Abstract
Objective
To evaluate the association of uric acid (UA) levels with a panel of markers of oxidative stress and inflammation.
Methods
Plasma UA levels, along with a panel of oxidative stress and inflammatory markers, were measured in 755 Chinese women.
Results
Plasma UA levels were inversely associated with urinary levels of the oxidative stress marker F2-isoprostanes and positively correlated to levels of inflammatory markers such as C-reactive protein and some proinflammatory cytokines (tumor necrosis factor-α and interleukin-6) in blood as well as prostaglandin E2 metabolites in urine.
Conclusions
Plasma UA levels correlate to oxidation and inflammation biomarkers in opposite directions in women.
Keywords: uric acid, inflammation, oxidative stress, biomarker
Introduction
In humans, oxidative stress is thought to be involved in the development of many diseases such as cancer (Halliwell, 2007), neurodegenerative disease (Valko, 2007), and cardiovascular disease (Ramond, 2011; Singh, 1995). Uric acid (UA), the end product of enzymatic degradation of xanthine in humans, is a potent endogenous antioxidant that effectively scavenges superoxide ions, singlet oxygen, hydroxyl radicals, and peroxynitrites and chelates transitional metal ions (Ames, 1981; Squadrito, 2000). For this reason, it has been hypothesized that higher UA may be beneficial to human aging (Ames, 1981). Higher circulating UA levels have been associated with a lower risk of some neurodegenerative diseases such as Parkinson’s disease (Chen, 2009; de Lau, 2005) and better clinical outcomes in patients with ischemic stroke (Chamorro, 2002), although results are not entirely consistent (Weir, 2003). On the other hand, it has been shown that UA is notoriously involved in chronic inflammation. UA can stimulate the release of interleukin-1β (IL-1β) and IL-6 and the synthesis of tumor necrosis factor (TNF)-α in animal models (Johnson, 2005), and upregulate C-reactive protein (CRP) expression in cultured human vascular cells (Kanellis, 2005; Kono, 2010). Higher UA levels have been associated with greater risk of many inflammatory diseases, including cardiovascular disease and diabetes (Culleton, 1999; Dehghan, 2008; Fang, 2000). Therefore, it appears that UA correlates to oxidation and inflammation in opposite directions. To our knowledge, there have been very few studies that have directly evaluated the association of UA with both inflammation and oxidative stress markers in humans (Dohi, 2007).
F2-isoprostanes (F2-IsoPs), products of free-radical-catalyzed peroxidation of arachidonic acid, have been accepted as an accurate and reliable biomarker of in vivo oxidative stress (Milne, 2007b; Roberts, 2000). F2-IsoPs are stable compounds and their levels are not affected by the lipid content of the diet. The main advantage of measurement of F2-IsoPs in urine is that the compounds are not formed ex vivo because urine does not contain high lipid contents (Roberts, 2000). Currently, mass spectrometric-based methods are considered to be the gold standard for F2-IsoPs quantification because of their high sensitivity and specificity (Milne, 2007b; Roberts, 2000). Several small observational studies have evaluated the association between UA and F2-IsoPs, yielding mixed results with an inverse association in one study (Polidori, 2004) and a null association in another (Hershfield, 2010). In this study, we evaluated the association of UA levels with a panel of markers of oxidative stress and inflammation in 755 middle-aged women.
Methods
Study participants
In this cross-sectional analysis, 755 women were originally selected for a nested case-control study of colorectal cancer from the Shanghai Women’s Health Study (SWHS). The SWHS is an ongoing prospective cohort study in Shanghai, China. Both selected cases and controls were cancer-free at baseline. The design and methods of the SWHS have been described in details elsewhere (Shu, 2004; Zheng, 2005). Briefly, at the baseline survey conducted between 1997 and 2000, 74 941 women aged 40–70 years from 7 typical urban communities of Shanghai were recruited (participation rate: 92.7%). All women completed a detailed baseline survey that collected information on demographic characteristics, lifestyle and dietary habits, medical history, and other exposures. Habitual dietary intake over the preceding 12 months was collected during in-person interviews using a validated food-frequency questionnaire (Shu, 2004). Anthropometric measurements, including weight, height, and circumferences of the waist and hips, were also taken according to a standard protocol (Zhang, 2007). A detailed assessment of physical activity was obtained using a validated questionnaire (Matthews, 2003). The study was approved by the institutional review boards for human research at the Shanghai Cancer Institute in China and the National Cancer Institute and the Vanderbilt University in the United States (IRB: 081406). Written informed consent was obtained from all study participants.
At study enrollment, 76% of cohort members donated blood and urine samples; an additional 12% provided a urine sample at the first follow-up survey approximately 2 years later after the baseline survey (Zheng, 2005). After collection, samples were kept at 4 °C and aliquoted into cryovials for long-term storage within 6 hours. Immediately after processing, all samples were stored at −70 °C until laboratory analyses were conducted.
Biomarker measurement
Levels of UA in plasma were measured by using the ACE® Uric Acid Reagent on an ACE® Clinical Chemistry System (Alfa Wassermann, Inc, West Caldwell, NJ) following the manufacturer’s protocol at the Vanderbilt Lipids Laboratory, Vanderbilt University, Nashville, USA. Hyperuricemia was defined as UA > 7.0 mg/dL (Villegas, 2012).
Urinary levels of F2-IsoPs and their major metabolite 2,3-dinor-5,6-dihydro-15-F2t-IsoP (F2-IsoP-M) were measured by gas chromatography/negative ion chemical ionization mass spectrometry (GC/NICI MS) in the Eicosanoid Core Laboratory at Vanderbilt University. Details of this method have been described elsewhere (Milne, 2007a). Urinary levels of the major urinary metabolite of prostaglandin E2 (PGE-M) were measured using the liquid chromatography/tandem MS method by the same lab, as reported (Cai, 2006). Concentrations of F2-IsoPs, F2-IsoP-M and PGE-M were expressed as ng/mg creatinine. The lower limits of sensitivity were 5 pg for F2-IsoPs and F2-IsoP-M and 40 pg for PGE-M. The precision was ±6% and the accuracy was 96% for F2-IsoPs and F2-IsoP-M (Milne, 2007a). The coefficient of variation for samples analyzed in multiple batches was 7.2% for PGE-M (Cai, 2006).
