Abstract
The complement system (C) present in circulating blood is an effective mechanism of host defense, responsible for the killing of pathogens and the production of potent anaphylatoxins. Inhibitors of the C have been described in the saliva of hematophagous arthropods that are involved in the protection of digestive tissues against C-mediated damage. Here we describe albicin, a novel inhibitor of the alternative pathway of complement from the salivary glands of the malaria vector, Anopheles albimanus. The inhibitor was purified from salivary gland homogenates by reverse phase HPLC, and identified by mass spectrometry as a small (13.4 kDa) protein related to the gSG7 protein of An. gambiae and An. stephensi. Recombinant albicin was produced in Escherichia coli and found to potently inhibit lysis of rabbit erythrocytes in assays of the alternative pathway while having no inhibitory effect on the classical or lectin pathways. Albicin also inhibited the deposition of complement components on agarose-coated plates, although it could not remove previously bound components. Antisera produced against recombinant albicin recognized both the native and recombinant inhibitors and also blocked their activities in in vitro assays. Using surface plasmon resonance and enzymatic assays, we found that albicin binds and stabilizes the C3-convertase complex (C3bBb) formed on a properdin surface, and inhibits the convertase activity of a reconstituted C3bBb complex in solution. The data indicate that albicin specifically recognizes the activated form of the complex allowing more efficient inhibition by an inhibitor whose quantity is limited.
Introduction
The complement system (C) is a crucial mediator of innate immunity, composed of about 30 proteins soluble in plasma or bound to the cell surface. It is responsible for the recognition, opsonization and lysis of invasive pathogens and altered cells of the organism. It is also involved in the production of inflammatory anaphylatoxins that are involved in the recruitment and activation of different types of leukocytes, including B and T lymphocytes, therefore promoting the adaptative immune response (1, 2).
The C is activated via three pathways that converge to a common point, the activation of the C3 component (3). The classical pathway (CP) is activated when its recognition molecule C1q binds to IgG or IgM antibodies forming immune complexes. The lectin pathway (LP) is triggered by particular carbohydrates usually associated with pathogen surface recognized by the mannan-binding-lectin (MBL), or other pattern recognition molecules. The alternative pathway (AP) starts with spontaneous hydrolysis of the C3 component, generating C3-H2O. Factor B binds to C3-H2O and is activated by factor D, generating C3-H2O-Bb. This complex can activate more C3 molecules, producing C3b that can recognize and covalently bind to the surface of the pathogen. On the non-self surface, factor B binds to C3b and is activated by factor D, forming C3bBb, the C3-convertase of the AP that is responsible for activating more C3 molecules (3). The AP C3-convertase is an unstable enzymatic complex, and utilizes properdin as a stabilizer, increasing its efficiency substantially (4). The final consequence of C is the assembly of the membrane attack complex (MAC) on the cell surface, forming pores that lead to cell death, and activating signaling pathways that result in resistance to lysis, proliferation or apoptosis (3).
Hematophagous arthropods have to contend with vertebrate host hemostatic responses during blood feeding, such as coagulation and vasoconstriction, as well as immune responses, including the C. To counteract these processes, arthropods inject pharmacologicaly active salivary molecules into the host skin, that facilitate acquisition of a blood meal and pathogen transmission (5, 6). Salivary anti-complement proteins were first described by Valenzuela et al (7), who identified and expressed ISAC, a salivary 18.5 kDa protein from the hard tick Ixodes scapularis that inhibits the AP. Later, two families of anti-complement proteins related to ISAC were identified in I. ricinus salivary glands, the IRACs (8) and IxACs (9), and both were shown to inhibit only the AP. Nunn et al (10) characterized OmCI, a salivary protein from the soft tick Ornithodoros moubata, that inhibits both the CP and AP. Anti-complement activity has also been reported in salivary gland homogenates of phlebotomine sandflies (11, 12) and triatomine bugs (13), highlighting the importance of the inhibitors for hematophagous arthropods. By inhibiting the C, the saliva of such arthropods is able to block the production of potent anaphylatoxins, diminishing the inflammatory response at the bite site and favoring a successful blood meal by the vector (14). The inhibitors are also important in the protection of the midgut from C-mediated damage that could lead to the death of midgut cells (13).
In this paper, we describe albicin (Anopheles albimanus complement inhibitor), a novel anti-complement protein from the salivary glands of the mosquito, Anopheles albimanus, the most important malaria vector in Central America. From salivary gland extracts of this species we have purified a protein related to the gSG7 protein of An. gambiae and An. stephensi that inhibits the AP by binding and inactivating its C3-convertase enzymatic complex.
Materials and Methods
Mosquitoes and salivary gland homogenates (SGH)
The salivary glands of 4-to-8-day-old non-blood fed females of Anopheles albimanus, An. dirus, An. freeborni, An. gambiae and An. stephensi were dissected and stored in PBS. The glands were then sonicated and centrifuged at 10,000 × g at 4°C for 10 minutes and the supernatants were used in the assays. The protein concentration in the salivary gland of each mosquito species was measured using the BCA method.
Hemolytic assays
In AP-mediated hemolysis assays, rabbit red blood cells (CompTech) were washed three times by centrifuging at 600 × g for 5 minutes, followed by discarding the supernatant and ressuspending the cells in 1 ml of Mg-EGTA solution (1 mM HEPES, 30 mM NaCl, 10 mM EGTA, 7 mM MgCl2, 3% glucose, 0.02% gelatin, pH 7.4). After the last wash, the cell concentration was adjusted to 1 × 108 cells/ml in Mg-EGTA solution.
In microcentrifuge tubes, 12.5 μl of PBS containing the desired concentration of SGH or recombinant protein were mixed with 25 μl of normal human serum (NHS) (CompTech) diluted 1:20 in Mg-EGTA solution. The final serum concentration in the assay was 2%. In the experiment with properdin-depleted serum (CompTech) the serum was diluted 1:2 in Mg-EGTA solution. Twenty five microliters of red blood cells were added to the tubes and incubated at 37°C for 30 minutes for complement activation. In the experiment with properdin-depleted serum, the tubes were incubated for 40 minutes. After incubation, 250 μl of cold PBS were added to stop the reaction and after centrifugation, 200 μl of the supernatant were transfered to an ELISA plate and read at 415 nm. The final serum concentration in the analyzed samples was 0.4%. An. albimanus SGH heated in boiling water for 30 minutes or treated with 0.2 μg of proteinase K at 37°C for 3 hours was tested in the same manner.
In each test, three types of controls were used: a total hemolysis control (TH), which had no serum or inhibitor and was stopped with distilled water, a negative control which had no serum or inhibitor and was stopped with cold PBS, and a positive control, which had serum but no inhibitor. Separate assays were used to demonstrate that the absorbance of assay wells having erythrocytes but no serum were not significantly different that than those containing serum and an excess of EDTA to inhibit activation of the C (supplemental data). The assays were done in duplicate, and in every test the mean of negative control was subtracted of the mean of the other results. The results were then normalized based on the positive control. ANOVA and Tukey’s test were used to detect statistical differences among the results.
For the assays with classical pathway-mediated hemolysis, antibody-sensitized sheep red blood cells (CompTech) were used. The assay was perfomed as described for the AP, but using NHS diluted 1:60 and red blood cells in a concentration of 2 × 108 cells/ml, both diluted in GVB2+ solution (145 mM NaCl, 5mM Veronal, 0.15mM CaCl2, 0,5mM MgCl2, 0,025% NaN3, 0,1% gelatin, pH 7.3) (CompTech).
Lectin pathway activation assay
ELISA plates (Costar) were coated with 50 μl of coating buffer (35 mM Na2CO3, 15 mM NaHCO3, pH 9.6) containing 100 μg/ml of mannan from Saccharomyces cerevisiae (Sigma) or 1% BSA (negative control) and incubated overnight at 4°C. After a one hour blockage of the wells with blocking buffer (1% BSA in PBS), 1% NHS diluted in GVB2+ was added together with different concentrations of An. albimanus SGH or recombinant protein dissolved in PBS (final volume of 100 μl/well) and incubated for 30 minutes at 37°C. Wells incubated with serum and no inhibitor were used as positive controls. The wells were washed with PBS-Tw (0.05% Tween-20 in PBS) and incubated for 60 minutes at room temperature with blocking buffer containing anti-C3 antibody diluted 1:1000. After two washes, 50 μl of blocking buffer containing conjugated antibody (Sigma) diluted 1:1500 were added to the wells. After two more washes, the wells were filled with 200 μL of developing buffer (50 mM Na3C6H5O7, 50 mM Na2HPO4, 1 mg/ml o-phenylenediamine (Sigma) and 0.075 % H2O2, pH 5.0) and read at 450 nm and 37°C for 10 min in the kinetic mode. The assays were done in duplicate and the means of the negative control were subtracted of the other means. The results were transformed in percentage of C3 activation, considering the positive control as 100% of activation and then analyzed using ANOVA and the Tukey test.
