −2.10 | 193 | 1.57 | 0.01 to 3.12 | ||||
| 24 | 189 | −3.47 | −5.04 to −1.90 | 176 | 1.90 | 0.29 to 3.52 | ||||
| Changes in serum uric acid from baseline (μmol/L) (weeks) | ||||||||||
| 4 | 197 | −21.41 | −39.05 to −3.77 | 197 | −28.01 | −45.65 to −10.38 | 76.96 | 57.44 to 96.49 | 61.07 | <0.001 |
| 12 | 194 | 7.85 | −2.15 to 17.85 | 191 | −72.75 | −82.82 to −62.67 | ||||
| 24 | 186 | 8.12 | −2.38 to 18.61 | 175 | −68.96 | −79.68 to −58.24 | ||||
| Changes in systolic blood pressure from baseline (mmHg) (weeks) | ||||||||||
| 4 | 200 | 0.44 | −0.63 to 1.52 | 199 | −0.84 | −1.92 to 0.24 | −0.07 | −2.99 to 2.86 | 0.84 | 0.361 |
| 12 | 196 | −0.17 | −1.47 to 1.12 | 189 | −0.53 | −1.84 to 0.79 | ||||
| 24 | 186 | 0.17 | −1.28 to 1.62 | 171 | −0.30 | −1.79 to 1.19 | ||||
| Changes in diastolic blood pressure from baseline (mmHg) (weeks) | ||||||||||
| 4 | 200 | −0.78 | −1.61 to 0.05 | 199 | −0.14 | −0.97 to 0.69 | −0.30 | −2.34 to 1.74 | 0.48 | 0.489 |
| 12 | 196 | −1.15 | −2.13 to −0.18 | 189 | −0.42 | −1.41 to 0.56 | ||||
| 24 | 186 | −0.24 | −1.25 to 0.77 | 171 | −0.46 | −1.50 to 0.58 | ||||
CI: Confidence interval; eGFR: Estimated glomerular filtration rate.
Figure 2.
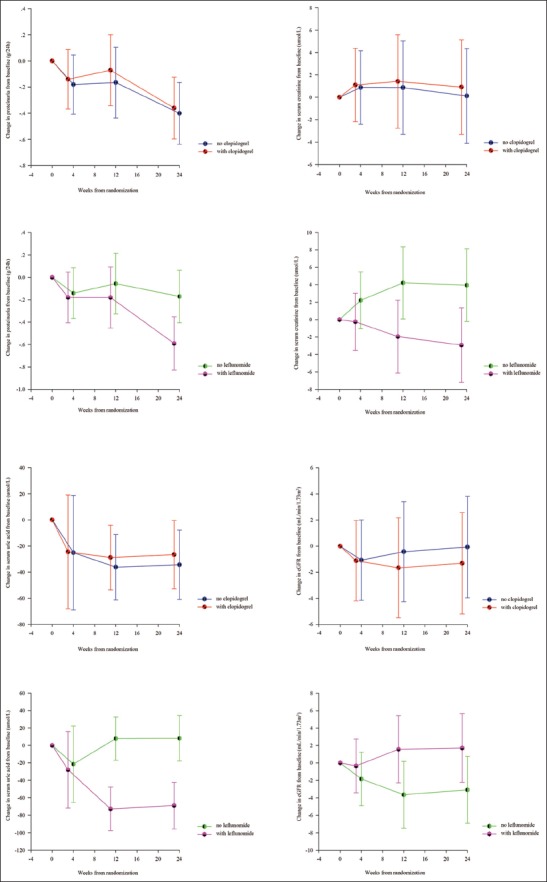
Estimated mean change curves for proteinuria, serum uric acid, serum creatinine, and estimated glomerular filtration rate according to treatment group (linear mixed-effect model). Error bars represent 95% confidence intervals.