Plasma levels of cytokines and their receptors were measured by using Millipore’s MILLIPLEX® MAP High Sensitivity Human Cytokine multiplex kit for IL-1β, TNF-α, and IL-6 and the MILLIPLEX® MAP Human Soluble Cytokine Receptor Panel multiplex kits for soluble IL-6 receptor (sIL-6R), soluble GP130 (sGP130, a regulator of IL-6/sIL-6R complex signaling), soluble TNF receptor 1 (sTNF-R1), and sTNF-R2. Assays were conducted in duplicate at the Vanderbilt Hormone Assay & Analytical Services Core. High-sensitivity CRP measurements were performed at the Vanderbilt Lipid Laboratory using the ACE® High Sensitivity C-Reactive Protein Reagent (ACI-22) for the first batch and the CRP (HS) Wide Range kit (Pointe Scientific, Canton, MI) for the other batches. Thus, we adjusted for batch in all analyses. Intra-assay coefficients of variation were 11.8% for TNF-α, 7.5% for sTNF-R1, 5.5% for sTNF-R2, 17.4% for IL 1β, 15.5% for IL-6, 3.6% for sGP130 and 3.8% for sIL-6R in this study; and inter-assay coefficients of variation were < 21%.
Statistical analysis
Log-transformation was conducted to normalize the distribution of biomarker data in parametric analyses. Correlations between UA and biomarkers were assessed by Pearson correlation, and correlations between UA and categorical lifestyle factors or health conditions were assessed by biserial correlation. Multivariable-adjusted geometric means of UA were obtained based on least square means estimated using a general linear model. We also used a restricted cubic spline linear regression analysis (Harrell, 2001) to evaluate the association between UA and selected biomarkers. Knots were placed at the 5th, 50th, and 95th percentiles of the distribution of UA levels. We also calculated odds ratio (OR) for hyperuricemia associated with certain chronic diseases using unconditional logistic regression models. Covariates adjusted for in multivariable models included age, education, occupation, physical activity, history of infectious or inflammation-related diseases (tuberculosis, gastritis, asthma, chronic hepatitis, cholelithiasis and chronic pancreatitis), cigarette smoking, alcohol consumption, regular use of aspirin or other non-steroidal anti-inflammatory drugs (NSAID), regular use of vitamin supplements, dietary intakes of total calories, fruits and vegetables, as well as assay batch. We further evaluated whether the association between UA levels and oxidative stress or inflammation markers differed by infectious or inflammatory diseases. All statistical tests were two-sided and were performed using SAS statistical software, version 9.3 (SAS Institute, Cary, NC).
Results
Study participants’ characteristics are presented in Table 1. The mean circulating level of UA was 5.2 ± 1.3 mg/dL. The prevalence of obesity (BMI ≥ 30 kg/m2, 6.8%), ever smoking (3.6%), ever drinking alcohol (2.9%), and ever using NSAIDs (3.6%) was very low. Approximately half of participants had a history of chronic disease, including hypertension (30.5%), diabetes (7.2%), and coronary heart disease (CHD, 10.1%). Women with higher levels of circulating UA were more likely to be old and obese and have a chronic disease such as diabetes, hypertension and CHD. We also found that UA levels were higher among regular users of NSAIDs and participants with higher education in a crude analysis. However, these differences disappeared after adjustment for age (r=0.04 for NSAID use [P=0.29] and r=0.001 for higher education [P=0.97]). Other low grade inflammation-related characteristics, such as physical activity, cigarette smoking, alcohol drinking, vitamin supplementation, or dietary antioxidant intake, were not associated with circulating levels of UA.
Table 1.
Correlation between selected factors and uric acid in 755 women
| Continuous variable | mean (SD) | Pearson r | P |
|---|---|---|---|
| Uric acid (mg/dL) | 5.2 (1.3) | - | - |
| Age (y) | 57.8 (9.1) | 0.338 | <0.0001 |
| Body mass index (kg/m2) | 24.7 (3.5) | 0.311 | <0.0001 |
| Physical activity (MET hours/week) | 109.3 (44.3) | −0.028 | 0.439 |
| Dietary intakes | |||
| Purine-rich food and nutrients (g/d) | |||
| Fish | 37.1 (36.8) | 0.023 | 0.52 |
| Shellfish | 17.8 (20.2) | 0.052 | 0.16 |
| Meat | 66.6 (48.3) | −0.051 | 0.17 |
| Protein | 66.6 (21.9) | 0.014 | 0.70 |
| Purine-rich vegetables* | 50.1 (34.7) | −0.006 | 0.88 |
| Dietary antioxidants (µg/d) | |||
| Vitamin A | 676.2 (369.0) | −0.043 | 0.24 |
| Vitamin C | 91.1 (50.4) | −0.012 | 0.74 |
| Vitamin E | 13.5 (5.9) | 0.026 | 0.47 |
| Selenium | 44.0 (22.6) | 0.002 | 0.95 |
| Categorical variable | % | Biserial r | P |
| Education, high school and above | 32.4 | −0.08 | 0.03 |
| Regularly smoked cigarettes | 3.6 | 0.06 | 0.11 |
| Regularly consumed alcohol | 2.9 | 0.03 | 0.41 |
| Regularly used NSAIDs | 3.6 | 0.08 | 0.03 |
| Regularly used antioxidant vitamin supplements |
17.2 | 0.05 | 0.14 |
| History of chronic disease† | 46.0 | 0.21 | <0.0001 |
| Diabetes | 7.2 | 0.14 | 0.0002 |
| Hypertension | 30.5 | 0.27 | <0.0001 |
| Coronary heart disease | 10.1 | 0.13 | 0.0002 |
Abbreviations: MET: metabolic equivalent; NSAID: non-steroidal anti-inflammatory drug.
Purine-rich vegetables: beans, peas, spinach, cauliflower, and mushrooms.
History of hypertension, diabetes and coronary heart disease.
