Abstract
Biofilm formation by Bacillus subtilis is largely governed by a circuit in which the response regulator Spo0A turns on the gene for the anti-repressor SinI. SinI, in turn, binds to and inactivates SinR, a dedicated repressor of genes for matrix production. Mutants of the genes ylbF, ymcA, and yaaT are blocked in biofilm formation, but the mechanism by which they act has been mysterious. A recent report attributed their role in biofilm formation to stimulating Spo0A activity. However, we detect no measurable effect on the transcription of sinI. Instead, we find that the block in biofilm formation is caused by an increase in the levels of SinR and of its mRNA. Evidence is presented that YlbF, YmcA and YaaT interact with, and control the activity of, RNase Y, which is known to destabilize sinR mRNA. We show that the processing of another target of RNase Y, cggR-gapA mRNA, similarly depends on YlbF and YmcA. Our work suggests that sinR mRNA stability is an additional posttranscriptional control mechanism governing the switch to multicellularity and raises the possibility that YlbF, YmcA, and YaaT broadly regulate mRNA stability as part of an RNase Y-containing, multi-subunit complex.
Keywords: Biofilm formation, B. subtilis, SinR, RNase Y, mRNA turnover
Introduction
Many bacteria are capable of forming multicellular communities known as biofilms in which the cells are held together by a self-produced extracellular matrix (Kolter & Greenberg, 2006). Wild isolates of the soil-dwelling, model bacterium Bacillus subtilis form highly structured biofilms on both solid surfaces and at the air/liquid interface in liquid medium (pellicles) (Branda et al., 2001, Kolter & Greenberg, 2006, Aguilar et al., 2007). The B. subtilis matrix is composed of an exopolysaccharide that is produced by enzymes of the fifteen-gene espA-O (henceforth eps) operon and an amyloid-like protein TasA, which is encoded by the tapA-sipW-tasA (henceforth tapA) operon (Branda et al., 2004, Chai et al., 2008, Chu et al., 2006). An ongoing challenge is to identify all of the genes involved in biofilm formation and to understand how they function. Here we report on two genes, ylbF and ymcA, whose function in biofilm formation has been mysterious.
The principal dedicated regulator of biofilm formation in B. subtilis is SinR (Kearns et al., 2005), a direct repressor of both the eps and tapA operons (Chu et al., 2006). A distinctive feature of SinR is that biofilm formation and matrix gene expression are hypersensitive to small perturbations in SinR levels. Thus, a small increase in SinR levels blocks biofilm formation and a small decrease results in hyper-wrinkly biofilms (Chai et al., 2011, Subramaniam et al., 2013). This hypersensitivity is a result of both the molecular titration of SinR by SinI as well as to the cooperative nature of SinR binding to DNA (Norman et al., 2013, Newman et al., 2013). Under biofilm-inducing conditions, matrix genes are derepressed through the production of the anti-repressor SinI (Fig. 1A), which binds to and antagonizes SinR (Bai et al., 1993). Production of the antirepressor also derepresses the gene for an additional antagonist of SinR (SlrR), amplifying the response to SinI (Chai et al., 2010, Chu et al., 2008).