Activation of C3 and factor B
AP-mediated hemolytic assays were performed as described above using NHS diluted 1:35 and amounts of SGH equivalent to four salivary glands of An. albimanus or recombinant albicin at a final concentration of 20 nM. Tubes containing only PBS without SGH or with gSG7-2 at the same final concentration were used as negative controls. At different times of incubation at 37°C (0, 30 and 60 min), the tubes were centrifuged and 5 μl of the supernatant were collected and subjected to SDS-PAGE. Five nanograms of purified C3a (CompTech) or 10 ng of purified Bb (CompTech) were used as controls, as well as diluted serum without inhibitors or red blood cells. The proteins in the gel were blotted onto nitrocellulose membranes, blocked for two hours with blocking buffer (0.05% Tween-20 and 10% dried non-fat milk in PBS), and incubated with goat anti-human factor B (CompTech) or rabbit anti-human C3a (CompTech) antibodies diluted 1:1000 in 0.05% Tween-20 and 1% BSA in PBS. The blots were washed three times with 0.05% Tween-20 in PBS and then incubated with anti-goat (Sigma) or anti-rabbit (Santa Cruz Biotechnology) antibody diluted 1:2000 or 1:3000, respectively, in the same buffer of primary antibodies. Both secondary antibodies were conjugated with peroxidase and the detection was performed using the Peroxidase Substrate DAB kit (Vector Laboratories).
In order to investigate the direct effect of the inhibitor on the components of the AP C3-convertase, 20 μl of Mg-EGTA buffer containing 200 ng of purified C3b (CompTech), 0.5 ng of purified factor D (CompTech) and 0.5 μg of purified factor B were incubated at 37°C with 20 μl of the same buffer containing An. albimanus SGH (4 gland equivalents) or recombinant albicin in a final concentration of 20 nM. Tubes incubated without SGH (only buffer) or with gSG7-2 were used as negative controls. At different times of incubation (0, 20 and 40 min), an aliquot of the supernatant was collected, mixed with sample buffer and reducing agent, heated and analyzed by SDS-PAGE. Activation of the purified factor B by factor D was detected by immunoblot as described above.
Purification and identification of An. albimanus salivary inhibitor of the C
Salivary glands (100 pairs) of 4-to-8-day-old non-blood fed females were dissected and stored in 100 μl of PBS. The glands were sonicated and centrifuged for 10 minutes at 10,000 × g at 4°C, the supernatant was loaded onto a Source 15 RPC column and eluted with a gradient of acetonitrile in water containing 0.1% trifluoroacetic acid. After drying under vacuum and dissolving in PBS, each fraction was tested in the AP-mediated hemolytic assay as described above. The fractions that showed anti-complement activity along with adjacent fractions were subjected to SDS-PAGE and silver stained using the SilverQuest kit (Invitrogen). A protein band between 6 and 14 kDa found exclusively in the active fractions was cut from the gel and submitted to trypsin digestion followed by nano liquid chromatography-tandem mass spectrometry (nano LC-MS/MS) analysis. The sequence was identified by comparison with an existing database for An. albimanus translation products on Vectorbase (http://www.vectorbase.org) and NCBI (http://www.ncbi.nlm.nih.gov). A signal peptide was identified using the SignalP 4.1 Server (http://www.cbs.dtu.dk/services/SignalP).
In order to confirm that the identified gene was transcribed in salivary glands, synthetic primers for the gene sequence were designed using the Primer3 software (http://primer3.ut.ee). The sequences were: albigsg7FOR1 (5′ GACAGTCATATTGCCGTTGG) and albigsg7REV3 (5′ AACATGCGCTTTGCATACAG). Fifty salivary glands from non-blood fed females were dissected, stored in 50 μl of Trizol reagent (Life Technologies) and homogenized. After RNA extraction, cDNA was produced using the QuantiTect Reverse Transcription kit (Qiagen), exchanging the random primer from the kit for the primer albigsg7REV3, specific for the sequence in interest. The resulting cDNA was amplified by PCR using the primers above with ultra pure water serving as the negative control. The PCR products were mixed with sample buffer (Invitrogen) and analyzed by agarose gel electrophoresis.
Expression and purification of recombinant proteins
Optimized synthetic cDNAs for An. albimanus albicin and gSG7-2 and An. darlingi gSG7 missing the signal peptide sequence and containing an initiator methionine codon directly 5′ to the mature coding sequence were cloned into pET17b and expressed in the BL21 pLys S strain of E. coli. Cultures were grown and inclusion bodies processed as described previously (15). The recombinant proteins were purified after concentration by two steps of gel filtration chromatography on Sephacryl S-100 (GE Healthcare), and Superdex 75 (GE Healthcare). Purity was assessed by SDS-PAGE with Comassie blue staining. The purified proteins were tested in the hemolytic assays, the LP assay, and western blot assays described previously to detect anti-complement activity.
Detection of bound complement components on an agarose-coated surface
Microtiter plates (Costar) were coated with 100 μl of 0.1% agarose (Sigma) diluted in distilled water dried overnight at 37°C (7). Then, 20% NHS diluted in HMEBN solution (5 mM HEPES, 7 mM MgCl2, 10 mM EGTA, 5 mg/ml BSA, 140 mM NaCl, pH 7.4) were added to the wells together with different concentrations of the recombinant proteins (final volume of 100 μl/well) and the plates were incubated at 37°C for 30 minutes. Wells incubated without serum were used as negative controls and wells incubated with serum and no inhibitor, as positive controls. The plates were washed with washing buffer (0.05% Tween-20 in PBS) and incubated with 50 μl of 10 mM HEPES and 140 mM NaCl solution containing anti-Human C3 (CompTech), anti-Human factor B (CompTech), anti-Human properdin (CompTech) or anti-Human C9 (CompTech) antibodies and incubated for 30 minutes at room temperature. After washing, a conjugated anti-goat antibody (Sigma) was added and incubated for 30 minutes. After two more washes, the plates were developed as described for the LP assays. The assays were performed in duplicate and in every independent experiment, the mean of the negative control was subtracted from the other mean values. The results were transformed to percentage of deposition, considering the positive control as 100% and then analyzed using ANOVA and the Tukey test.
In order to check if the recombinant protein could reverse the binding of previously bound complement components, a similar assay was performed. First, the agarose-coated plates were incubated at 37°C for 30 minutes with 100 μl of 4 % NHS in HMEBN solution. After two washes using the same buffer, the wells were incubated with 100 μl of HMEBN containing albicin at 20 nM final concentration. Wells incubated with buffer alone or with 100 μg of purified factor H (CompTech) in 100 μl of HMEBN (6.45 μM final concentration), were used as controls. After two more washes, anti-human factor B, anti-human C3 or anti-human properdin antibodies were added to the wells and the rest of the assay followed as described for the deposition assays.
Immunoblot and hemolytic assays in the presence of anti-albicin IgG
Antibodies against albicin were produced by Spring Valley Laboratories (Woodbine, Maryland, USA) in rabbits immunized three times with the protein together with Freund’s adjuvant. IgG from the rabbit anti-serum were purified on a protein A column (GE Healthcare) following the manufacturers instructions. Amounts of SGH equivalent to 20 salivary glands from females An. albimanus and 0.2 μg of recombinant albicin were electrophoresed by SDS-PAGE and blotted onto nitrocellulose membranes as described above and analyzed by immunoblot as described above.
To evaluate if the anti-albicin could block the anti-complement activity, hemolytic assays were performed as described above, using SGH amounts equivalent to 1 salivary gland or recombinant albicin at a final concentration of 5 nM. Prior to the assay, 12.5 μl of PBS with SGH or recombinant inhibitor were mixed with 12.5 μl of PBS containing anti-albicin at different dilutions (from 1:10 to 1:1,000). NHS and rabbit red blood cells were then added (final volume 75 μl/tube) and the tubes incubated at 37°C for 30 minutes. Tubes containing anti-albicin 1:10 and no serum were used as controls to detect any IgG-mediated red blood cell lysis. The rest of the assay was performed as described above. For data analysis, the results were normalized considering the positive control as 100% of hemolysis. For statistical analysis, ANOVA followed by Tukey test were used.
ELISA assays to detect direct binding of complement components to albicin
Wells of an ELISA plate (Costar) were coated overnight at 4°C with 50 μl of the same coating buffer cited above containing 0.5 μg of albicin or gSG7-2 (or 1% BSA as negative controls). The wells were blocked for one hour at room temperature with 200 μl of blocking buffer (1% BSA in PBS) and then 50 μl of PBS with 0.2 μg of each complement component (factor B, Bb, C3, C3b, factor D and properdin) were added to the wells and incubated for 30 minutes at room temperature. After washing the plates two times with 200 μl of PBS-Tw, polyclonal primary antibody specific to each component (CompTech) was added to the wells in a 1:5,000 dilution in blocking buffer and incubated for 30 minutes at room temperature (polyclonal anti-C3 was used to detect C3 and C3b, and polyclonal anti-factor B was used to detect factor B and Bb). After two more washes, the wells were treated with peroxidase conjugated secondary antibody diluted 1:3,000 in blocking buffer for 30 minutes at room temperature. The wells were developed as described for the LP assay and read in an ELISA plate reader at 450 nm after 5 minutes of incubation at 37°C. The assays were done in duplicate and the means of absorbance for each complement component were statistically compared using ANOVA and Tukey test.