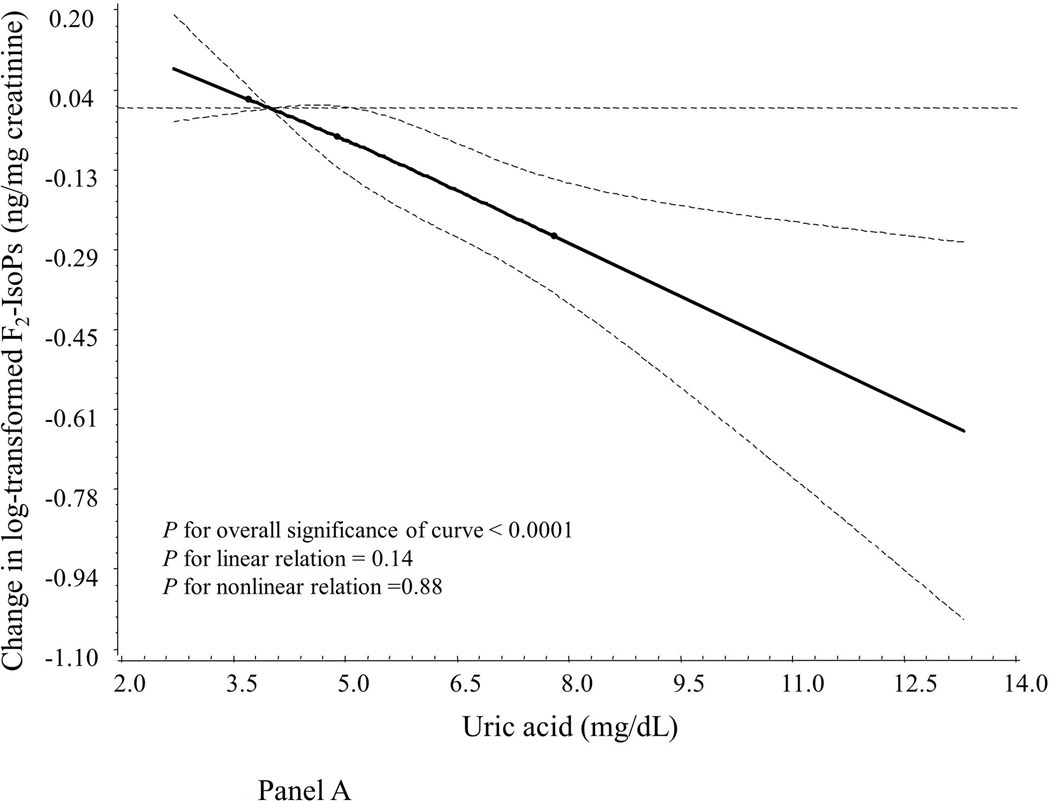
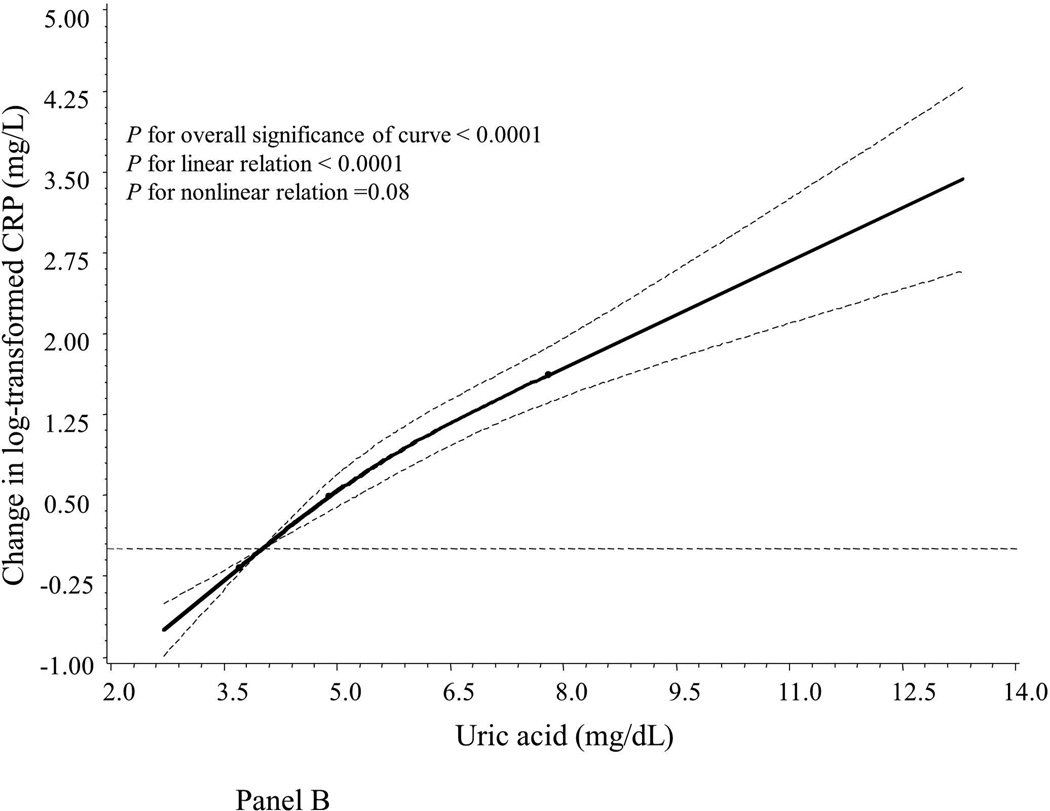
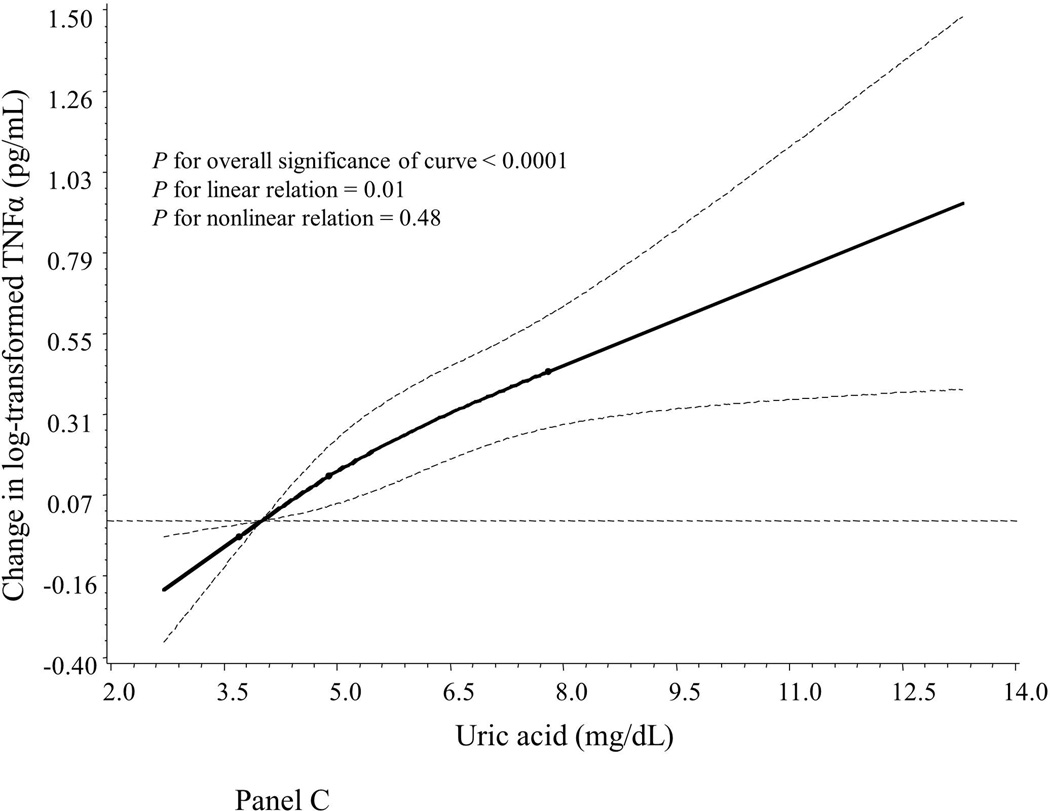
Compared to women with normal UA levels (UA ≤ 7.0 mg/dL), hyperuricemic women had significantly lower levels of the oxidative stress marker F2-IsoPs but higher levels of inflammatory markers CRP, IL-6, TNF-α and sTNF-R2 (Table 2). Similarly, we found that, after adjustment for age and assay batch (Table 3), UA concentrations were inversely correlated to levels of F2-IsoPs (Pearson r = −0.18, P < 0.0001) but positively correlated to levels of inflammatory markers CRP, IL-6, TNF-α, sTNF-R2, and PGE-M, with Pearson r’s ranging from 0.12 for PGE-M to 0.42 for CRP (all P < 0.05). UA levels were not correlated to F2-IsoP-M levels (r = 0.01, P = 0.75). We also used a restricted cubic spline linear regression model to account for possible non-linear effects. As illustrated graphically, associations between UA and F2-IsoPs, CRP and TNF-α appeared monotonic and approximately linear (Figure 1, Panels A, B and C). In addition, UA levels were positively correlated to age (Pearson r = 0.34) and BMI (r = 0.31) in multivariable models (both P < 0.05; data not shown in tables).
Table 2.
Geometric concentrations of biomarkers in non-hyperuricemic and hyperuricemic women
| Biomarkers | All participants, mean (SD) |
Without hyperuricemia (uric acid ≤ 7 mg/dL), mean (SD) |
With hyperuricemia (uric acid > 7 mg/dL), mean (SD) |
|---|---|---|---|
| Uric acid (mg/dL) | 5.03 (1.26) | 4.81 (1.20) | 8.11 (1.12)* |
| F2-IsoPs (ng/mg) | 1.48 (1.65) | 1.51 (1.64) | 1.21 (1.69)* |
| F2-IsoP-M (ng/mg) | 0.59 (1.64) | 0.59 (1.63) | 0.61 (1.77) |
| CRP (mg/L) | 1.03 (3.67) | 0.92 (3.59) | 3.46 (2.20)* |
| PGE-M (ng/mg) | 6.73 (2.37) | 6.60 (2.36) | 8.21 (2.42) |
| IL-6 (pg/mL) | 4.04 (4.26) | 3.89 (4.36) | 5.92 (3.10)* |
| sGP130 (pg/mL) | 169393.01 (1.88) | 167929.58 (1.93) | 184259.43 (1.37) |