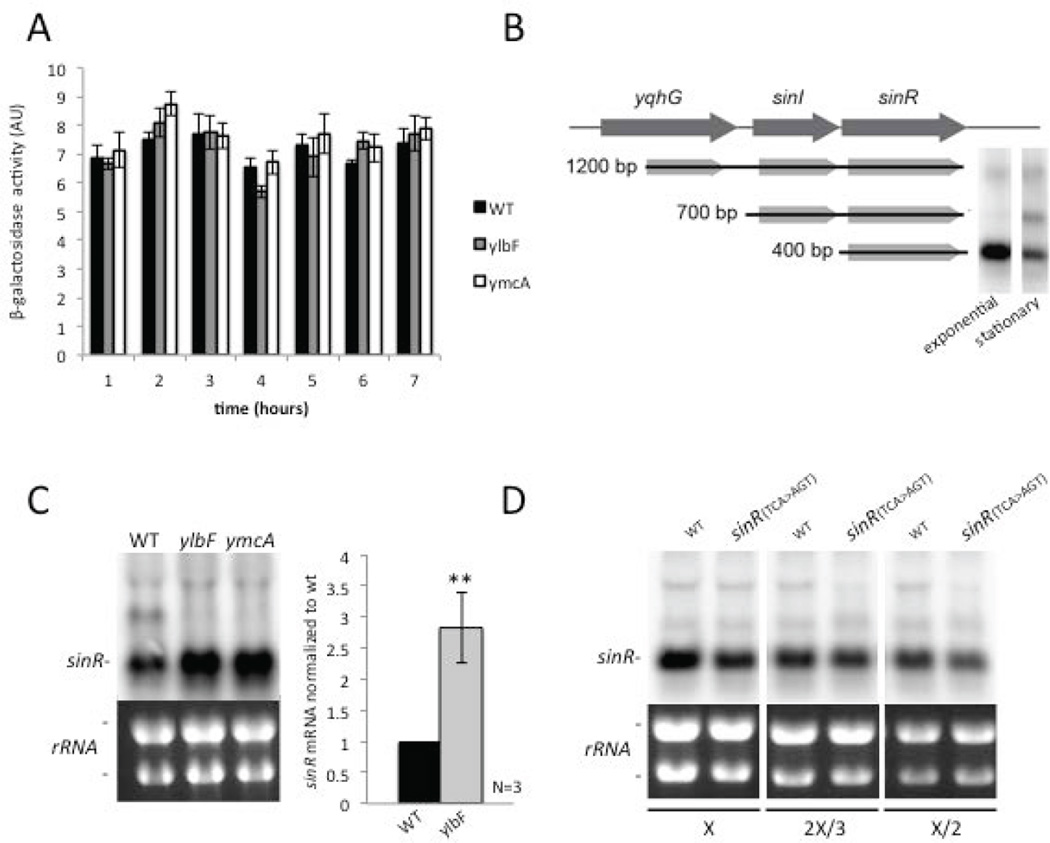
Figure 1. YlbF, YmcA and YaaT function independently of Spo0A.
Panel (A) Diagram of the Spoo0A-controlled regulatory circuit governing biofilm formation. Dashed line indicates protein-protein interaction and solid lines indicate transcription regulation. Panel (B) Synthesis of β-galactosidase under the control of the sinI promoter (PsinI -lacZ) in liquid shaking MSgg. Shown is a comparison of synthesis by the wild type (◆)(RL4610) with that of ylbF (■)(AJD95), ymcA (●)(AJD96), and spo0A (▲)(AJD97) mutants (averages plotted for 3 biological replicates). Panel (C) Comparison of SinI levels by western blotting carried out as previously described (Chai et al., 2008). Shown is a comparison of levels of the wild type (wt) (RL3852) with that of ylbF (RL4168) and ymcA (RL4169) mutants in liquid shaking MSgg during stationary phase. A western blot with an antibody against σA, a component of RNA polymerase, was used as a loading control. The same samples were loaded at two concentrations (left 1×, right 0.5×) to confirm linearity of the blot. Panel (D) Same as B except the synthesis of β-galactosidase was under the control of the abrB promoter (PabrB –lacZ). Wild type (◆)(AJD99) is compared to mutants of ylbF (■)(AJD100), yaaT (▲)(AJD102), and spo0A (✕) (AJD101). For this panel the β-galactosidase activity is reported as a percentage of the activity at the initial, 2 hour time point. Panel (E) Shown are growth curves for the wild type (■) (RL3852), and the mutants ylbF (□) (RL4168), ymcA (Δ) (RL4169), and spo0A (◆) (RL4620) in LB medium. The times in panels B, C and D are hours after inoculation of the culture, corresponding to an OD600 of ~0.05.