Surface plasmon resonance (SPR) and other mechanistic studies
The assays were conducted on a Biacore T100 instrument (GE Healthcare). The recombinant albicin was diluted to 20 μg/ml in 10 mM sodium acetate buffer pH 5.0, were immobilized on a CM5 sensor chip (GE Healthcare) using the amine coupling method (690 RU). Assays were performed at 25°C with different concentrations of properdin (4.7 nM to 300 nM) in HBS-N buffer (0.01 M HEPES, 0.15 M NaCl, pH 7.4) flowing at 10μL/min. The chip surface was regenerated with 10 s pulses of 10 mM glycine pH 2.0. The properdin response amplitude values at the end of the injection period were fit to a hyperbolic equation using the Biacore evaluation software. C3b and properdin were immobilized to the levels given in the captions of Fig. 7 and 8 using the methods of Hourcade (16) on CM5 chips. Analytes were passed over the surface in HBS-N buffer containing 2 mM MgCl2. The immobilized properdin surface could be regenerated using 10–15 s pulses of 10 mM glycine, pH 2.5. Bound factor B, Bb and albicin could be removed from the C3b surface using 0.5 M NaCl. The properdin coated C3b surface could be regenerated using 4 s pulses of 10 mM glycine pH 2.5. In this case factor B, Bb and albicin could be removed, but the bound properdin remained largely intact.
To detect inhibition of the C3bBb convertase in solution, the complex was formed by mixing 200 nM C3b, 100 nM factor B and 50 nM factor D in HBS-N containing 2 mM MgCl2 and incubating for 2 min at room temperature as described by Rooijakkers et al. (17). After stopping the formation of factor Bb by adding 5 mM EDTA, purified C3 (CompTech) was added to the complex after the addition of 1 μM albicin or an equal volume of buffer. After 20 min the reaction mixture was separated by SDS-PAGE and blotted to nitrocellulose. The blot was incubated with the anti-human C3a as described above and C3a was detected using an anti-rabbit IgG-alkaline phosphatase conjugate (Sigma-Aldrich) as a secondary antibody.
Statistical analysis
Statistical calculations and graphs were done with GraphPad Prism 5.0 software. All the assays were performed at least three times. Normality of the results was assessed by Kolmogorov-Smirnov test. Significance was determined at p < 0.05.
Results
Assay of anti-complement activity in salivary homogenates
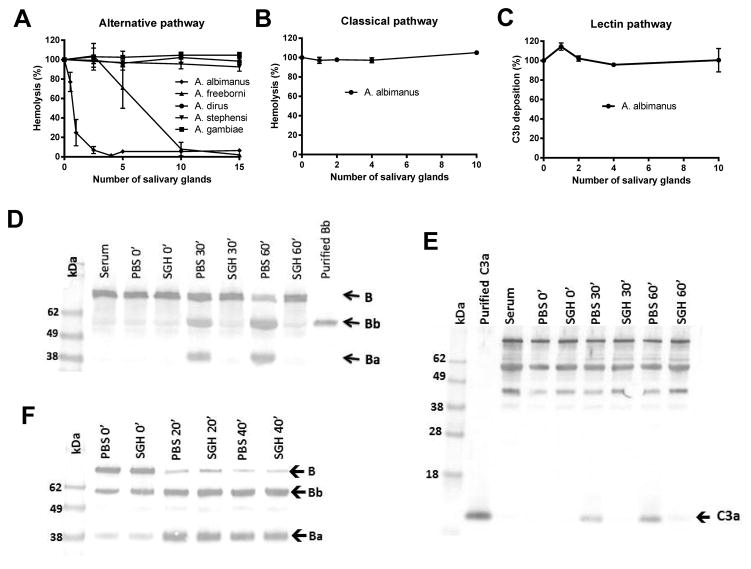
The female SGH of five Anopheles species, An. albimanus, An. dirus, An. freeborni, An. gambiae and An. stephensi were tested in an AP mediated-hemolysis assay using rabbit red blood cells. Protein concentrations in the SGH were similar and ranged from 0.56 to 0.83 μg/gland. An. albimanus and An. freeborni SGH inhibited hemolysis in a dose-dependent way when compared to a positive control lacking SGH (Fig. 1A). An. albimanus possessed a more potent activity, and was able to inhibit hemolysis with amounts of SGH equivalent to one salivary gland (p < 0.0001), while An. freeborni required more than five salivary gland equivalents (p < 0.05) for similar levels of inhibition. Unlike An. albimanus, SGH of An. dirus, An. gambiae and An. stephensi showed no inhibitory effect on the alternative pathway (Fig. 1A). When An. albimanus SGH was subjected to denaturing treatments such as heat and proteinase K, the inhibitory activity was lost, suggesting that the factor is a protein (data not shown). When tested in CP-mediated hemolytic assays and the LP-C3b deposition assay, An. albimanus SGH did not show inhibitory activity even with amounts of SGH equivalent to 10 salivary glands (Fig. 1, B and C).
Figure 1.
An. albimanusSGH inhibits the alternative pathway. (A) Effect of SGH from five Anopheles species on the AP-mediated lysis of rabbit red blood cells. Amounts of SGH equivalent to one gland of An. albimanus was sufficient to inhibit approximately 80% of hemolysis (p < 0.0001) while five gland equivalents of An. freeborni SGH were required to produce detectable inhibition (p < 0.05). An. dirus, An. gambiae and An. stephensi SGE did not show an inhibitory effect. (B, C), An. albimanus SGH showed no effect on either CP-mediated hemolysis (B) or LP-C3b deposition (C), even with amounts of SGH equivalent to 10 salivary glands. Bars indicate SD. (D, E) Supernatants of the AP-hemolytic assay were collected at different times of incubation, separated by SDS-PAGE, and blotted on to nitrocellulose membranes. Using anti-factor B or anti-C3a antibodies, inhibition of factor B (D) and C3 (E) cleavage in the presence of the SGH was observed, even at incubation times of up to 60 min. (F) An. albimanus SGH does not inhibit assembly of the C3 convertase. Purified C3b (200 ng), factor B (0.5 μg) and factor D (0.5 ng) were mixed and incubated at 37°C. Aliquots were collected at different times of incubation (0, 20 and 40 min) and analyzed by western blot to detect factor B activation. In both the presence and absence of SGH, factor B was activated as indicated by a loss of intact factor B and an increase in the quantity of the Ba fragment.
Inhibition of complement factor activation by SGH
The inhibitory effect of An. albimanus SGH on the AP was further assessed by immunoblot assays to detect cleavage of the C components factor B and C3. AP-mediated hemolytic assays were performed in the presence or absence of SGH and aliquots of the supernatant were collected after different incubation times, separated by SDS-PAGE and blotted onto nitrocellulose membranes. Using anti-factor B antibody as a probe, no formation of the factor B cleavage product, Bb, was detected in the presence of SGH, at incubation times of up to 60 minutes, while activation was readily apparent in the absence of SGH (Fig. 1D). Probing with the anti-C3a antibody showed that C3 activation was also inhibited in the presence of SGH, as we did not observe a band corresponding to C3a after 30 and 60 minutes of incubation at 37°C (Fig. 1E).
In order to determine if An. albimanus SGH inhibited C3-convertase assembly, we formed this enzymatic complex by combining purified C3b, factor B and factor D in the presence and absence of SGH, and analyzed factor B activation by immunoblot after different times of incubation. The SGH did not affect factor B activation, as we detected Ba and Bb bands after 20 and 40 minutes of incubation in both the presence and absence of the inhibitor, indicating that SGH did not inhibit assembly of the C3-convertase (Fig. 1F).