| sIL-6R (pg/mL) | 19699.93 (2.1) | 19516.2 (2.15) | 21575.2 (1.61) |
| IL-1β (pg/mL) | 1.04 (5.83) | 1.05 (5.68) | 0.95 (7.55) |
| TNF-α (pg/mL) | 6.26 (2.16) | 6.08 (2.17) | 8.48 (1.96)* |
| sTNF-R1 (pg/mL) | 1110.34 (1.79) | 1102.68 (1.70) | 1187.14 (2.56) |
| sTNF-R2 (pg/mL) | 4292.87 (1.47) | 4228.49 (1.47) | 4969.13 (1.41)* |
P < 0.05, derived from t-test between individuals with and without hyperuricemia.
Abbreviations: CRP: C-reactive protein; F2-IsoPs: 15-F2t-isoprostanes; F2-IsoP-M: 2,3-dinor-5,6-dihydro-15-F2t-isoprostanes; IL-1β: interleukin 1β; IL-6: interleukin 6; PGE-M: prostaglandin E2 metabolite; sGP130: soluble GP130; sIL-6R: soluble IL-6 receptor; sTNF-R1: soluble tumor necrosis factor receptor 1; sTNF-R2: soluble tumor necrosis factor receptor 2; TNF-α: tumor necrosis factor α.
Table 3.
Age, batch-adjusted Pearson correlations between log-transformed concentrations of uric acid and markers of oxidative stress and inflammation
| Biomarkers | n | r | P |
|---|---|---|---|
| F2-IsoPs (ng/mg) | 657 | −0.18 | <0.0001 |
| F2-IsoP-M (ng/mg) | 630 | 0.01 | 0.75 |
| CRP (mg/L) | 755 | 0.42 | <0.0001 |
| PGE-M (ng/mg) | 394 | 0.12 | 0.02 |
| IL-6 (pg/mL) | 658 | 0.13 | <0.0001 |
| sGP130 (pg/mL) | 618 | 0.04 | 0.38 |
| sIL-6R (pg/mL) | 618 | 0.06 | 0.16 |
| IL-1β (pg/mL) | 658 | −0.002 | 0.96 |
| TNF-α (pg/mL) | 658 | 0.19 | <0.0001 |
| sTNF-R1 (pg/mL) | 618 | 0.06 | 0.17 |
| sTNF-R2 (pg/mL) | 618 | 0.14 | <0.0001 |
Abbreviations: CRP: C-reactive protein; F2-IsoPs: 15-F2t-isoprostanes; F2-IsoP-M: 2,3-dinor-5,6-dihydro-15-F2t-isoprostanes; IL-1β: interleukin 1β; IL-6: interleukin 6; PGE-M: prostaglandin E2 metabolite; sGP130: soluble GP130; sIL-6R: soluble IL-6 receptor; sTNF-R1: soluble tumor necrosis factor receptor 1; sTNF-R2: soluble tumor necrosis factor receptor 2; TNF-α: tumor necrosis factor α.