The synthesis of SinI is controlled by the response regulator Spo0A, which in its phosphorylated form (Spo0A~P) turns on the sinI gene (Molle et al., 2003). The phosphorylation of Spo0A is governed by the histidine kinases KinA, KinB, KinC, and KinD (Jiang et al., 2000, McLoon et al., 2011), which feed phosphoryl groups into a phosphorelay consisting of the phosphotransfer proteins, Spo0B and Spo0F (Tzeng et al., 1998). A second regulatory protein, AbrB, a global repressor of many stationary phase genes, contributes to the control of the eps and tapA operons. In this case, AbrB is a repressor of matrix genes and Spo0A~P relieves this repression by repressing the gene for AbrB (Chu et al., 2008, Molle et al., 2003, Banse et al., 2008, Hamon & Lazazzera, 2001, Hamon et al., 2004).
The ylbF and ymcA genes were revealed in a genetic screen for mutants that were strongly blocked in biofilm formation and matrix gene expression (Branda et al., 2004, Kearns et al., 2005). Subsequent, pull down experiments both by Carabetta et al. (2013) and independently by us (as reported here) revealed that YlbF and YmcA are in a complex with each other and with a third protein YaaT. A mutant lacking YaaT is also impaired in biofilm formation. Additional phenotypes have also been attributed to mutants lacking YlbF, YmcA, and YaaT, including impaired genetic competence and impaired sporulation (Carabetta et al., 2013, Tortosa et al., 2000).
What is the function of this complex and why is it required for biofilm formation and other aspects of B. subtilis growth and development? Carabetta et al. reported that the YlbF-YmcA-YaaT complex works by accelerating the phosphorylation of Spo0A (Carabetta et al., 2013). However, as we report here, no significant defect in Spo0A~P-directed gene expression, including transcription of sinI, accumulation of SinI protein and repression of abrB, was detected under biofilm-inducing conditions in mutants of the complex. At the same time, we observe phenotypes, such a growth defect in rich medium that are not exhibited by a Spo0A mutant. A further indication that the complex has a role outside of the phosphorelay is that all three proteins are co-conserved in Gram-positive bacteria that lack Spo0A and components of the phosphorelay.
Here, we present evidence that mutants lacking YlbF or YmcA exhibit an increase in SinR levels and that this increase is sufficient to explain the block in biofilm formation. We also present evidence that the YlbF-YmcA-YaaT complex exerts its effects at the level of the mRNA for SinR and does so via direct interaction with the endoribonuclease RNase Y.
Results
Spo0A activity is not dependent on YlbF, YmcA, and YaaT under biofilm-inducing conditions
A possible explanation for the block in biofilm formation observed in mutants lacking YlbF, YmcA, or YaaT is that these proteins are needed for efficient phosphorylation of Spo0A, which, in turn, activates the gene for SinI. To investigate this possibility we measured sinI expression using a lacZ fusion in wild (biofilm proficient) cells (strain 3610) and cells of mutant derivatives lacking YlbF or YmcA. In liquid, shaking biofilm-inducing medium (MSgg), the pattern of induction of PsinI-lacZ in stationary phase was indistinguishable between the mutants and the wild type (Fig. 1B) As a control, and as expected, little or no expression was observed in a mutant lacking Spo0A (Fig. 1B). Also, the absence of YlbF or YmcA did not affect the levels of SinI protein as judged by immunoblot analysis using anti-SinI antibodies (Fig. 1C).
Spo0A~P also contributes to biofilm formation by repressing the gene for AbrB, which is expressed at high levels during exponential phase growth and repressed in stationary phase in a Spo0A-dependent manner. Using a lacZ fusion to the promoter for abrB (PabrB–lacZ), we observed little difference in the pattern of PabrB–lacZ expression between the wild type and cells lacking YlbF or YaaT (Fig. 1D). In toto, the results are inconsistent with the idea that the block in biofilm formation observed in the mutants is due to impaired Spo0A activity.