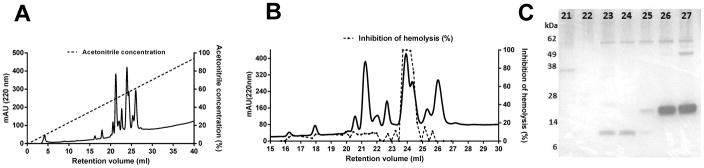
Purification and identification of An. albimanus salivary inhibitor
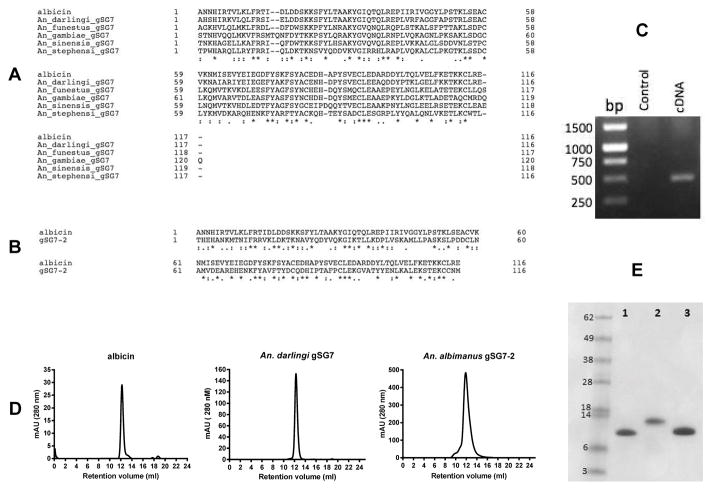
Reverse phase chromatography was used to purify the complement inhibitor from An. albimanus SGH. A cluster of peaks measured by absorbance at 220 nm, was eluted from a Source-15 polystyrene column at a gradient concentration of approximately 50% acetonitrile (Fig. 2A). An aliquot of each fraction was tested in the AP-mediated hemolytic assay and four contiguous fractions (23, 24, 25 and 26) showed strong anti-complement activity (Fig. 2B). The active fractions matched a specific peak of absorbance having an elution volume of approximately 24 mL (Fig. 2B). Analysis by SDS-PAGE revealed two protein bands in the active fractions; one of approximately 62 kDa, that also appeared in adjacent non-active fractions, and a second small band between 6 and 14 kDa, that appeared only in the active fractions (Fig. 2C). Analysis by mass spectrometry (MS) identified the smaller polypeptide as a form of gSG7 (VectorBase, AALB001854-PA), a 13 kDa protein previously described from other anopheline species (18–22). In the genomic sequence of An. albimanus (23), a tandemly duplicated pair of secreted gSG7-like proteins are encoded that we initially designated as gSG7 and gSG7-2. All of the fragment peptides identified by MS analysis match only the predicted gSG7 sequence, thereby indentifying it as the putative anticomplement factor, to which we have given the name albicin (An. albimanus complement inhibitor). The coding regions of both genes contain a predicted signal sequence, and can be aligned with gSG7 sequences obtained from published salivary gland transcriptomes of other Anopheles species. The albicin sequence is most similar to its homolog from the salivary gland transcriptome of An. darlingi (24), as would be expected from the phylogenetic placement of both species in the new-world subgenus Nyssorhynchus (Fig. 3A). An. gambiae, An. funestus, and An. stephensi belong to the subgenus Cellia and An. sinensis belongs to subgenus Anopheles. The gSG7 sequences from the Cellia and Anopheles subgenera show approximately 45 % amino acid identity with albicin, while albicin and its An. darlingi homolog are 75 % identical. After removal of its putative signal peptide sequence, the albicin polypeptide is composed of 116 amino acids, with a molecular weight of 13.4 kDa and a predicted isoelectric point of 5.4 (Fig. 3B). The mature gSG7-2 protein is also composed of 116 amino acids, with a molecular weight of 13.4 kDa and a predicted isoelectric point of 7.7 (Fig. 3B). The two proteins share 33.3% amino acid identity (Fig. 3B).
Figure 2.
Purification of the An. albimanus salivary C inhibitor. (A) SGE equivalent to 200 salivary glands was applied to a Source-15 polystyrene column and eluted with a gradient of acetonitrile containing 0.1% TFA. The fractions were tested using the AP-mediated hemolysis assay and fractions 23, 24, 25 and 26 were found to contain anti-complement activity. (B) The active material was associated with a peak of absorbance centered at a retention volume of 24 mL. (C) Fractions showing anti-complement activity, along with several adjacent fractions, were analyzed by SDS-PAGE and silver stained. A band migrating between 6 and 14 kDa was detected exclusively in the active fractions (23, 24 and 25). The band was cut from the gel and analyzed by mass spectrometry.
Figure 3.
Sequence analysis and recombinant expression of albicin and its relatives. (A) The amino acid sequence alignment of albicin with gSG7 family members from Anopheles darlingi (GenBank: ACI30142.1), An. funestus (GenBank: ABI83776.1), An. gambiae (GenBank: CAC35523.1), An. sinensis (GenBank: KFB36874.1) and An. stephensi (GenBank: AAO06828.1) was done on UniProt (http://www.uniprot.org/align) and revealed 75.5 % identity with that of An. darlingi gSG7 but only approximately 45 % identity with old world Anopheles species. (B) The sequences of albicin and gSG7-2 from An. albimanus showed only 33% identity. In both A and B, identical residues are indicated by asterisks. (C) Detection of the albicin transcript in a preparation of salivary gland mRNA by RT-PCR. The size of the product is consistent with the size of the albicin coding sequence. (D) Recombinant albicin, gSG7-2 and An. darlingi gSG7 were expressed in E. coli and analyzed by gel filtration chromatography. The Superdex-75 gel filtration chromatogram of the last purification step showed a single peak for each protein having a retention volume consistent with a monomer. (E) The purity of albicin (1), gSG7-2 (2) and An. darlingi gSG7 (3) was confirmed by SDS-PAGE with Coomassie blue staining.
To confirm the presence of the albicin transcript in An. albimanus salivary glands, the sequence was amplified by RT-PCR using primers specific for the termini of the coding region, with a sample of salivary gland mRNA as template. Electrophoretic analysis showed a single product of the size predicted by the sequence of albicin, indicating that the message is present in the salivary gland (Fig. 3C).
Activity of recombinant proteins
To study the anti-complement activity of albicin, An. darlingi gSG7 and gSG7-2, recombinant forms of the mature proteins were produced as inclusion bodies in E. coli, refolded and purified. The Superdex-75 gel filtration chromatogram of the last purification step showed a single peak for each protein having a retention volume consistent with a monomer (Fig. 3D). The purity of the protein was confirmed on Coomassie blue stained SDS-PAGE gels, indicating a high degree of purity (Fig. 3E).
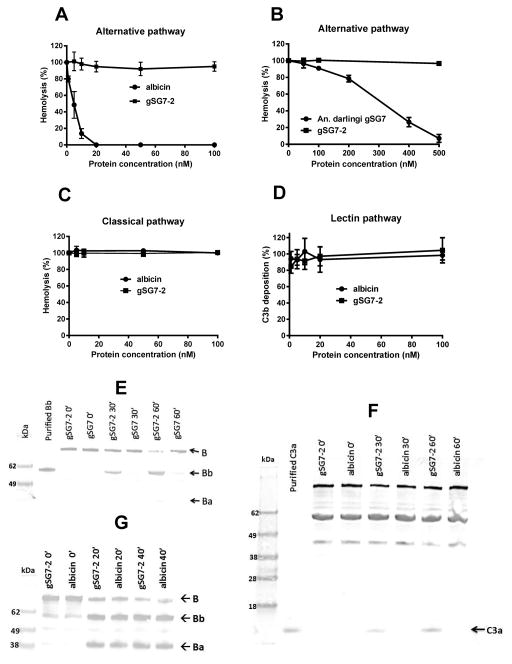
As shown in Figure 4A, recombinant albicin was able to potently inhibit AP-mediated hemolysis, confirming it to be the salivary inhibitor from An. albimanus. An. darlingi gSG7 also inhibited the AP, although higher concentrations of the protein were required (Fig. 4B). Conversely, recombinant gSG7-2 showed no inhibitory effect in the hemolytic assay at concentrations up to 500 nM (Fig. 4, A and B), verifying the results of the mass spectral studies of SGH in which albicin was the only gSG7-like protein associated with anti-complement activity. Consistent with the results from experiments using SGH, albicin had no inhibitory effect on the CP or LP (Fig. 4, C and D). In immunoblot assays, inhibition of both C3 and factor B activation was observed in the presence of albicin, but not gSG7-2 (Fig. 4, E and F). However, when the purified components C3b, factor B and factor D were used, neither of the recombinant proteins showed inhibitory effects on factor B activation, indicating that like the SGH, recombinant albicin does not inhibit assembly of the C3-convertase complex (Fig. 4G).
Figure 4.
Recombinant albicin and An. darlingi gSG7 inhibit the AP. (A) Albicin caused potent inhibition of AP-mediated hemolysis. (B) The recombinant protein from An. darlingi also inhibited the AP, but a higher concentration was required. (C, D) Albicin showed no effect on CP-mediated hemolysis (C) or LP-mediated C3b deposition (D) and gSG7-2 had no inhibitory effect on any of the complement pathways (A, C and D). The mean of at least three independent experiments performed in duplicate is presented with bars indicating SD. (E–G) The supernatants of the AP-mediated hemolytic assays were collected at different times of incubation (0, 30 and 60 minutes) at 37°C, separated by SDS-PAGE and transferred to nitrocellulose membranes. The products of factor B and C3 activation, Bb and C3a, respectively, were not detected in the presence of albicin (E and F). When purified factor B, C3b and factor D were mixed and incubated at 37°C in the presence of albicin or gSG7-2, no inhibitory effect was detected, as the Bb and Ba bands were observed in both the presence and absence of albicin even after 40 minutes of incubation (G).