Figure 1.
Smoothed plot of logarithmically transformed concentrations of oxidative stress and inflammatory markers according to plasma UA levels. The value of the studied markers at the median level of the first quartile of UA was treated as the reference point. The difference in log-transformed concentrations of the markers by UA levels were estimated by restricted cubic-spline linear regression analyses with knots placed at the 5th (3.7 mg/dL), 50th (4.9 mg/dL), and 95th (7.8 mg/dL) percentiles of UA levels after adjustment for age and assay batch. Point estimates are indicated by a solid line and 95% CIs by dashed lines. CRP: C-reactive protein; F2-IsoPs: 15-F2t-isoprostanes; TNF-α: tumor necrosis factor α.
Table 4 presents the relationship between plasma UA levels and prevalent medical conditions. After adjustment for age and assay batch, UA levels were significantly higher among participants with a history of diabetes, hypertension, or CHD (all P < 0.05). This association was attenuated after further adjusting for lifestyle factors, dietary and supplement antioxidants, NSAIDs, and other inflammatory diseases. We also calculated odds ratio for having hyperuricemia (UA > 7.0 mg/dL). Compared with non-diabetic individuals, patients with diabetes were nearly 2.4-fold more likely to have hyperuricemia (95% CI 1.09–5.31).
Table 4.
Associations between uric acid concentrations and health conditions
| Age, batch-adjusted model | Multivariable-adjusted model* | ||||||
|---|---|---|---|---|---|---|---|
| Health condition | n | Geometric mean |
Standard error |
P | Geometric mean |
Standard error |
P |
| Diabetes | |||||||
| No | 701 | 5.01 | 1.01 | 0.03 | 5.01 | 1.01 | 0.16 |
| Yes | 54 | 5.35 | 1.03 | 5.24 | 1.03 | ||
| Hypertension | |||||||
| No | 525 | 4.89 | 1.01 | <0.0001 | 4.93 | 1.01 | <0.001 |
| Yes | 230 | 5.36 | 1.01 | 5.27 | 1.01 | ||
| Coronary heart disease | |||||||
| No | 679 | 5.00 | 1.01 | 0.04 | 5.02 | 1.01 | 0.29 |
| Yes | 76 | 5.28 | 1.03 | 5.16 | 1.02 | ||
Adjusted for age, cigarette smoking, alcohol consumption, body mass index, education, physical activity, regular NSAID use, regular antioxidant vitamin use, dietary intakes of fish and shellfish, meat and antioxidant vitamins (A, C and E) and selenium, history of other infectious/inflammation-related diseases, and assay batch using linear regression models.
We further evaluated whether the association between UA levels and oxidative stress or inflammation differed by prevalent health conditions. No significant effect modification by the status of these health conditions was suggested (all P for interaction > 0.05). In addition, exclusion of participants with a history of renal disease (n = 4) did not markedly change the results.
Discussion
In this population-based sample of 755 Chinese women, we found that circulating UA levels were inversely correlated to levels of the oxidative stress marker F2-IsoPs and positively correlated to levels of inflammatory markers such as CRP, IL-6, TNF-α, sTNF-R2, and PGE-M. In addition, UA levels were positively associated with BMI, and a higher UA level was associated with a higher prevalence of hypertension and diabetes after adjustment for age and other potential confounding factors.
We were faced with the paradoxical fact that UA is a potent endogenous antioxidant and a risk factor for some chronic diseases such as CHD (Feig, 2008), in which oxidative stress plays an important pathophysiological role. UA can scavenge various free radicals (Ames, 1981; Squadrito, 2000). Some prospective cohort studies suggest that UA may be a protective factor against Parkinson’s disease (Chen, 2009; de Lau, 2005), a neurodegenerative disease where oxidative stress has been implicated. An inverse association between F2-IsoPs and UA levels was also suggested in a small study of patients with congestive heart failure (Polidori, 2004). Moreover, administration of UA to healthy individuals during acute high-intensity aerobic exercise has been found to reduce oxidative stress (Waring, 2003).
However, increased levels of UA may act as a pro-oxidant that activates a complex vicious cycle involving mechanisms related to inflammation and oxidative stress and negatively influences the health. Cumulative evidence from animal studies suggests that UA stimulates inflammatory responses, characterized by increased production of IL-1β, IL-6, and TNFα (Johnson, 2003) and upregulation of cyclooxygenase-2 activity and vascular CRP (Johnson, 2003; Watanabe, 2002). Higher levels of UA have been repeatedly observed in patients with inflammation-related diseases, such as metabolic syndrome, diabetes and CHD (Choi, 2005; Culleton, 1999; Dehghan, 2008). As observed in our study and two other studies (Frohlich, 2000; Ruggiero, 2006), UA levels were positively correlated to levels of inflammatory markers as well as a history of chronic inflammation-related diseases (Dorjgochoo, 2011a; Fang, 2000; Hershfield, 2010; Kodama, 2009; Sundstrom, 2005; Wu, 2013).
A potential dual effect of UA on neuronal tissues has been recently reported in a prospective follow-up study of ischemic stroke (Seet, 2010). The study found a U-shaped relationship between UA levels and clinical outcomes of stroke. Patients with low and high levels of UA were associated with poor functional outcomes, compared with those in the median level group. Kanellis et al suggested that whether UA is an anti- or pro-oxidant may depend on the cellular environment (Kanellis, 2005). In patients with ischemic stroke, serum urate is significantly lower in the acute phase and replacement of urate lowers levels of malondialdehyde, a marker of lipid peroxidation (Amaro, 2007). On the other hand, an in vivo experimental study recently found no significant changes in plasma F2-IsoP concentrations during treatment with pegloticase, a very potent urate-lowering therapy, among refractory gout patients (Hershfield, 2010). In the study, levels of UA before treatment in patients with refractory gout were positively associated with levels of plasma F2-IsoPs, which is in contrast to the finding of an inverse correlation between UA and urinary F2-IsoPs observed in our study conducted among apparently healthy individuals with a very low prevalence of hyperuricemia (8.6%). There is no clear explanation for the discrepancy. We speculate that UA-mediated oxidative stress and redox homeostasis in refractory gout patients with significantly elevated levels of UA may not reflect the impact of UA on oxidative stress in healthy individuals. A positive relationship between UA and oxidative stress in refractory gout patients suggests that the pro-oxidant effect of UA is predominant over the antioxidant effect. On the other hand, antioxidant effect of UA may be more evident among individuals with normal UA levels such as our study population. Further investigation is clearly needed to clarify the association between UA and oxidative stress in humans.