Estimated differences in the changes in proteinuria, serum creatinine, eGFR, serum uric acid, and systolic and diastolic blood pressure between no clopidogrel and clopidogrel groups (linear mixed-effects model) during the 24-week treatment period are presented in Table 3 and Figure 2. Changes in continuous outcomes were not statistically different in telmisartan combined with clopidogrel. The effects of clopidogrel by leflunomide interaction on changes in continuous outcomes were not clinically relevant. Clopidogrel and leflunomide were not associated with changes in hematuria grade.
Table 3.
Estimated differences in the changes in proteinuria, serum creatinine, estimated glomerular filtration rate, serum uric acid, and systolic and diastolic blood pressure between no clopidogrel and clopidogrel groups during the 24-week treatment period (linear mixed-effects model)
| Characteristic | No clopidogrel | Clopidogrel | Differences | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | 95% CI | n | Mean | 95% CI | Mean | 95% CI | F | P | |
| Changes in proteinuria from baseline (g/24 h) (weeks) | ||||||||||
| 4 | 200 | −0.18 | −0.27 to −0.09 | 199 | −0.14 | −0.23 to −0.05 | −0.07 | −0.26 to 0.12 | 0.77 | 0.380 |
| 12 | 195 | −0.17 | −0.27 to −0.06 | 195 | −0.07 | −0.18 to 0.04 | ||||
| 24 | 182 | −0.40 | −0.50 to −0.31 | 183 | −0.36 | −0.46 to −0.26 | ||||
| Changes in serum creatinine from baseline (μmol/L) (weeks) | ||||||||||
| 4 | 200 | 0.87 | −0.45 to 2.19 | 199 | 1.09 | −0.23 to 2.42 | 3.09 | −0.35 to 6.53 | 0.13 | 0.716 |
| 12 | 195 | 0.85 | −0.82 to 2.53 | 195 | 1.39 | −0.29 to 3.07 | ||||
| 24 | 182 | 0.58 | −1.13 to 2.28 | 183 | 0.83 | −0.88 to 2.53 | ||||
| Changes in eGFR from baseline (ml·min−1·1.73 m−2) (weeks) | ||||||||||
| 4 | 200 | −1.06 | −2.30 to 0.18 | 199 | −1.11 | −2.35 to 0.13 | −1.19 | −4.42 to 2.03 | 0.81 | 0.368 |
| 12 | 195 | −0.41 | −1.96 to 1.13 | 195 | −1.65 | −3.20 to −0.11 | ||||
| 24 | 182 | −0.30 | −1.90 to 1.29 | 183 | −1.27 | −2.86 to 0.33 | ||||
| Changes in serum uric acid from baseline (μmol/L) (weeks) | ||||||||||
| 4 | 196 | −24.99 | −42.67 to −7.30 | 198 | −24.44 | −42.03 to −6.85 | −5.99 | −27.33 to 15.36 | 0.54 | 0.461 |
| 12 | 191 | −36.11 | −46.20 to −26.03 | 194 | −28.78 | −38.78 to −18.79 | ||||
| 24 | 178 | −34.24 | −44.90 to −23.57 | 183 | −26.61 | −37.15 to −16.07 | ||||
| Changes in systolic blood pressure from baseline (mmHg) (weeks) | ||||||||||
| 4 | 200 | −0.21 | −1.28 to 0.87 | 199 | −0.19 | −1.27 to 0.89 | −0.88 | −3.85 to 2.09 | 0.05 | 0.829 |
| 12 | 193 | −0.42 | −1.72 to 0.88 | 192 | −0.28 | −1.58 to 1.02 | ||||
| 24 | 177 | −0.24 | −1.71 to 1.23 | 180 | 0.11 | −1.36 to 1.57 | ||||
| Changes in diastolic blood pressure from baseline (mmHg) (weeks) | ||||||||||
| 4 | 200 | −0.19 | −1.02 to 0.64 | 199 | −0.73 | −1.56 to 0.10 | 1.07 | −1.00 to 3.15 | 2.93 | 0.090 |
| 12 | 193 | −0.44 | −1.41 to 0.54 | 192 | −1.14 | −2.13 to −0.16 | ||||
| 24 | 177 | 0.45 | −0.58 to 1.47 | 180 | −1.14 | −2.17 to −0.12 | ||||
CI: Confidence interval; eGFR: Estimated glomerular filtration rate.