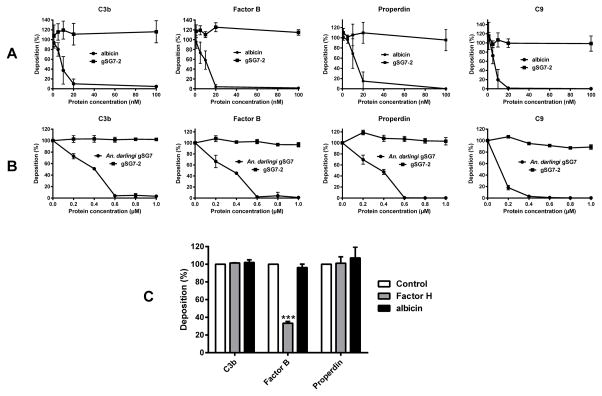
The anti-complement activity of albicin and An. darlingi gSG7 was also demonstrated in deposition assays using agarose-coated plates. The recombinant proteins were able to inhibit the deposition of C3b, factor B, properdin and C9 in a dose-dependent manner (Fig. 5, A and B). No inhibition was observed when gSG7-2 was tested in the same way. Recombinant albicin was not effective in removing previously bound C3b, factor B or properdin from the agarose-coated wells, indicating that it does not act in a factor H-like manner by displacing factor B from C3b (Fig. 5C).
Figure 5.
Inhibition of the deposition and removal of C components from agarose plates. (A, B) Recombinant albicin and An. darlingi gSG7 inhibit the deposition of complement components on agarose-coated plates. Albicin (A) and An. darlingi gSG7 (B) significantly inhibited the deposition of C3b, factor B, Properdin and C9 on an agarose surface. In both sets of experiments, An. albimanus gSG7-2 was used as a negative control. (C) Albicin does not remove previously bound C3b, factor B or properdin. Agarose-coated plates were incubated at 37°C with 4% NHS. After a wash, albicin, factor H or buffer alone (control) were added and incubated again at 37°C. C components remaining after washing were detected using antisera specific for each component. The results are expressed as the mean of the percentage of each component deposited after incubation with the inhibitors. Bars indicate SD, and asterisks indicate statistical significance at the p<0.0005 level, ANOVA and Tukey test.
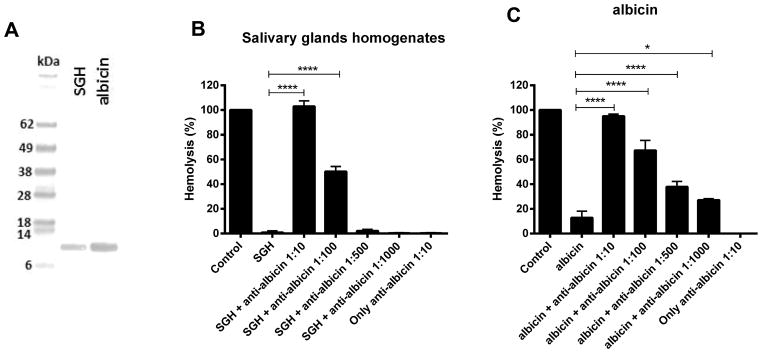
As shown in Figure 6A, purified IgG from a rabbit immunized with recombinant albicin was able to recognize a protein band of the predicted size of albicin in samples of SGH and recombinant albicin when analyzed by immunoblotting. Besides recognizing both preparations of the protein, the antibody also blocked the activity of both in the hemolytic assay. IgG diluted 1:10 and 1:100 was able to block the anti-complement activity of the SGH in a statistically significant manner (p < 0.0001) (Fig. 6B). Purified IgG also blocked the activity of the recombinant albicin in a concentration dependent manner (Fig. 6C). The IgG itself caused no damage to the red blood cells, as it did not lead to hemolysis in the absence of NHS (Fig. 6, B and C).
Figure 6.
Polyclonal antibodies to recombinant albicin recognize and block the activity of native and recombinant albicin. (A) Immunoblot of salivary gland homogenate and recombinant albicin probed with rabbit anti-albicin recognizes albicin present in SGH (amount equivalent to 20 glands) as well as the recombinant protein (0.2 μg) blotted after SDS-PAGE onto a nitrocellulose membrane. (B, C) Inhibition of C-mediated hemolysis in the presence of anti-albicin antisera. AP-mediated hemolytic assays were performed in the presence of SGH (one gland) or recombinant albicin (10 nM final concentration) and different dilutions of anti-albicin antiserum. The antibody was able to block the activity of both native (B) and recombinant inhibitors (C). The mean of at least three independent experiments performed in duplicate is presented with bars indicating SD (**** p<0.0001; * p<0.05, ANOVA and Tukey test).
Mechanistic studies of albicin function
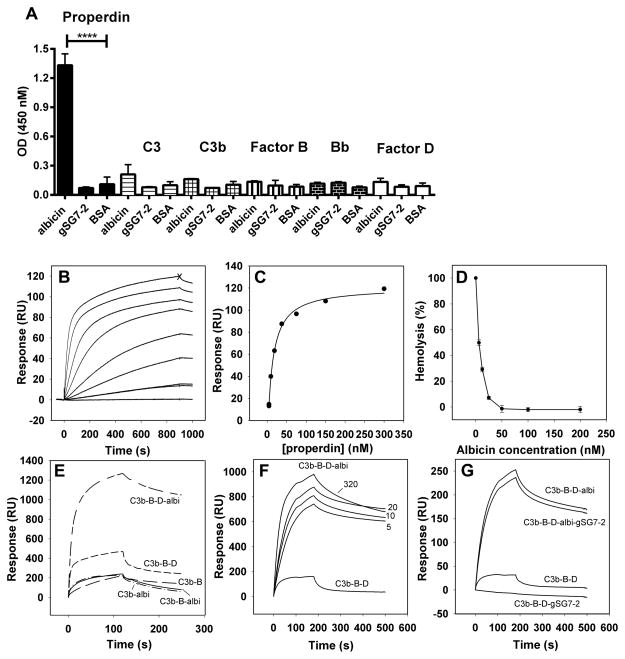
An ELISA was performed to detect direct binding of different complement components to albicin. Plate wells coated with albicin, gSG7-2 or BSA were incubated with properdin, C3, C3b, factor B, Bb or factor D, and antibodies specific to each component were used to detect binding (Fig. 7A). Properdin binding with recombinant albicin-coated wells was highly significant (P<0.0001) relative to the binding to gSG7-2 or BSA coated wells (Fig. 7A). No significant binding was observed with C3, C3b, factor B, Bb or factor D indicating that properdin binds an albicin surface selectively, but does not appear to interact with other individual components of the C (Fig. 7A).
Figure 7.
Analysis of albicin binding to components of the alternative C3 convertase, and inhibition of the alternative pathway in properdin-depleted serum. (A) ELISA plates were coated with recombinant albicin, gSG7-2 or BSA and incubated with purified components of the C. Properdin binding to albicin was significantly greater than that to the gSG7-2 and BSA controls (p < 0.0001). (B) SPR analysis of properdin binding to an immobilized albicin surface. Albicin was immobilized by amine coupling (690 RU) and properdin was passed over the surface at concentrations of 0, 4.7 (x2), 9.4, 18.7, 37.5, 75, 150 and 300 nM. The expected maximal binding of properdin would be approximately 2800 RU. Maximal values significantly lower than this were consistently obtained, possibly due to multivalent interaction with the albicin surface or steric hindrance. (C) Fit of the maximal response values (at time point X indicated on the top curve of panel A) to a rectangular hyperbolic equation. From this the half-maximal binding concentration (K1/2) of 11.6 nM was obtained. (D) AP-mediated hemolysis of rabbit erythrocytes by properdin-depleted serum. (E) The binding of alternative C3 convertase components to a properdin surface (5475 RU) and the effect of albicin on binding. The various treatments are indicated on the graph and in all cases the concentration of C3b was 20 μg/mL (114 nM), factor B was 20 μg/mL (215 nM) and factor D was 2 μg/mL (83 nM). When indicated, albicin (albi) was present at a concentration of 1 μM. (F) Concentration dependence of the stabilization of C3 convertase formation by albicin on a properdin surface (the same surface as in panel E). The treatments are shown on the graph and in all curves the concentration of C3b was 5 μg/mL, the concentration of factor B was 5 μg/mL and factor D was 0.5 μg/mL. The concentrations (nM) of albicin are shown on the graph. (G) Effect of gSG7-2 on complex assembly and on stabilization by albicin on a properdin surface (2200 RU). In all curves the concentration of C3b was 5 μg/mL, the concentration of factor B was 5 μg/mL and factor D was 0.5 μg/mL. The concentration of albicin and gSG7-2 was 100 nM.
When analyzed by surface plasmon resonance (SPR), properdin was again found to bind to an immobilized albicin surface in a concentration-dependent manner (Fig. 7B). Fitting the concentration-dependent maximal resonance unit values to a hyperbolic function gave a value for the albicin concentration at half-saturation (K1/2) of 11.6 nM for this interaction (Fig. 7C). As expected from the ELISA results, properdin showed no detectable interaction with a surface of gSG7-2 immobilized in the same manner (data not shown). Additionally, when the hemolysis assay was performed using properdin-depleted serum in place of NHS, inhibition of C was seen, and the concentration-dependence of this inhibition was similar to that observed with NHS, despite the longer incubation times required for lysis (Fig. 7D). These results indicate that properdin is not essential for inhibition, but may play a role when present.