The absence of YlbF, YmcA or YaaT, but not Spo0A, impairs growth in LB medium
Also distinguishing the phenotype of mutant derivatives of the wild strain (3610) lacking YlbF, YmcA, and YaaT from a mutant lacking Spo0A is a growth defect in LB medium. Unlike the wild type, cultures of ylbF, ymcA, and yaaT mutant cells exhibited a conspicuous and reproducible dip in growth at the onset of stationary phase measured by the OD600 in liquid shaking medium. Specifically, the mutants grew at the same rate as the wild type until the cultures reached an OD600 of about 1.0 at which point the mutants exhibited a short period of reduced optical density and then resumed growth, ultimately reaching a density comparable to that of wild-type (Fig. 1E). (By contrast, little growth impairment was seen in the biofilm-inducing medium MSgg.) Importantly, no such growth impairment was observed for mutants lacking Spo0A or the phosphorelay proteins Spo0B and Spo0F (Supporting information Fig. S1A). Live/dead staining indicated that impaired growth was associated with cell death in ylbF, ymcA, and yaaT mutants. Again, no evidence of cell death was observed for a spo0A mutant or mutants lacking the other phosphorelay components (Supporting information Fig. S1B). Another phenotype that distinguishes the mutants of ylbF, ymcA, and yaaT from mutants of components of the phosphorelay is colony morphology on solid LB agar (1.5%). The ylbF, ymcA, and yaaT mutants produced colonies that were smaller than those of the wild type, flat, and smooth, whereas mutants lacking Spo0A, produced colonies that were very large with a distinct mucoid texture (Supporting information Fig. S1C). The results so far indicate that YlbF, YmcA, and YaaT have a function that is distinct from the phosphorelay.
A final indication that YlbF, YmcA, and YaaT have a distinct function is based on gene conservation (Supporting information SF23). Homologs of ylbF, ymcA, and yaaT can be found in many Gram-positive bacteria, including most of the Firmicutes. These species lack homologs of spo0A and other genes for components of the phosphorelay.
SinR levels are higher in ylbF and ymcA mutants
Because, as we have shown, ylbF, ymcA and yaaT mutations do not act by impairing Spo0A activity or the production of SinI, we next considered the possibility that they exert their effect by influencing the levels of SinR. It is known that even a single extra copy of sinI is sufficient to block biofilm formation (Chai et al., 2011). Also, in previous work we found that under biofilm-inducing conditions four of the six synonymous serine codons (TCN), henceforth called serine-sensitive codons, allow for less efficient translation of the sinR mRNA than the other two (AGC or AGT), henceforth called serine-insensitive codons. A strain in which several of the serine-sensitive codons were switched to serine-insensitive codons was found to have slightly higher levels of SinR (approximately 1.6 fold higher) and to be completely defective in biofilm formation, representing independent evidence that biofilm formation is hypersensitive to SinR levels (Subramaniam et al., 2013).
We therefore carried out immunoblot analysis to investigate the relative levels of SinR in wild type cells and in mutant cells lacking YlbF and YmcA using antibodies against SinR that we had employed in previous studies with SinR (Chai et al., 2008, Chai et al., 2010, Chai et al., 2011). Protein was extracted from stationary phase cells grown under biofilm-inducing conditions and tested for SinR levels at three different concentrations of protein. SinR levels were found to be 1.8 ± 0.07 and 1.7 ± 0.3 fold higher, respectively, in mutants lacking YlbF and YmcA than in the wild type. In contrast, SinR levels were unaltered in a spo0A mutant, a result that indicates that the phosphorelay does not influence SinR levels (Fig. 2A and 2B). For each strain the quantification of the SinR band was normalized to the quantification of the SigA band, used as a loading control. As a control, the band identified as SinR was absent from a lysate of a mutant lacking sinR (Fig. 2D).
Figure 2. Mutants of the complex have heightened levels of SinR protein.