Safety analysis
Adverse events according to treatment group are shown in Table 4. Two patients in Group A and one patient in Group C were diagnosed with nephrotic syndrome during routine visits at 30 days, 84 days, and 96 days, respectively. One patient in Group B and two patients in Group C demonstrated hypotension (<90/60 mmHg) at 30 days, 30 days, and 84 days, respectively. Two patients in Group D withdrew from therapy because of abnormal liver function at 114 and 129 days. One patient in Group A was diagnosed with upper gastrointestinal bleeding 23 days after enrollment. One patient in Group D was diagnosed with skin purpura at 129 days. No serious adverse events occurred.
Table 4.
Adverse events according to each treatment group, n
| Adverse events | Group A* (n = 100) | Group B* (n = 100) | Group C* (n = 100) | Group D*,† (n = 99) | P |
|---|---|---|---|---|---|
| Death | 0 | 0 | 0 | 0 | 1.000 |
| Patient withdrew due to adverse events | 1 | 1 | 2 | 3 | 0.663 |
| Any adverse event | 4 | 7 | 4 | 9 | 0.353 |
| Any serious adverse event‡ | 0 | 0 | 0 | 0 | 1.000 |
| Abnormal liver function | 0 | 3 | 1 | 3 | 0.267 |
| Hypotension | 0 | 1 | 2 | 0 | 0.298 |
| Hyperkalemia | 1 | 2 | 1 | 0 | 0.571 |
| Neutropenia | 1 | 0 | 0 | 2 | 0.292 |
| Rash | 0 | 0 | 0 | 2 | 0.107 |
| Skin purpura | 0 | 0 | 0 | 1 | 0.386 |
| Upper gastrointestinal bleeding | 0 | 0 | 0 | 1 | 0.386 |
| Herpes zoster | 1 | 0 | 0 | 0 | 0.392 |
| Urinary tract infection | 0 | 1 | 0 | 0 | 0.392 |
| Upper respiratory tract infection | 1 | 0 | 0 | 0 | 0.392 |
*Group A: Telmisartan 80 mg/d + placebo, Group B: Telmisartan 80 mg/d + 50 mg/d clopidogrel, Group C: Telmisartan 80 mg/d + 20 mg/d leflunomide, Group D: Telmisartan 80 mg/d + 50 mg/d clopidogrel + 20 mg/d leflunomide; †One patient did not receive the allocated intervention and was not included in the primary analysis; ‡Severe adverse events refer to adverse events that cause initial or prolonged hospitalization, handicaps, employment handicaps, congenital malformation, life-threatening health events, or death.
DISCUSSION
This 24-week RCT of 399 IgAN patients indicates that telmisartan combined with leflunomide is effective for reducing proteinuria, serum creatinine, and uric acid and is effective for increasing eGFR. However, telmisartan combined with clopidogrel turns out to be ineffective.