Previous studies have shown that assembly of the C3bBb complex can be observed using SPR (16). Specifically, the binding of factor B to a C3b surface is enhanced when factor D is added, suggesting the formation of a stable C3bBb complex (16). Similarly, immobilized properdin has also been found to support the assembly of C3bBb (16). To determine the effect of albicin on the assembly of the C3bB and C3bBb complexes we examined the binding of components of the complex to an immobilized properdin SPR surface in the presence and absence of the inhibitor. As previously shown, a mixture of C3b and factor B assembled into a complex on the surface, and the interaction was more pronounced in the presence of factor D (Fig. 7E). Surprisingly, we found that addition of albicin to the ternary mixture produced a large additional increase in complex formation (Fig. 7E). If factor D was not included, the binding of the C3b-factor B-albicin mixture was very similar to that of the C3b-factor B mixture without albicin, indicating that the increase is not due to albicin binding to the properdin surface, and that albicin does not enhance the formation of C3bB (Fig. 7E). Albicin also did not increase the binding of C3b in binary mixtures (Fig. 7E). Additionally, factor B and factor D showed no interaction with the properdin surface when tested by themselves or mixed with albicin (data not shown).
The concentration dependence of complex stabilization by albicin on the properdin surface was examined by adding varying concentrations of albicin to a fixed concentration of C3b, factor B and factor D (Fig. 7F). As little as 5 nM albicin greatly increased the quantity of bound complex, and only small additional increases in binding were observed at concentrations of 10, 20 and 320 nM. These results suggest that albicin binds selectively to the activated C3bBb complex formed on a properdin surface at concentrations consistent with its inhibitory activity in hemolysis assays. Unexpectedly, addition of the inactive gSG7-2 also affected complex formation on the surface, but in this case binding of the C3b-B-D mixture was reduced. Nevertheless, when albicin and gSG7-2 were added together at a concentration of 100 nM each, the complex bound to the surface at a level very similar to that seen with 100 nM albicin alone, indicating that gSG7-2 does not diminish or enhance the stabilizing effect of albicin (Fig. 7G).
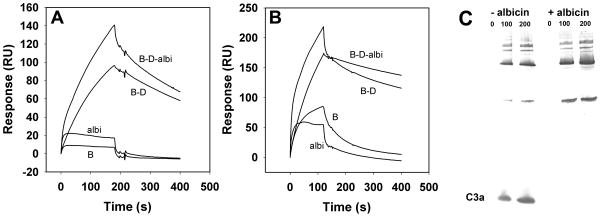
When C3b was immobilized, the effect of albicin on complex formation with factor B was less dramatic than on properdin surfaces. Formation of a C3bBb complex in the presence of factor D was observed, but addition of 100 nM albicin to the mixture, resulted in a smaller increase in amplitude of the binding response than seen on the properdin surface (Fig. 8A). The smaller degree of complex formation may be the result of higher inherent stability due to the covalent binding of one of its components (C3b) to the chip surface, but still indicates an interaction of the complex with albicin. Coating of a C3b surface with properdin prior to injection of a factor B-D is also known to further stabilize the apparent C3bBb complex formed in the presence of factor D. When albicin was added to a C3bBbP complex formed in this manner, a small but significant change was observed in the binding and stabilization of the complex, further suggesting an interaction with albicin (Fig. 8B).
Figure 8.
Effect of albicin on the formation of the alternative C3 convertase on immobilized C3b surfaces. (A) SPR analysis of factor B binding to immobilized C3b in the absence and presence of factor D and albicin. C3b was immobilized by amine coupling (3170 RU) and C3b (5 μg/mL), factor B (5 μg/mL), factor D (0.5 μg/mL) and albicin (100 nM) were passed over the surface in the combinations indicated in the graph. (B) SPR analysis of factor B binding to immobilized C3b (5720 RU) coated with properdin (additional 1200 RU) in the absence and presence of factor D and albicin. The components were passed over the surface individually and in the combinations indicated on the graph. (C) Inhibition of C3bBb convertase activity in solution. Purified C3b, factor B and factor D were mixed in HBS-N containing 2 mM MgCl2 and after stopping the reaction, C3 was added to 0, 100 and 200 nM (as indicated in the figure) in the presence and absence of 1 μM albicin. After incubation (20 min), the reaction mixtures were separated by SDS-PAGE, transferred to nitrocellulose and C3a (labeled on figure) was detected using rabbit polyclonal anti-C3a, and anti-rabbit IgG alkaline phosphatase conjugate. C3a was detected only in the absence of albicin (− albicin), while in the presence of albicin (+ albicin) no C3a was detected at any concentration of C3.
Inhibition of convertase activity of the C3bBb complex in solution was evaluated by adding purified C3, in the presence and absence of albicin, to C3bBb formed by mixing purified C3b, factor B and factor D. After an incubation period, the mixture was separated by SDS-PAGE, transfered to nitrocellulose, and probed with anti-C3a. The formation of C3a was observed in the absence of albicin, but not in the presence of 1 μM albicin, demonstrating that albicin inhibits the convertase activity of the C3bBb complex in the absence of any other component (Fig. 8C).
Discussion
The C in plasma is a potent weapon of the innate immune system against invasive pathogens. Once activated, it can recruit and activate phagocytes and form pores on the cell membrane, leading to cell death. It is not surprising that hematophagous arthropods contain complement inhibitors in their saliva or midgut, that act to inhibit the C present in ingested blood (14). It has been demonstrated that the C remains intact in the blood meal and can be activated inside the midgut lumen of the mosquito (25). Furthermore, if the C is not inactivated by salivary or intestinal inhibitors, it can cause midgut cell damage (13, 25). Additionally, anaphylatoxins released through complement activation in the skin surrounding the bite would lead to mast cell degranulation, histamine release and plasma extravasation. In general these processes are thought to be a hindrance to blood feeding.
In this work, we have shown that anti-complement activity is present in An. albimanus and An. freeborni salivary gland homogenates. Both SGHs inhibited the AP, but An. albimanus contained a more potent activity, requiring amounts of saliva equivalent to one salivary gland to inhibit approximately 80% of AP-mediated hemolysis (Fig. 1A). In contrast to this, An. albimanus SGH had no effect on the CP and LP (Fig. 1, B and C). When exposed to denaturing treatments such as heat and digestion by proteinase K, the SGH lost activity, indicating that the inhibitor is a protein. We confirmed the occurence of anti-AP activity in An. albimanus SGH using immunoblot assays, showing that in the presence of the SGH, there is no activation of the C3 or factor B components (Fig. 1, D and E). SGH did not directly block the assembly of the AP C3-convertase, as it did not inhibit factor B activation when the assay was performed with purified factor B, factor D and C3b in the absence of properdin (Fig. 1F).
Curiously, the SGH of old world species of Anopheles did not have any effect on AP-mediated hemolysis, even at high concentrations of salivary protein (Fig. 1A). It was recently demonstrated that An. gambiae and An. stephensi mosquitoes bind factor H, a negative regulator of the C, in the midgut epithelium, and inactivate the C at the intestinal level (25). This could explain why old world mosquito species do not contain salivary inhibitors, as the intestinal inhibition would be enough to protect the midgut. Complement inhibitors have been described in the midgut of triatomine bugs (13), sand flies (12), tse-tse flies (26) and scabies mites (27). The presence of intestinal inhibitors in An. albimanus and other new world anophelines remains to be investigated.
The An. albimanus salivary inhibitor was identified by mass spectrometry as belonging to the gSG7 protein family that is widely distributed in the salivary secretions of anopheline mosquitoes. This group of proteins exhibits little sequence similarity to any non-anopheline protein group and has not been structurally characterized. The inactive gSG7 proteins from old world anophelines show approximately 45% amino acid sequence identity with An. albimanus gSG7, while the gSG7 from the new world species An. darlingi is approximately 80% identical to the An. albimanus form and shows similar anti-complement activity. It seems clear that the anticomplement activity of gSG7 family members is characteristic of new world anophelines, and has evolved after the divergence of old and new world forms.
Arthropod salivary proteins having anti-complement activity have been reported from the hard ticks Ixodes scapularis and I. ricinus. I. scapularis possesses ISAC (7) and Salp20 (28), proteins that inhibit solely the AP. Two homologous families of anti-AP proteins were also found in I. ricinus: the IRACs (8) and IxACs (9). Salp20 and the IxACs inhibit the AP by binding to properdin (9, 28). These proteins inhibit the binding of properdin to the AP C3-convertase, destabilizing the enzymatic complex and thus, blocking the AP cascade. Like these proteins, albicin can inhibit the deposition of factor B and other complement components on activating surfaces but, unlike ISAC and Salp20, is not able to remove previously bound factor B and properdin (Fig. 5C). We can conclude that albicin affects the activity of the C3 convertase, but does not dissociate it, as been described for the IxACs from Ixodes ricinus (9) and the SMIPPs, complement inhibitors from Sarcoptes scabiei mites that inhibit the AP by binding properdin, although this protein is not specific for the AP (27).