Panel (A) Comparison of SinR levels by western blotting using an antibody against SinR. Shown is a comparison of levels of the wild type (wt) (RL3852) with that of ylbF (RL4168), ymcA (RL4169), and spo0A (RL4620) mutants in liquid shaking MSgg during stationary phase. The levels are compared in a dilution series as indicated. Note that the signal intensity for SinR varied from blot to blot, but in each case the wild type and the mutant were included on the same blot. The ratio of SinR/SigA signal was then normalized to wild type for quantification. Panel (B) The densitometry of SinR bands was normalized against the densitometry of σA bands in panel A. The ratio of SinR/σA was then normalized to wild type (average fold change plotted for three biological replicates). Panel (C) Colony morphology phenotypes of wt (3610) and ylbF (RL4168), ymcA (RL4169), and sinR (TCA>AGT) (YC923) mutants grown for 72 hrs on MSgg 1.5 % agar plates. (Matrix production is indicated by wrinkly morphology and colony spreading. All images are at the same scale) Panel (D). Comparison of SinR levels between wt (RL3852) and ylbF (RL4168), ymcA (RL4169), and sinR(TCA>AGT) (YC923) mutants using an antibody against SinR. Panel (E). Colony morphology phenotypes of the wild strain (3610), a mutant of ylbF (RL4168), a mutant of sinR with increased number of serine-sensitive codons sinR(AGT>TCA) (YC1173), and double mutant of ylbF sinR(AGT>TCA) (AJD205) grown for 72 hrs on MSgg 1.5% agar (all images are at the same scale). For Panels A and E, a western blot against σA was used as a loading control.
To show that the increase in SinR levels in the cells lacking YlbF and YmcA was sufficient to block biofilm formation, the level of SinR was compared between the mutants and a strain harboring mutations causing synonymous switches to serine-insensitive codons (TCA>AGT) in sinR that cause a complete block in biofilm formation. The increase in SinR levels in cells lacking YlbF and YmcA was comparable to the increase seen with the synonymous mutations, and the biofilm phenotype defect (flat, unwrinkled colonies that do not spread as much as the wild type) was approximately the same in all cases (Fig. 2C, D) (Subramaniam et al., 2013).
As a further test of whether this enhanced level of SinR could be responsible for the block in biofilm formation, we again turned to the effect of switching serine codons to see if we could restore biofilm formation to mutants of ylbF. This time we took advantage of a strain in which serine-insensitive codons in sinR were switched to serine-sensitive codons, which was known to result in a small decrease in SinR levels and the production of hyper-wrinkled biofilms (Subramaniam et al., 2013). We therefore introduced the serine-sensitive-codon-containing gene into a YlbF mutant. The results show that the mutant sinR gene was able to suppress the biofilm phenotype caused by the ylbF mutation, restoring a wrinkled colony phenotype (Fig. 2E).
sinR mRNA levels are higher in ylbF and ymcA mutants
As we have shown, the levels of SinR are higher in cells lacking YlbF and YmcA during stationary phase. One explanation could be an increase in the transcription of sinR, which is thought to be transcribed constitutively. To investigate whether the mutations were increasing the transcription of sinR we measured expression using a fusion of the promoter immediately upstream of sinR to lacZ. The results confirmed that sinR is transcribed constitutively, revealing little or no effect of the ylbF and ymcA mutations on expression levels (Fig. 3A).
Figure 3. Mutants of the complex have heightened levels of sinR mRNA.
Panel (A) Comparison of β-galactosidase synthesis under the control of the sinR promoter (PsinR -lacZ) in liquid shaking MSgg between the wild type (wt) (AJD30) and mutants of ylbF (AJD31) and ymcA (AJD32) (average plotted for 2biological replicates). Panel (B) Schematic depicting the transcription of the sinI/sinR operon and a northern blot with a probe against sinR mRNA with RNA samples from exponential and stationary phase cells in MSgg medium. Panel (C) Northern blot with a probe against the sinR mRNA of samples collected in stationary phase in MSgg medium for the wild type (wt) (RL3852) as well as for mutants of ylbF (RL4168) and ymcA (RL4169). Also shown is quantification of the densitometry of the sinR mRNA normalized to wild type (average plotted for 3 biological replicates) Panel (D) Northern blot with a probe against the sinR mRNA of samples collected in stationary phase in MSgg medium for wt (RL3852) and sinR(TCA>AGT) (YC923). Also in included in Panels C and D as loading controls are the two predominant rRNAs (16S and 23S) as visualized by ethidium bromide staining. RNA samples for the northern blots in C and D were loaded at the indicated dilutions.