IgAN is the most common CKD, which has an overall prevalence of 10.8% in China.[14] Effective treatment of IgAN is critical for reducing the incidence of end-stage renal disease in China.[15] Many studies have identified severe proteinuria as a risk factor associated with deterioration to end-stage renal disease.[16,17,18,19]
Although many clinical studies have shown that angiotensin-converting enzyme inhibitor/angiotensin II type 1 receptor blockers (ACEI/ARBs) therapy can effectively reduce the proteinuria and protect renal function in IgAN patients, many individuals with clinical proteinuria cannot achieve effective control with ACEI/ARB therapy alone, and some patients with hypotension cannot be tolerated ACEI/ARB therapy. KDIGO suggests the use of corticosteroid therapy in patients who are unable to achieve a proteinuria reduction of <1.0 g/d after 3–6 months of ACEI/ARB treatment. The use of immunosuppressive agents for IgAN treatment remains controversial based on English-language MEDLINE search (until May 2016). A multicenter RCT of 207 IgAN patients in Italy and Switzerland found no statistical difference between prednisone and prednisone plus low-dose azathioprine in decreasing the proteinuria or improving renal function.[20] A new study also showed that the addition of immunosuppressive therapy to intensive supportive care in patients with high-risk IgAN did not significantly improve the full clinical remission, and during the 3-year study phase, more adverse effects were observed among the patients who received immunosuppressive therapy, with no change in the rate of decrease in the eGFR.[21] Conversely, a small-sample British RCT reported that low-dose cyclophosphamide followed by azathioprine was associated with a much higher survival (72%) compared with the control treatment (6%).[22] Both of the two Chinese RCT found that cyclosporine A in combination with prednisone remarkably reduced the levels of proteinuria and ameliorated the renal function in the IgAN.[23,24] A Japanese RCT found that the rate of proteinuria disappearance in IgAN patients receiving a combination of prednisone, azathioprine, warfarin, and dipyridamole (92%) was much better than that in patients receiving only hormone therapy (74%).[25] Previous investigations have revealed that mycophenolate mofetil effectively decreases proteinuria in IgAN patients;[26,27] however, another study reported no such effect.[28] The study findings are currently too heterogeneous to support the use of mycophenolate mofetil for IgAN.[29] Leflunomide is effective for treating systemic lupus erythematosus and lupus nephritis.[30,31] Its effectiveness in the treatment of nephrotic syndrome[32] and IgAN[33,34] has also been reported with small samples. Our findings confirm the reported effectiveness of leflunomide in reducing the proteinuria in IgAN patients. Proteinuria reduction is not a goal by itself but a surrogate endpoint for placing the disease in remission or slowing the progression to end-stage renal disease. The immunomodulating effects of leflunomide may be related to its selective inhibition of de novo pyrimidine synthesis. Leflunomide inhibits dihydroorotate dehydrogenase and thereby blocks the de novo pathway of pyrimidine nucleotide synthesis, which is crucial for T-cell activation and proliferation. Leflunomide is also an inhibitor of protein tyrosine kinases which play a fundamental role in the intracellular signal transduction triggered by cytokines.[35] These immunomodulatory properties may confer a beneficial effect against IgAN because inflammation and circulating immune complexes play an important role in the onset and progression of IgAN. At low therapeutically applicable doses, the active metabolite of leflunomide (A771726) reversibly inhibits dihydroorotate dehydrogenase, which is the rate-limiting enzyme in de novo pyrimidine synthesis. Evidence suggests that the observed anti-inflammatory effects of A771726 may be related to its ability to suppress interleukin-1, and the tumor necrosis factor downregulates the glycosylation of adhesion molecules while reducing the T lymphocyte/monocyte contact activation during inflammation.[36] We also found that the serum creatinine and uric acid levels statistically decreased in IgAN patients taking leflunomide, whereas the eGFR was statistically increased. A previous study also showed that leflunomide reduced the uric acid levels in rheumatoid arthritis patients.[37] A meta-analysis demonstrated a positive association between the serum uric acid levels and risk of CKD.[38] Furthermore, uric acid-lowering therapy with allopurinol may hinder CKD progression.[39] Therefore, the leflunomide-associated reduction in uric acid may have contributed to the improvements in IgAN in our study.
Blood coagulation and platelet activation are also involved in IgAN progression.[40] Some RCTs have demonstrated that anticoagulant/antiplatelet therapy reduces urinary protein in IgAN patients.[40,41,42] We found that the ARB irbesartan combined with clopidogrel had renal protective effects in CKD animal models.[11] In the present study, clopidogrel did not reduce the proteinuria or protect renal function in IgAN patients.