The specificity of albicin for the AP indicates a likely targeting of the alternative C3 convertase complex or one of its components. Properdin binds tightly to albicin coated surfaces, suggesting that it may be important in the inhibitory mechanism. However, albicin-mediated inhibition of the C is not lost in properdin-depleted serum, indicating that a simple blockade of properdin binding is not the mechanism of action. Albicin also does not block activation of factor B when it is reconstituted with purified C3b, factor B and factor D, demonstrating that it is not an inhibitor of factor D and does not block factor B cleavage by binding to it.
Previous studies have shown an enhancement of the assembly of C3b and factor B on properdin SPR surfaces in the presence of factor D, presumably due to an increased stability of the C3bBb complex (16). These observations have led to the proposal that properdin plays an active role in recruiting C3b to target surfaces and serves as a site for complex assembly (29, 30). Additional studies have shown that properdin is a pattern recognition molecule that binds to dangerous nonself surfaces where it orchestrates assembly of complement complexes (29, 30). Here we found that addition of albicin to the C3b-factor B-factor D mixture led to a very large additional enhancement of complex formation on properdin surfaces that was dependent on the presence of factor D. The increase was near maximal at concentrations as low as 5 nM albicin, showing that the effect is operating at concentrations similar to those needed to inhibit the AP in hemolysis assays. This result suggests that albicin recognizes only the activated C3bBb complex and has a stabilizing effect on the complex under some assembly conditions. A smaller increase in the quantity of complex forming on an immobilized C3b surface was also seen when albicin was passed over the surface as a mixture with factor B and factor D, suggesting that albicin stabilizes complexes containing covalently bound C3b.
Albicin-mediated inhibition of reconstituted C3bBb convertase activity in solution provides evidence that stabilized complexes formed on surfaces are inactivated. The inhibition of early noncovalent complexes appearing prior to amplification may be an adaptive mechanism for utilization of a limited amount of salivary protein against a target that becomes very abundant as the response proceeds. It is also likely that targeting of only activated complexes is necessary to avoid the consumption of inhibitor by nonproductive binding to abundant precursor molecules. The role of properdin in albicin-mediated inhibition is not completely clear since the activation of C in properdin-depleted serum can be inhibited, and inhibition can be observed with reconstituted complexes in the absence of properdin. The amounts of properdin in depleted serum are below the level of detection by sensitive immunodiffusion methods, again indicating that albicin can block convertase function in the near absence of this protein.
The proposed mechanism of albicin inhibition has similarity to that of the staphylococcal C3 convertase inhibitor SCIN (17). This inhibitor promotes the formation of convertase dimers by forming contacts with elements of both monomers, as shown in the crystal structure of the complex. Each molecule of SCIN binds the C3b and Bb portions of one complex monomer and the C3b portion of a second monomer, by interacting with a number of domain regions through several contact points on the inhibitor surface. Convertase activity is inhibited in the complex, but active chimeric SCIN derivatives that bind only a single convertase complex monomer show that dimerization is not essential for inhibitor binding. Additionally, SCIN does not block the catalytic site of of Bb, but rather is thought to interfere with substrate binding or important conformational changes in the complex (17). In the case of albicin-mediated inhibition, formation of convertase oligomers would be consistent with the observed stabilization of binding on properdin surfaces in the presence of the inhibitor, since at least two properdin binding sites would be present on each oligomer.
We have demonstrated that IgG antibodies directed at recombinant albicin can recognize and block the activity of both the salivary and recombinant inhibitor (Fig. 6). If an anopheline mosquito takes a bloodmeal in an albicin-immunized host, the antibodies could block the inhibitor, resulting in activation of C and damage to the insect midgut. This being the case, albicin could have potential as a transmission blocking vaccine component for malaria. Also, C-mediated auto-immune diseases have recently been in the spotlight because of their severity, including the atypical hemolytic uremic syndrome (aHUS) and paroxysmal nocturnal hemoglobinuria (PNH), diseases that lead to death in a few years if not treated (31–33). The cause of these life-threatening diseases is disregulation of the AP for different reasons (34), resulting in inappropriate activation and damage to host tissues. The current available drug for treating aHUS and PNH is an anti-C5 antibody (eculizumab), that blocks the activation of the C5 component, preventing MAC formation on normal cell surface (35). However, since C5 is a component of the terminal C pathways, its inhibition causes susceptibility of the host to invasive pathogens, especially life-threatening Neisseria infections (35). Thus, albicin, a small protein that binds to the activated C3bBb complex and inhibits solely the AP, could be a candidate for tests on a new drug for AP-mediated auto-immune diseases, such as aHUS, lowering the risk of infections during the treatment, as the CP and LP would still be active against invasive pathogens. However, its feasibility as a drug would depend on its immunogenicity, which remains to be investigated.
Supplementary Material
Acknowledgments
The authors thank Carl Hammer, Raynaldo Martin and Renee Olano for electrophoretic and mass spectral analyses.
This work was supported by the intramural research program of the NIAID, National Institutes of Health. AFMS was supported by CAPES, the Brazilian Government foundation for the qualification of human resources (Grant 23038.005604/2012-62).
References
- 1.Kolev M, Le Friec G, Kemper C. Complement — tapping into new sites and effector systems. Nat Rev Immunol. 2014;14:811–820. doi: 10.1038/nri3761. [DOI] [PubMed] [Google Scholar]
- 2.Villiers MB, Villiers CL, Laharie AM, Marche PN. Different stimulating effects of complement C3b and complete Freund’s adjuvant on antibody response. Immunopharmacology. 1999;42:151–157. doi: 10.1016/s0162-3109(99)00017-x. [DOI] [PubMed] [Google Scholar]
- 3.Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010;11:785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fearon D, Austen F. Properdin: binding to C3b and stabilization of the C3b-dependent C3 convertase. J Exp Med. 1975;142:856–863. doi: 10.1084/jem.142.4.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Titus RG, Bishop JV, Mejia JS. The immunomodulatory factors of arthropod saliva and the potential for these factors to serve as vaccine targets to prevent pathogen transmission. Parasite Immunol. 2006;28:131–41. doi: 10.1111/j.1365-3024.2006.00807.x. [DOI] [PubMed] [Google Scholar]
- 6.Schneider B, Higgs S. The enhancement of arbovirus transmission and disease by mosquito saliva is associated with modulation of the host immune response. Trans R Soc Trop Med Hyg. 2009;102:400–408. doi: 10.1016/j.trstmh.2008.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valenzuela JG, Charlab R, Mather TN, Ribeiro JM. Purification, cloning, and expression of a novel salivary anticomplement protein from the tick, Ixodes scapularis. J Biol Chem. 2000;275:18717–18723. doi: 10.1074/jbc.M001486200. [DOI] [PubMed] [Google Scholar]
- 8.Daix V, Schroeder H, Praet N, Georgin J-P, Chiappino I, Gillet L, de Fays K, Decrem Y, Leboulle G, Godfroid E, Bollen a, Pastoret P-P, Gern L, Sharp PM, Vanderplasschen a. Ixodes ticks belonging to the Ixodes ricinus complex encode a family of anticomplement proteins. Insect Mol Biol. 2007;16:155–166. doi: 10.1111/j.1365-2583.2006.00710.x. [DOI] [PubMed] [Google Scholar]
- 9.Couvreur B, Beaufays J, Charon C, Lahaye K, Gensale F, Denis V, Charloteaux B, Decrem Y, Prévôt PP, Brossard M, Vanhamme L, Godfroid E. Variability and action mechanism of a family of anticomplement proteins in Ixodes ricinus. PLoS One. 2008;3:e1400. doi: 10.1371/journal.pone.0001400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nunn Ma, Sharma A, Paesen GC, Adamson S, Lissina O, Willis AC, Nuttall P. Complement inhibitor of C5 activation from the soft tick Ornithodoros moubata. J Immunol. 2005:2084–2091. doi: 10.4049/jimmunol.174.4.2084. [DOI] [PubMed] [Google Scholar]