Next, we investigated whether the mutations were influencing the level of the sinR mRNA by carrying out northern blot analysis using a probe against the sinR message. The gene for sinR is transcribed from three promoters, resulting in three different sized transcripts (Fig. 3B)(Lehnik-Habrink et al., 2011b). The predominant transcript during exponential phase growth originates from a promoter just upstream of sinR. This 400-base transcript contains the sinR coding sequence but not the coding sequence for the adjacent, upstream sinI gene (Supporting information Fig S2). The second transcript, which is 700-base, contains both sinR and sinI mRNAs and originates from a promoter under the control of Spo0A~P just upstream of sinI (Lehnik-Habrink et al., 2011b). The third transcript originates from within the gene (yqhG) upstream of sinI resulting in a transcript of 1.2 kb. This transcript is thought to be constitutively expressed at a low level and is present during both exponential growth and stationary phase. We found that the level of the sinR-only transcript was increased in cells lacking YlbF or YmcA during stationary phase, approximately 2.8 fold in the case of YlbF (Fig. 3C). As further control, we again turned to the use of the mutant sinR in which serine-sensitive codons were changed to serine-insensitive codons [sinR(AGY>TCA)]. Given that the effect of synonymous serine codons is exerted by affecting translation, we expected that the levels of sinR mRNA in the synonymous mutant would be unaltered. The results of Fig. 3D show that this was the case.
YlbF, YmcA and YaaT are associated and co-conserved with the endoribonuclease RNase Y
How does the absence of YlbF and YmcA cause sinR mRNA levels to increase? We and Dubnau and colleagues (Carabetta et al, 2013) have independently carried out pull-down experiments to identify binding partners for YlbF and YmcA. We used YlbF in our experiments, which identified YmcA and YaaT as binding partners (Supporting information Table S1). Carabetta et al (2013) similarly obtained compelling evidence, that YlbF, YmcA and YaaT are in a complex, carrying out their pull down experiments with all three proteins and carrying out size exclusion chromatography. Interestingly, however, these pull down experiments revealed several additional proteins, one of which was the endoribonuclease RNase Y (Rny). RNase Y was seen in our pull down experiment with YlbF (Supporting information Table S1) and in the pull-down experiment with YaaT carried out by Carabetta et al. (2013; Fig S3 in the supporting materials).
RNase Y provided an intriguing explanation for the increased levels of sinR mRNA in the mutants for several reasons. First, and most importantly, not only does RNase Y play a general role in mRNA turnover (Durand et al., 2012), but it has already been implicated in destabilizing the message for sinR in an unbiased analysis for RNAs whose levels increased in a strain depleted for RNase Y (Lehnik-Habrink et al., 2011b). Second, the gene that encodes RNase Y (rny) is near to the gene for YmcA (being separated by 5 genes), and this synteny is conserved among gram-positive bacteria that have homologs of ymcA (Supporting information SF3).
Finally, we note that none of the components of the phosphorelay were detected in the pull down experiments described here as well those of Carabetta et al. (2013).
An RNase Y mutant mimics the phenotypes of YlbF and YmcA mutants
If YlbF, YmcA, and YaaT are acting with Rny to reduce SinR levels, we would predict that a strain lacking RNase Y would be blocked for biofilm formation. The rny gene had been believed to be essential and indeed the investigation of Lehnik-Habrink et al. (2011b) used a construct in which the levels of RNase Y could be depleted rather than using a mutation. In light of recent work showing that the gene for RNase Y is not required for viability (Figaro et al., 2013), we sought to investigate the effect of a null mutation in the biofilm-proficient, wild strain 3610. Also, because the gene for RNase Y is the upstream member of a two-gene operon, it was important to confirm that the observed effects were not due to a polar effect on the downstream member of the operon. A markerless deletion of the gene for RNase Y (kindly provided by Byoung Mo Koo and Carol Gross from whom we first learned that the gene is not needed for viability) was created in a 3610 background. The resulting strain was in fact completely defective for biofilm formation, producing small, flat colonies (Fig 4A). In addition, the mutation blocked expression of the SinR-repressed promoter for the matrix operon eps as judged using a lacZ reporter (Fig. 4D). Importantly, biofilm formation was restored by the presence of a copy of the gene for RNase Y under the control of its native promoter at an ectopic locus (Fig. 4A).