This study has some limitations. First, a 24-week randomized controlled study is not sufficient for evaluating the long-term efficacy of leflunomide for managing IgAN. Second, since this study began before the KDIGO 2012 guidelines were issued, our treatment protocol conflicted with these guidelines. The KDIGO guidelines do not support the use of antiplatelet or immunosuppressive agents, except in cases of crescentic IgAN with rapid deterioration. Third, a wash-in period of 4 weeks was too short and a fixed dose of telmisartan was used rather than up-titration dosage. Lastly, considering the reported discrepancies in the effects of immunosuppressive agents (specifically mycophenolate mofetil) between Chinese and European IgAN patients, our study findings only apply to Chinese patients until they can be confirmed in subjects of other ancestries.
In conclusion, this multicenter, prospective, double-dummy RCT confirms the efficacy and safety of telmisartan combined with leflunomide in reducing proteinuria of IgAN patients. However, telmisartan combined with clopidogrel turns out to be ineffective. This results may provide an option for IgAN patients in whom telmisartan alone is insufficient for lowering proteinuria. However, the long-term efficacy and safety of telmisartan combined with leflunomide in IgAN patients remain to be determined.
Financial support and sponsorship
This work is supported by the grants from National Key Technology Research and Development Program (No. 2011BAI10B00); from National High Technology Research and Development Program of China (863 Program No. 2012AA02A512); two grants from National Clinical Research Center for Kidney Disease (No. 2013BAI09B05 and No. 2015BAI12B06), and from the Science and Technology Project of Beijing, China (No. D09050704310904, No. D131100004713003).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Li-Min Chen
REFERENCES
- 1.D’Amico G. The commonest glomerulonephritis in the world: IgA nephropathy. Q J Med. 1987;64:709–27. [PubMed] [Google Scholar]
- 2.Li LS, Liu ZH. Epidemiologic data of renal diseases from a single unit in China: Analysis based on 13,519 renal biopsies. Kidney Int. 2004;66:920–3. doi: 10.1111/j.1523-1755.2004.00837.x. doi: 10.1111/j.1523-1755.2004.00837.x. [DOI] [PubMed] [Google Scholar]
- 3.Donadio JV, Grande JP. IgA nephropathy. N Engl J Med. 2002;347:738–48. doi: 10.1056/NEJMra020109. doi: 10.1056/NEJMra.020109. [DOI] [PubMed] [Google Scholar]
- 4.Reich HN, Troyanov S, Scholey JW, Cattran DC Toronto Glomerulonephritis Registry. Remission of proteinuria improves prognosis in IgA nephropathy. J Am Soc Nephrol. 2007;18:3177–83. doi: 10.1681/ASN.2007050526. doi: 10.1681/ASN.2007050526. [DOI] [PubMed] [Google Scholar]
- 5.Mestecky J, Raska M, Julian BA, Gharavi AG, Renfrow MB, Moldoveanu Z, et al. IgA nephropathy: Molecular mechanisms of the disease. Annu Rev Pathol. 2013;8:217–40. doi: 10.1146/annurev-pathol-011110-130216. doi: 10.1146/annurev-pathol-011110-130216. [DOI] [PubMed] [Google Scholar]
- 6.Floege J. The pathogenesis of IgA nephropathy: What is new and how does it change therapeutic approaches? Am J Kidney Dis. 2011;58:992, 1004. doi: 10.1053/j.ajkd.2011.05.033. doi: 10.1053/j.ajkd.2011.05.033. [DOI] [PubMed] [Google Scholar]