- 11.Cavalcante RR, Pereira MH, Gontijo NF. Anti-complement activity in the saliva of phlebotomine sand flies and other haematophagous insects. Parasitology. 2003;127:87–93. doi: 10.1017/s0031182003003329. [DOI] [PubMed] [Google Scholar]
- 12.Mendes-Sousa AF, Nascimento AAS, Queiroz DC, Vale VF, Fujiwara RT, Araújo RN, Pereira MH, Gontijo NF. Different host complement systems and their interactions with saliva from Lutzomyia longipalpis (Diptera, Psychodidae) and Leishmania infantum promastigotes. PLoS One. 2013;8:e79787. doi: 10.1371/journal.pone.0079787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barros VC, Assumpção JG, Cadete AM, Santos VC, Cavalcante RR, Araújo RN, Pereira MH, Gontijo NF. The role of salivary and intestinal complement system inhibitors in the midgut protection of triatomines and mosquitoes. PLoS One. 2009;4:e6047. doi: 10.1371/journal.pone.0006047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schroeder H, Skelly PJ, Zipfel PF, Losson B, Vanderplasschen A. Subversion of complement by hematophagous parasites. Dev Comp Immunol. 2009;33:5–13. doi: 10.1016/j.dci.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alvarenga PH, Francischetti IMB, Calvo E, Sá-Nunes A, Ribeiro JMC, Andersen JF. The function and three-dimensional structure of a thromboxane A2/cysteinyl leukotriene-binding protein from the saliva of a mosquito vector of the malaria parasite. PLoS Biol. 2010;8:e1000547. doi: 10.1371/journal.pbio.1000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hourcade DE. The role of properdin in the assembly of the alternative pathway C3 convertases of complement. J Biol Chem. 2006;281:2128–2132. doi: 10.1074/jbc.M508928200. [DOI] [PubMed] [Google Scholar]
- 17.Rooijakkers SHM, Wu J, Ruyken M, van Domselaar R, Planken KL, Tzekou A, Ricklin D, Lambris JD, Janssen BJC, van Strijp JAG, Gros P. Structural and functional implications of the alternative complement pathway C3 convertase stabilized by a staphylococcal inhibitor. Nat Immunol. 2009;10:721–727. doi: 10.1038/ni.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lanfrancotti A, Lombardo F, Santolamazza F, Veneri M, Castrignanò T, Coluzzi M, Arcà B. Novel cDNAs encoding salivary proteins from the malaria vector Anopheles gambiae. FEBS Lett. 2002;517:67–71. doi: 10.1016/s0014-5793(02)02578-4. [DOI] [PubMed] [Google Scholar]
- 19.Calvo E, Dao A, Pham VM, Ribeiro JMC. An insight into the sialome of Anopheles funestus reveals an emerging pattern in anopheline salivary protein families. Insect Biochem Mol Biol. 2007;37:164–175. doi: 10.1016/j.ibmb.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Calvo E, V, Pham M, Marinotti O, Andersen JF, Ribeiro JMC. The salivary gland transcriptome of the neotropical malaria vector Anopheles darlingi reveals accelerated evolution of genes relevant to hematophagy. BMC Genomics. 2009;10:57. doi: 10.1186/1471-2164-10-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valenzuela JG, I, Francischetti MB, Pham VM, Garfield MK, Ribeiro JMC. Exploring the salivary gland transcriptome and proteome of the Anopheles stephensi mosquito. Insect Biochem Mol Biol. 2003;33:717–732. doi: 10.1016/s0965-1748(03)00067-5. [DOI] [PubMed] [Google Scholar]
- 22.Zhou D, Zhang D, Ding G, Shi L, Hou Q, Ye Y, Xu Y, Zhou H, Xiong C, Li S, Yu J, Hong S, Yu X, Zou P, Chen C, Chang X, Wang W, Lv Y, Sun Y, Ma L, Shen B, Zhu C. Genome sequence of Anopheles sinensis provides insight into genetics basis of mosquito competence for malaria parasites. BMC Genomics. 2014;15:42. doi: 10.1186/1471-2164-15-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neafsey DE, Waterhouse RM, Abai MR, Aganezov SS, Alekseyev MA, Allen JE, Amon J, Arca B, Arensburger P, Artemov G, Assour LA, Basseri H, Berlin A, Birren BW, Blandin SA, Brockman AI, Burkot TR, Burt A, Chan CS, Chauve C, Chiu JC, Christensen M, Costantini C, Davidson VLM, Deligianni E, Dottorini T, Dritsou V, Gabriel SB, Guelbeogo WM, Hall AB, Han MV, Hlaing T, Hughes DST, Jenkins AM, Jiang X, Jungreis I, Kakani EG, Kamali M, Kemppainen P, Kennedy RC, Kirmitzoglou IK, Koekemoer LL, Laban N, Langridge N, Lawniczak MKN, Lirakis M, Lobo NF, Lowy E, MacCallum RM, Mao C, Maslen G, Mbogo C, McCarthy J, Michel K, Mitchell SN, Moore W, Murphy KA, Naumenko AN, Nolan T, Novoa EM, O’Loughlin S, Oringanje C, Oshaghi MA, Pakpour N, Papathanos PA, Peery AN, Povelones M, Prakash A, Price DP, Rajaraman A, Reimer LJ, Rinker DC, Rokas A, Russell TL, Sagnon N, Sharakhova MV, Shea T, Simao FA, Simard F, Slotman MA, Somboon P, Stegniy V, Struchiner CJ, Thomas GWC, Tojo M, Topalis P, Tubio JMC, Unger MF, Vontas J, Walton C, Wilding CS, Willis JH, Wu Y-C, Yan G, Zdobnov EM, Zhou X, Catteruccia F, Christophides GK, Collins FH, Cornman RS, Crisanti A, Donnelly MJ, Emrich SJ, Fontaine MC, Gelbart W, Hahn MW, Hansen IA, Howell PI, Kafatos FC, Kellis M, Lawson D, Louis C, Luckhart S, Muskavitch MAT, Ribeiro JM, Riehle MA, Sharakhov IV, Tu Z, Zwiebel LJ, Besansky NJ. Highly evolvable malaria vectors: The genomes of 16 Anopheles mosquitoes. Science (80-) 2015;347:1258522–1258522. doi: 10.1126/science.1258522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calvo E, Andersen J, Francischetti IM, de Capurro ML, deBianchi AG, James AA, Ribeiro JMC, Marinotti O. The transcriptome of adult female Anopheles darlingi salivary glands. Insect Mol Biol. 2004;13:73–88. doi: 10.1111/j.1365-2583.2004.00463.x. [DOI] [PubMed] [Google Scholar]
- 25.Khattab A, Barroso M, Miettinen T, Meri S. Anopheles Midgut Epithelium Evades Human Complement Activity by Capturing Factor H from the Blood Meal. PLoS Negl Trop Dis. 2015;9:e0003513. doi: 10.1371/journal.pntd.0003513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ooi CP, Haines LR, Southern DM, Lehane MJ, Acosta-Serrano A. Tsetse GmmSRPN10 Has Anti-complement Activity and Is Important for Successful Establishment of Trypanosome Infections in the Fly Midgut. PLoS Negl Trop Dis. 2015;9:e3448. doi: 10.1371/journal.pntd.0003448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bergström FC, Reynolds S, Johnstone M, Pike RN, Buckle AM, Kemp DJ, Fischer K, Blom AM. Scabies mite inactivated serine protease paralogs inhibit the human complement system. J Immunol. 2009;182:7809–7817. doi: 10.4049/jimmunol.0804205. [DOI] [PubMed] [Google Scholar]
- 28.Tyson KR, Elkins C, de Silva aM. A Novel Mechanism of Complement Inhibition Unmasked by a Tick Salivary Protein That Binds to Properdin. J Immunol. 2008;180:3964–3968. doi: 10.4049/jimmunol.180.6.3964. [DOI] [PubMed] [Google Scholar]
- 29.Spitzer D, Mitchell LM, Atkinson JP, Hourcade DE. Properdin can initiate complement activation by binding specific target surfaces and providing a platform for de novo convertase assembly. J Immunol. 2007;179:2600–2608. doi: 10.4049/jimmunol.179.4.2600. [DOI] [PubMed] [Google Scholar]
- 30.Kemper C, Atkinson JP, Hourcade DE. Properdin: Emerging Roles of a Pattern-Recognition Molecule. Annu Rev Immunol. 2010;28:131–155. doi: 10.1146/annurev-immunol-030409-101250. [DOI] [PubMed] [Google Scholar]
- 31.Ricklin D, Lambris JD. Progress and Trends in Complement Therapeutics. Adv Exp Med Biol. 2013;734:1–22. doi: 10.1007/978-1-4614-4118-2_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Botto M, Kirschfink M, Macor P, Pickering MC, Würzner R, Tedesco F. Complement in human diseases: Lessons from complement deficiencies. Mol Immunol. 2009;46:2774–2783. doi: 10.1016/j.molimm.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 33.DeZern AE, Brodsky Ra. Paroxysmal Nocturnal Hemoglobinuria. Hematol Oncol Clin North Am. 2015;29:479–494. doi: 10.1016/j.hoc.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roumenina LT, Loirat C, Dragon-Durey MA, Halbwachs-Mecarelli L, Sautes-Fridman C, Fremeaux-Bacchi V. Alternative complement pathway assessment in patients with atypical HUS. J Immunol Methods. 2011;365:8–26. doi: 10.1016/j.jim.2010.12.020. [DOI] [PubMed] [Google Scholar]
- 35.Brodsky Ra, Young NS, Antonioli E, Risitano AM, Schrezenmeier H, Gaya A, Coyle L, De Castro C, Fu C, Maciejewski JP, Bessler M. Multicenter phase 3 study of the complement inhibitor eculizumab for the treatment of patients with paroxysmal nocturnal hemoglobinuria. Assessment. 2008;111:1840–1847. doi: 10.1182/blood-2007-06-094136. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.