Figure 4. An RNase Y mutant phenocopies mutants of the complex.
Panel (A) Colony morphology phenotypes of wild type (WT) (3610), an rny mutant (AJD204), and an rny mutant bearing a complementation construct for rny (amyE::Prny-rny) (AJD211) grown for 72 hrs on MSgg 1.5% agar plates. Panel (B) Northern blot with a probe against sinR of samples collected in stationary phase in MSgg medium for wt (RL3852) and an rny mutant (AJD204). As a loading control, the two predominant rRNAs (16S and 23S) were visualized by ethidium bromide staining. Panel (C) Biofilms produced by wt (3610), a mutant of rny (AJD204), a muant of sinR with increased serine-sensitive codons sinR(AGT>TCA) (YC1173), and a double mutant an rny mutant combined with sinR(AGT>TCA) (AJD210) grown for 72 hrs on MSgg 1.5% agar (images at the same scale). Panel (D) Synthesis of β-galactosidase under the control of the epsA promoter (PepsA –lacZ) in liquid shaking MSgg. Shown is a comparison of synthesis by the wt (■) (RL4547) with that of ylbF (△) (AJD6), ymcA (✕) (AJD7), and rny (○) (AJD209) mutants. The lines depicting the activity in the ylbF, ymcA, and rny mutants are overlapping. Panel (E) Growth curve in LB medium. Shown are the growth curves for the wild type (◆) (RL3852), and the mutants ylbF (●) (RL4168), ymcA (▲) (RL4169), and rny (■) (AJD204).
Next, we investigated the effect of the rny null mutation on sinR mRNA levels. We found that the mutation caused a similar increase in the levels of the sinR-only transcript to that seen in a ylbF or ymcA mutant (Fig 4B). To determine if the biofilm defect could be attributed to increased levels of SinR, we introduced an altered sinR enriched in serine-sensitive codons (as employed above for ylbF) into an RNase Y mutant. The mutant sinR was in fact capable of restoring biofilm formation to the rny mutant (Fig 4C).
Finally, we investigated whether the RNase Y mutation would mimic the growth defect exhibited by YlbF and YmcA mutants in LB medium. Indeed, in a 3610 background cells lacking RNase Y showed the same impaired growth in liquid LB as mutants lacking YlbF and YmcA (Fig. 4E).
YlbF and YmcA interact directly and independently with RNase Y
To further investigate whether YlbF and YmcA interact directly with RNaseY, we carried out bacterial two-hybrid experiments using as “bait” fusions of YlbF or YmcA to λ CI repressor and as “prey” a fusion of the cytosolic domain of RNase Y to the N-terminal domain of the α subunit of RNA polymerase (Deighan et al., 2008). We used the cytosolic domain because RNase Y is an integral membrane protein with a 25-residue long transmembrane domain at its N-terminus, and we reasoned that if YlbF and YmcA interact with RNase Y, it would likely have to be with the cytosolic domain of the endoribonuclease. As a positive control, we used the β-flap of RNA polymerase as bait and σ70 as prey (Kuznedelov et al., 2002, Deighan et al., 2008, Deaconescu et al., 2006). As negative controls we used either λ CI without a bait fused to it tested against the indicated prey proteins or α without a fused prey tested against the indicated bait proteins. As further negative controls, we used λ CI fused to β-flap tested against RNase Y or α fused with σ70 tested against Rny, YlbF and YmcA. The results show that both YlbF and YmcA interacted with the cytosolic domain of RNase Y, with the YlbF interaction being particularly robust (Fig. 5). We also observed strong interaction of YlbF with YmcA, in keeping with the finding that these proteins interact with each other, and of RNase Y with itself.
Figure 5. Bacterial two-hybrid assays show that YlbF and YmcA interact directly and independently with RNase Y.