- 7.Brown CB, Wilson D, Turner D, Cameron JS, Ogg CS, Chantler C, et al. Combined immunosuppression and anticoagulation in rapidly progressive glomerulonephritis. Lancet. 1974;2:1166–72. doi: 10.1016/s0140-6736(74)90810-1. [DOI] [PubMed] [Google Scholar]
- 8.Frascà GM, Martello M, Sestigiani E, Canova C, Vangelista A, Bonomini V. Effects of defibrotide treatment in patients with IgA nephropathy and reduced renal function. Nephrol Dial Transplant. 1996;11:392–3. doi: 10.1093/oxfordjournals.ndt.a027280. [DOI] [PubMed] [Google Scholar]
- 9.Savage JM, Postlethwaite RJ, Lendon M, Houston IB, Evans DI. Combined immunosuppressive and anticoagulant treatment in children with glomerulonephritis and declining renal function. Int J Pediatr Nephrol. 1982;3:167–73. [PubMed] [Google Scholar]
- 10.Robson AM, Cole BR, Kienstra RA, Kissane JM, Alkjaersig N, Fletcher AP. Severe glomerulonephritis complicated by coagulopathy: Treatment with anticoaguland and immunosuppresive drugs. J Pediatr. 1977;90:881–92. doi: 10.1016/s0022-3476(77)80554-4. [DOI] [PubMed] [Google Scholar]
- 11.Radhakrishnan J, Cattran DC. The KDIGO practice guideline on glomerulonephritis: Reading between the (guide) lines –Application to the individual patient. Kidney Int. 2012;82:840–56. doi: 10.1038/ki.2012.280. doi: 10.1038/ki.2012.280. [DOI] [PubMed] [Google Scholar]
- 12.Katafuchi R, Kiyoshi Y, Oh Y, Uesugi N, Ikeda K, Yanase T, et al. Glomerular score as a prognosticator in IgA nephropathy: Its usefulness and limitation. Clin Nephrol. 1998;49:1–8. [PubMed] [Google Scholar]
- 13.Stevens LA, Claybon MA, Schmid CH, Chen J, Horio M, Imai E, et al. Evaluation of the chronic kidney disease epidemiology collaboration equation for estimating the glomerular filtration rate in multiple ethnicities. Kidney Int. 2011;79:555–62. doi: 10.1038/ki.2010.462. doi: 10.1038/ki.2010.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang L, Wang F, Wang L, Wang W, Liu B, Liu J, et al. Prevalence of chronic kidney disease in China: A cross-sectional survey. Lancet. 2012;379:815–22. doi: 10.1016/S0140-6736(12)60033-6. doi: 10.1016/S0140-6736(12)60033-6. [DOI] [PubMed] [Google Scholar]
- 15.Cai GY, Chen XM. Immunoglobulin A nephropathy in China: Progress and challenges. Am J Nephrol. 2009;30:268–73. doi: 10.1159/000225563. doi: 10.1159/000225563. [DOI] [PubMed] [Google Scholar]
- 16.Moriyama T, Tanaka K, Iwasaki C, Oshima Y, Ochi A, Kataoka H, et al. Prognosis in IgA nephropathy: 30-year analysis of 1,012 patients at a single center in Japan. PLoS One. 2014;9:e91756. doi: 10.1371/journal.pone.0091756. doi: 10.1371/journal.pone.0091756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Komatsu H, Fujimoto S, Hara S, Fukuda A, Fukudome K, Yamada K, et al. Recent therapeutic strategies improve renal outcome in patients with IgA nephropathy. Am J Nephrol. 2009;30:19–25. doi: 10.1159/000197116. doi: 10.1159/000197116. [DOI] [PubMed] [Google Scholar]
- 18.Le W, Liang S, Hu Y, Deng K, Bao H, Zeng C, et al. Long-term renal survival and related risk factors in patients with IgA nephropathy: Results from a cohort of 1155 cases in a Chinese adult population. Nephrol Dial Transplant. 2012;27:1479–85. doi: 10.1093/ndt/gfr527. doi: 10.1093/ndt/gfr527. [DOI] [PubMed] [Google Scholar]