We did not observe an association between UA and urinary F2-IsoP-M in this study. F2-IsoP-M is a β-oxidation metabolite of F2-IsoPs, with a moderate correlation to the level of the parent compound in urine (Dorjgochoo, 2011b). It has been shown that the strength of the association with oxidative stress differs by different F2-IsoP measurements (Dorjgochoo, 2011b; Seet, 2011). Chronic cigarette smoking is associated with higher levels of F2-IsoPs but not the metabolite F2-IsoP-M in urine (Dorjgochoo, 2011b; Seet, 2011). However, it appears that urinary F2-IsoP-M is a more sensitive biomarker for obesity than its parent compound F2-IsoPs (Dorjgochoo, 2011b; Wu, 2013). Therefore, it is reasonable to take advantage of multiple rather than single F2-IsoP markers when studying diverse health conditions or disease mechanisms.
This study has several notable strengths. To our knowledge, this is the largest study to date to examine the association of UA with both oxidative stress and inflammation in a population-based sample. Concentrations of F2-IsoPs were measured by a GC/NICI MS-based assay. Several methodological limitations of this study should be also considered. The study was cross-sectional in design; thus, causal relationships cannot be inferred. We could not completely rule out the possibility of residual confounding due to unmeasured or inadequately measured covariates. For example, we did not consider gout treatments in the analysis because of relevant information unavailable in the study. Another concern is that biomarkers were measured in a single sample. We evaluated the intra-person variation of F2-IsoPs and PGE-M measurements in a validation study (Wu, 2010). Four spot urine samples were collected in each season over a 1-year period. The intraclass correlation coefficients for F2-IsoPs, F2-IsoP-M and PGE-M were 0.69, 0.76 and 0.67, respectively, similar to that for serum cholesterol levels that is generally accepted as being measured reasonably well by a single blood sample. Moreover, although lipid peroxidation products F2-isoprostanes have been accepted as an accurate and reliable biomarker of in vivo oxidative stress (Milne, 2007b; Roberts, 2000), inclusion of other oxidative stress parameters such as markers of DNA and protein oxidative damage may provide additional information on the association of UA and in vivo oxidation in future studies.
Conclusions
This study found that circulating UA levels are inversely correlated to oxidative stress and positively correlated to inflammatory markers, BMI and prevalence of hypertension and diabetes in women. Because of the potential dual effect of UA as an anti and pro-oxidant, both low and high UA levels could be associated with various comorbidities (Alvarez-Lario, 2011). Further investigations are certainly warranted to fully characterize the dual effect of UA and understand its health effects.
Acknowledgments
We are grateful to the participants and research staff of the Shanghai Women’s Health Study for their contributions to the study. We also thank Regina Courtney and Rodica Cal-Chris for sample preparation. The plasma and urine sample preparation was performed at the Survey and Biospecimen Shared Resource, which is supported in part by the Vanderbilt-Ingram Cancer Center (P30 CA68485).
This study was, in part, supported by USPHS grants and contracts from the National Institutes of Health, including R01CA122364 (to GY), R37CA070867 (to WZ), R01HL095931 (to XLZ), and N02 CP1101066 (to XOS).
Abbreviations
- BMI
body mass index
- CHD
Coronary heart disease
- CI
confidence interval
- CRP
C-reactive protein
- F2-IsoPs
15-F2t-isoprostanes
- F2-IsoP-M
2,3-dinor-5,6-dihydro-15-F2t-isoprostanes
- GC/NICI MS
gas chromatography/negative ion chemical ionization mass spectrometry
- MET
metabolic equivalent values
- NSAID
non-steroidal anti-inflammatory drug
- NHANES III
National Health and Nutrition Examination Survey
- PGE-M
prostaglandin E2 metabolite
- SD
standard deviation
- sGP130
soluble GP130
- sIL-6R
soluble IL-6 receptor
- sTNF-R1
soluble tumor necrosis factor receptor 1
- sTNF-R2
soluble tumor necrosis factor receptor 2
- SWHS
Shanghai Women’s Health Study
- TNF-α
tumor necrosis factor-α
- UA
Uric acid
Footnotes
Declaration of interest
The authors report no declarations of interest.