- 19.Alamartine E, Sabatier JC, Guerin C, Berliet JM, Berthoux F. Prognostic factors in mesangial IgA glomerulonephritis: An extensive study with univariate and multivariate analyses. Am J Kidney Dis. 1991;18:12–9. doi: 10.1016/s0272-6386(12)80284-8. [DOI] [PubMed] [Google Scholar]
- 20.Pozzi C, Andrulli S, Pani A, Scaini P, Del Vecchio L, Fogazzi G, et al. Addition of azathioprine to corticosteroids does not benefit patients with IgA nephropathy. J Am Soc Nephrol. 2010;21:1783–90. doi: 10.1681/ASN.2010010117. doi: 10.1681/ASN.2010010117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rauen T, Eitner F, Fitzner C, Sommerer C, Zeier M, Otte B, et al. Intensive supportive care plus immunosuppression in IgA nephropathy. N Engl J Med. 2015;373:2225–36. doi: 10.1056/NEJMoa1415463. doi: 10.1056/NEJMoa1415463. [DOI] [PubMed] [Google Scholar]
- 22.Ballardie FW, Roberts IS. Controlled prospective trial of prednisolone and cytotoxics in progressive IgA nephropathy. J Am Soc Nephrol. 2002;13:142–8. doi: 10.1681/ASN.V131142. [DOI] [PubMed] [Google Scholar]
- 23.Xu L, Liu ZC, Guan GJ, Lv XA, Luo Q. Cyclosporine A combined with medium/low dose prednisone in progressive IgA nephropathy. Kaohsiung J Med Sci. 2014;30:390–5. doi: 10.1016/j.kjms.2014.04.002. doi: 10.1016/j.kjms.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu H, Xu X, Fang Y, Ji J, Zhang X, Yuan M, et al. Comparison of glucocorticoids alone and combined with cyclosporine a in patients with IgA nephropathy: A prospective randomized controlled trial. Intern Med. 2014;53:675–81. doi: 10.2169/internalmedicine.53.1136. doi: 10.2169/internalmedicine.53.1136. [DOI] [PubMed] [Google Scholar]
- 25.Yoshikawa N, Honda M, Iijima K, Awazu M, Hattori S, Nakanishi K, et al. Steroid treatment for severe childhood IgA nephropathy: A randomized, controlled trial. Clin J Am Soc Nephrol. 2006;1:511–7. doi: 10.2215/CJN.01120905. doi: 10.2215/CJN.01120905. [DOI] [PubMed] [Google Scholar]
- 26.Tang SC, Tang AW, Wong SS, Leung JC, Ho YW, Lai KN. Long-term study of mycophenolate mofetil treatment in IgA nephropathy. Kidney Int. 2010;77:543–9. doi: 10.1038/ki.2009.499. doi: 10.1038/ki.2009.499. [DOI] [PubMed] [Google Scholar]
- 27.Tang S, Leung JC, Chan LY, Lui YH, Tang CS, Kan CH, et al. Mycophenolate mofetil alleviates persistent proteinuria in IgA nephropathy. Kidney Int. 2005;68:802–12. doi: 10.1111/j.1523-1755.2005.00460.x. doi: 10.1111/j.1523-1755.2005.00460.x. [DOI] [PubMed] [Google Scholar]
- 28.Frisch G, Lin J, Rosenstock J, Markowitz G, D’Agati V, Radhakrishnan J, et al. Mycophenolate mofetil (MMF) vs placebo in patients with moderately advanced IgA nephropathy: A double-blind randomized controlled trial. Nephrol Dial Transplant. 2005;20:2139–45. doi: 10.1093/ndt/gfh974. doi: 10.1093/ndt/gfh974. [DOI] [PubMed] [Google Scholar]
- 29.Wyatt RJ, Julian BA. IgA nephropathy. N Engl J Med. 2013;368:2402–14. doi: 10.1056/NEJMra1206793. doi: 10.1056/NEJMra1206793. [DOI] [PubMed] [Google Scholar]
- 30.Zhang FS, Nie YK, Jin XM, Yu HM, Li YN, Sun Y. The efficacy and safety of leflunomide therapy in lupus nephritis by repeat kidney biopsy. Rheumatol Int. 2009;29:1331–5. doi: 10.1007/s00296-009-0861-3. doi: 10.1007/s00296-009-0861-3. [DOI] [PubMed] [Google Scholar]