References
- Alvarez-Lario B, Macarron-Vicente J. Is there anything good in uric acid? QJM. 2011;104(12):1015–1024. doi: 10.1093/qjmed/hcr159. [DOI] [PubMed] [Google Scholar]
- Amaro S, Soy D, Obach V, Cervera A, Planas AM, Chamorro A. A pilot study of dual treatment with recombinant tissue plasminogen activator and uric acid in acute ischemic stroke. Stroke; a journal of cerebral circulation. 2007;38(7):2173–2175. doi: 10.1161/STROKEAHA.106.480699. [DOI] [PubMed] [Google Scholar]
- Ames BN, Cathcart R, Schwiers E, Hochstein P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc. Natl. Acad. Sci. U. S. A. 1981;78(11):6858–6862. doi: 10.1073/pnas.78.11.6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Q, Gao YT, Chow WH, Shu XO, Yang G, Ji BT, Wen W, Rothman N, Li HL, Morrow JD, Zheng W. Prospective study of urinary prostaglandin E2 metabolite and colorectal cancer risk. J Clin. Oncol. 2006;24(31):5010–5016. doi: 10.1200/JCO.2006.06.4931. [DOI] [PubMed] [Google Scholar]
- Chamorro A, Obach V, Cervera A, Revilla M, Deulofeu R, Aponte JH. Prognostic significance of uric acid serum concentration in patients with acute ischemic stroke. Stroke; a Journal of Cerebral Circulation. 2002;33(4):1048–1052. doi: 10.1161/hs0402.105927. [DOI] [PubMed] [Google Scholar]
- Chen H, Mosley TH, Alonso A, Huang X. Plasma urate and Parkinson's disease in the Atherosclerosis Risk in Communities (ARIC) study. Am. J Epidemiol. 2009;169(9):1064–1069. doi: 10.1093/aje/kwp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi HK, Mount DB, Reginato AM. Pathogenesis of gout. Annals of Internal Medicine. 2005;143(7):499–516. doi: 10.7326/0003-4819-143-7-200510040-00009. [DOI] [PubMed] [Google Scholar]
- Culleton BF, Larson MG, Kannel WB, Levy D. Serum uric acid and risk for cardiovascular disease and death: the Framingham Heart Study. Annals of Internal Medicine. 1999;131(1):7–13. doi: 10.7326/0003-4819-131-1-199907060-00003. [DOI] [PubMed] [Google Scholar]
- de Lau LM, Koudstaal PJ, Hofman A, Breteler MM. Serum uric acid levels and the risk of Parkinson disease. Annals of Neurology. 2005;58(5):797–800. doi: 10.1002/ana.20663. [DOI] [PubMed] [Google Scholar]
- Dehghan A, van HM, Sijbrands EJ, Hofman A, Witteman JC. High serum uric acid as a novel risk factor for type 2 diabetes. Diabetes care. 2008;31(2):361–362. doi: 10.2337/dc07-1276. [DOI] [PubMed] [Google Scholar]
- Dohi Y, Takase H, Sato K, Ueda R. Association among C-reactive protein, oxidative stress, and traditional risk factors in healthy Japanese subjects. Int J Cardiol. 2007;115(1):63–66. doi: 10.1016/j.ijcard.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Dorjgochoo T, Gao YT, Chow WH, Shu XO, Yang G, Cai Q, Rothman N, Cai H, Li H, Deng X, Shrubsole MJ, Murff H, Milne G, Zheng W, Dai Q. Obesity, age, and oxidative stress in middle-aged and older women. Antioxid. Redox. Signal. 2011a;14(12):2453–2460. doi: 10.1089/ars.2010.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorjgochoo T, Gao YT, Chow WH, Shu XO, Yang G, Cai Q, Rothman N, Cai H, Li H, Deng X, Shrubsole MJ, Murff H, Milne G, Zheng W, Dai Q. Obesity, age, and oxidative stress in middle-aged and older women. Antioxid. Redox. Signal. 2011b;14(12):2453–2460. doi: 10.1089/ars.2010.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang J, Alderman MH. Serum uric acid and cardiovascular mortality the NHANES I epidemiologic follow-up study, 1971–1992. National Health and Nutrition Examination Survey. JAMA : the journal of the American Medical Association. 2000;283(18):2404–2410. doi: 10.1001/jama.283.18.2404. [DOI] [PubMed] [Google Scholar]
- Feig DI, Kang DH, Johnson RJ. Uric acid and cardiovascular risk. N. Engl. J Med. 2008;359(17):1811–1821. doi: 10.1056/NEJMra0800885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohlich M, Imhof A, Berg G, Hutchinson WL, Pepys MB, Boeing H, Muche R, Brenner H, Koenig W. Association between C-reactive protein and features of the metabolic syndrome: a population-based study. Diabetes care. 2000;23(12):1835–1839. doi: 10.2337/diacare.23.12.1835. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Oxidative stress and cancer: have we moved forward? Biochem. J. 2007;401(1):1–11. doi: 10.1042/BJ20061131. [DOI] [PubMed] [Google Scholar]
- Harrell FJ., Jr . Regression modeling strategies: with applications to linear models, logistic regression, survival analysis. New York, NY: Springer-Verlag; 2001. [Google Scholar]
- Hershfield MS, Roberts LJ, Ganson NJ, Kelly SJ, Santisteban I, Scarlett E, Jaggers D, Sundy JS. Treating gout with pegloticase, a PEGylated urate oxidase, provides insight into the importance of uric acid as an antioxidant in vivo. Proc. Natl. Acad. Sci. U. S. A. 2010;107(32):14351–14356. doi: 10.1073/pnas.1001072107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RJ, Kang DH, Feig D, Kivlighn S, Kanellis J, Watanabe S, Tuttle KR, Rodriguez-Iturbe B, Herrera-Acosta J, Mazzali M. Is there a pathogenetic role for uric acid in hypertension and cardiovascular and renal disease? Hypertension. 2003;41(6):1183–1190. doi: 10.1161/01.HYP.0000069700.62727.C5. [DOI] [PubMed] [Google Scholar]
- Johnson RJ, Rodriguez-Iturbe B, Kang DH, Feig DI, Herrera-Acosta J. A unifying pathway for essential hypertension. Am. J Hypertens. 2005;18(3):431–440. doi: 10.1016/j.amjhyper.2004.08.035. [DOI] [PubMed] [Google Scholar]
- Kanellis J, Kang DH. Uric acid as a mediator of endothelial dysfunction, inflammation, and vascular disease. Semin. Nephrol. 2005;25(1):39–42. doi: 10.1016/j.semnephrol.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Kodama S, Saito K, Yachi Y, Asumi M, Sugawara A, Totsuka K, Saito A, Sone H. Association between serum uric acid and development of type 2 diabetes. Diabetes care. 2009;32(9):1737–1742. doi: 10.2337/dc09-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono H, Chen CJ, Ontiveros F, Rock KL. Uric acid promotes an acute inflammatory response to sterile cell death in mice. J. Clin. Invest. 2010;120(6):1939–1949. doi: 10.1172/JCI40124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews CE, Shu XO, Yang G, Jin F, Ainsworth BE, Liu D, Gao YT, Zheng W. Reproducibility and validity of the Shanghai Women's Health Study physical activity questionnaire. Am. J Epidemiol. 2003;158(11):1114–1122. doi: 10.1093/aje/kwg255. [DOI] [PubMed] [Google Scholar]