- 31.Remer CF, Weisman MH, Wallace DJ. Benefits of leflunomide in systemic lupus erythematosus: A pilot observational study. Lupus. 2001;10:480–3. doi: 10.1191/096120301678416033. doi: 10.1191/096120301678416033. [DOI] [PubMed] [Google Scholar]
- 32.Yang S, Xie L, Xue W, Yin A, Lu W. Leflunomide plus oral prednisone in treatment of idiopathic membranous nephropathy: A retrospective clinical study of efficacy and safety. Nephrology (Carlton) 2013;18:615–22. doi: 10.1111/nep.12143. doi: 10.1111/nep.12143. [DOI] [PubMed] [Google Scholar]
- 33.Lou T, Wang C, Chen Z, Shi C, Tang H, Liu X, et al. Randomised controlled trial of leflunomide in the treatment of immunoglobulin A nephropathy. Nephrology (Carlton) 2006;11:113–6. doi: 10.1111/j.1440-1797.2006.00547.x. doi: 10.1111/j.1440-1797.2006.00547.x. [DOI] [PubMed] [Google Scholar]
- 34.Cheng G, Liu D, Margetts P, Liu L, Zhao Z, Liu Z, et al. Valsartan combined with clopidogrel and/or leflunomide for the treatment of progressive immunoglobulin A nephropathy. Nephrology (Carlton) 2015;20:77–84. doi: 10.1111/nep.12359. doi: 10.1111/nep.12359. [DOI] [PubMed] [Google Scholar]
- 35.Teschner S, Burst V. Leflunomide: A drug with a potential beyond rheumatology. Immunotherapy. 2010;2:637–50. doi: 10.2217/imt.10.52. doi: 10.2217/imt.10.52. [DOI] [PubMed] [Google Scholar]
- 36.Breedveld FC, Dayer JM. Leflunomide: Mode of action in the treatment of rheumatoid arthritis. Ann Rheum Dis. 2000;59:841–9. doi: 10.1136/ard.59.11.841. doi: 10.1136/ard.59.11.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perez-Ruiz F, Nolla JM. Influence of leflunomide on renal handling of urate and phosphate in patients with rheumatoid arthritis. J Clin Rheumatol. 2003;9:215–8. doi: 10.1097/01.rhu.0000081470.31167.8b. doi: 10.1097/01.rhu.0000081470.31167.8b. [DOI] [PubMed] [Google Scholar]
- 38.Zhu P, Liu Y, Han L, Xu G, Ran JM. Serum uric acid is associated with incident chronic kidney disease in middle-aged populations: A meta-analysis of 15 cohort studies. PLoS One. 2014;9:e100801. doi: 10.1371/journal.pone.0100801. doi: 10.1371/journal.pone.0100801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Feig DI. Serum uric acid and the risk of hypertension and chronic kidney disease. Curr Opin Rheumatol. 2014;26:176–85. doi: 10.1097/BOR.0000000000000033. doi: 10.1097/BOR.0000000000000033. [DOI] [PubMed] [Google Scholar]
- 40.Liu XJ, Geng YQ, Xin SN, Huang GM, Tu XW, Ding ZR, et al. Antithrombotic drug therapy for IgA nephropathy: A meta analysis of randomized controlled trials. Intern Med. 2011;50:2503–10. doi: 10.2169/internalmedicine.50.5971. doi: 10.2169/internalmedicine.50.5971. [DOI] [PubMed] [Google Scholar]
- 41.Taji Y, Kuwahara T, Shikata S, Morimoto T. Meta-analysis of antiplatelet therapy for IgA nephropathy. Clin Exp Nephrol. 2006;10:268–73. doi: 10.1007/s10157-006-0433-8. doi: 10.1007/s10157-006-0433-8. [DOI] [PubMed] [Google Scholar]
- 42.Chen X, Qiu Q, Tang L, Liu S, Cai G, Liu H, et al. Effects of co-administration of urokinase and benazepril on severe IgA nephropathy. Nephrol Dial Transplant. 2004;19:852–7. doi: 10.1093/ndt/gfh069. doi: 10.1093/ndt/gfh069. [DOI] [PubMed] [Google Scholar]