- Milne GL, Sanchez SC, Musiek ES, Morrow JD. Quantification of F2-isoprostanes as a biomarker of oxidative stress. Nat. Protoc. 2007a;2(1):221–226. doi: 10.1038/nprot.2006.375. [DOI] [PubMed] [Google Scholar]
- Milne GL, Yin H, Brooks JD, Sanchez S, Jackson RL, Morrow JD. Quantification of F2-isoprostanes in biological fluids and tissues as a measure of oxidant stress. Methods in enzymology. 2007b;433:113–126. doi: 10.1016/S0076-6879(07)33006-1. [DOI] [PubMed] [Google Scholar]
- Polidori MC, Pratico D, Savino K, Rokach J, Stahl W, Mecocci P. Increased F2 isoprostane plasma levels in patients with congestive heart failure are correlated with antioxidant status and disease severity. J Card Fail. 2004;10(4):334–338. doi: 10.1016/j.cardfail.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Ramond A, Godin-Ribuot D, Ribuot C, Totoson P, Koritchneva I, Cachot S, Levy P, Joyeux-Faure M. Oxidative stress mediates cardiac infarction aggravation induced by intermittent hypoxia. Fundam. Clin. Pharmacol. 2011 doi: 10.1111/j.1472-8206.2011.01015.x. [DOI] [PubMed] [Google Scholar]
- Roberts LJ, Morrow JD. Measurement of F(2)-isoprostanes as an index of oxidative stress in vivo. Free Radic. Biol. Med. 2000;28(4):505–513. doi: 10.1016/s0891-5849(99)00264-6. [DOI] [PubMed] [Google Scholar]
- Ruggiero C, Cherubini A, Ble A, Bos AJ, Maggio M, Dixit VD, Lauretani F, Bandinelli S, Senin U, Ferrucci L. Uric acid and inflammatory markers. Eur. Heart J. 2006;27(10):1174–1181. doi: 10.1093/eurheartj/ehi879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seet RC, Kasiman K, Gruber J, Tang SY, Wong MC, Chang HM, Chan YH, Halliwell B, Chen CP. Is uric acid protective or deleterious in acute ischemic stroke? A prospective cohort study. Atherosclerosis. 2010;209(1):215–219. doi: 10.1016/j.atherosclerosis.2009.08.012. [DOI] [PubMed] [Google Scholar]
- Seet RC, Lee CY, Loke WM, Huang SH, Huang H, Looi WF, Chew ES, Quek AM, Lim EC, Halliwell B. Biomarkers of oxidative damage in cigarette smokers: which biomarkers might reflect acute versus chronic oxidative stress? Free Radic. Biol. Med. 2011;50(12):1787–1793. doi: 10.1016/j.freeradbiomed.2011.03.019. [DOI] [PubMed] [Google Scholar]
- Shu XO, Yang G, Jin F, Liu D, Kushi L, Wen W, Gao YT, Zheng W. Validity and reproducibility of the food frequency questionnaire used in the Shanghai Women's Health Study. Eur. J Clin. Nutr. 2004;58(1):17–23. doi: 10.1038/sj.ejcn.1601738. [DOI] [PubMed] [Google Scholar]
- Singh N, Dhalla AK, Seneviratne C, Singal PK. Oxidative stress and heart failure. Mol. Cell Biochem. 1995;147(1–2):77–81. doi: 10.1007/BF00944786. [DOI] [PubMed] [Google Scholar]
- Squadrito GL, Cueto R, Splenser AE, Valavanidis A, Zhang H, Uppu RM, Pryor WA. Reaction of uric acid with peroxynitrite and implications for the mechanism of neuroprotection by uric acid. Arch. Biochem. Biophys. 2000;376(2):333–337. doi: 10.1006/abbi.2000.1721. [DOI] [PubMed] [Google Scholar]
- Sundstrom J, Sullivan L, D'Agostino RB, Levy D, Kannel WB, Vasan RS. Relations of serum uric acid to longitudinal blood pressure tracking and hypertension incidence. Hypertension. 2005;45(1):28–33. doi: 10.1161/01.HYP.0000150784.92944.9a. [DOI] [PubMed] [Google Scholar]
- Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem. Cell Biol. 2007;39(1):44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Villegas R, Xiang YB, Elasy T, Xu WH, Cai H, Cai Q, Linton MF, Fazio S, Zheng W, Shu XO. Purine-rich foods, protein intake, and the prevalence of hyperuricemia: the Shanghai men's health study. Nutr. Metab Cardiovasc. Dis. 2012;22(5):409–416. doi: 10.1016/j.numecd.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waring WS, Convery A, Mishra V, Shenkin A, Webb DJ, Maxwell SR. Uric acid reduces exercise-induced oxidative stress in healthy adults. Clin. Sci (Lond) 2003;105(4):425–430. doi: 10.1042/CS20030149. [DOI] [PubMed] [Google Scholar]
- Watanabe S, Kang DH, Feng L, Nakagawa T, Kanellis J, Lan H, Mazzali M, Johnson RJ. Uric acid, hominoid evolution, and the pathogenesis of salt-sensitivity. Hypertension. 2002;40(3):355–360. doi: 10.1161/01.hyp.0000028589.66335.aa. [DOI] [PubMed] [Google Scholar]
- Weir CJ, Muir SW, Walters MR, Lees KR. Serum urate as an independent predictor of poor outcome and future vascular events after acute stroke. Stroke; a journal of cerebral circulation. 2003;34(8):1951–1956. doi: 10.1161/01.STR.0000081983.34771.D2. [DOI] [PubMed] [Google Scholar]
- Wu SH, Shu XO, Chow WH, Xiang YB, Zhang X, Cai Q, Li HL, Milne G, Wen W, Ji BT, Rothman N, Gao YT, Zheng W, Yang G. Adiposity and fat distribution in relation to inflammation and oxidative stress in a relatively lean population of Chinese women. Dis. Markers. 2013;34(4):279–293. doi: 10.3233/DMA-130969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Cai H, Xiang YB, Cai Q, Yang G, Liu D, Sanchez S, Zheng W, Milne G, Shu XO. Intra-person variation of urinary biomarkers of oxidative stress and inflammation. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2010;19(4):947–952. doi: 10.1158/1055-9965.EPI-10-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Shu XO, Yang G, Li H, Cai H, Gao YT, Zheng W. Abdominal adiposity and mortality in Chinese women. Archives of Internal Medicine. 2007;167(9):886–892. doi: 10.1001/archinte.167.9.886. [DOI] [PubMed] [Google Scholar]
- Zheng W, Chow WH, Yang G, Jin F, Rothman N, Blair A, Li HL, Wen WQ, Ji BT, Li Q, Shu XO, Gao YT. The Shanghai Women's Health Study: Rationale, study design, and baseline characteristics. American Journal of Epidemiology. 2005;162(11):1123–1131. doi: 10.1093/aje/kwi322. [DOI] [PubMed] [Google Scholar]