The β-galactosidase activity for the indicated pairs of proteins tested in the reporter strain E. coli FW102 OL2–62 in which lacZ expression is dependent on a direct interaction (average plotted for 2 biological replicates). The strains tested are AJD221-241, for details see supporting information.
Finally, we asked whether we could detect an interaction of YlbF or YmcA with the phosphorelay protein Spo0B, as previously reported (Carabetta et al., 2013). The results failed to reveal such an interaction (Fig. 5).
Cleavage of a well-studied target of RNase Y depends on YlbF and YmcA
The central glycolytic gene repressor cggR is co-transcribed with the adjacent downstream gene gapA to yield a 2.2 kb, cggR-gapA transcript (as well as longer transcript extending further downstream). Previous studies have shown that RNase Y cleaves the 2.2. kb transcript between cggR and gapA, producing a 1.0 kb transcript that contains most of cggR and a 1.2 kb transcript containing gapA (Fig. 6A)(Ludwig et al., 2001, Commichau et al., 2009). If, as we posit, RNase Y function depends on YlbF and YmcA, then little of the 1.0 kb transcript cleavage should be seen in mutants lacking either protein. Using probes against cggR and gapA we indeed found that the 2.2 kb cggR-gapA transcript is processed into 1.0 kb and 1.2 kb transcripts in the wild type and that this cleavage was strongly dependent on YlbF and YmcA (Fig. 6B). Thus, a minimum of two previously described targets of RNase Y (sinR and cggR-gapA) depend on YlbF and YmcA for their cleavage.
Figure 6. The cleavage of cggR-gapA mRNA by RNase Y is dependent on YlbF and YmcA.
Panel (A) Schematic depicting the transcription of the cggR-gapA operon and transcripts resulting from the cleavage by RNase Y. Panel (B) Northern blot with a probe against the cggR mRNA and against gapA mRNA of samples collected in stationary phase in MSgg medium for wt (RL3852), and the mutants of ylbF (RL4168) and ymcA(RL4169). The rRNA bands were visualized by methylene blue staining and imaged with a Sony digital camera.
Discussion
A principal contribution of this investigation is evidence that the dependence of biofilm formation on the YlbF-YmcA-YaaT complex occurs at the level of sinR mRNA. We have shown that mutants of the three proteins cause an increase in the abundance of SinR, that this increase is sufficient to explain the block in biofilm formation, and that this increase is due to an increase in sinR mRNA.
We have also obtained compelling evidence that the complex controls the levels of sinR mRNA through a functional interaction with the ribonuclease Rny, which is known to destabilize the sinR mRNA (Lehnik-Habrink et al., 2011b). This conclusion is supported by the following: First, an rny mutant mimics the phenotypes of mutants of the complex, including causing a block in biofilm formation and a growth defect in a rich medium. Also, like mutants of ylbF and ymcA, a mutant of rny has been reported to be impaired for genetic competence and sporulation (Figaro et al., 2013). Second, RNase Y was found to be associated with the complex in independent pull-down experiments carried out using YlbF in the present work and by Carabetta et al. (2013) using YaaT. Third, Rny and YaaT have similar patterns of subcellular localization. RNase Y is known to be a membrane protein (it has a transmembrane segment at its N-terminus) and it has been reported to localize to the periphery of the cell and to form foci at the division septum (Burmann et al., 2012, Lehnik-Habrink et al., 2011a). Meanwhile, YaaT (which does not have a predicted transmembrane domain) has also been reported to localize to the cell periphery and to the division septum (Hosoya et al., 2002). Fourth, and importantly, bacterial two-hybrid experiments revealed that YlbF and YmcA interact directly and independently with RNase Y. Finally, the processing of a well characterized target of RNase Y, the cggR-gapA operon, was found to be dependent on YlbF and YmcA. This is a striking result as the processing of the cggR-gapA mRNA is independent of both biofilm formation and the phosphorelay. In light of these considerations, we propose that YlbF, YmcA, and YaaT be renamed RcsA, RcsB, and RcsC, respectively, for RNase Y-containing complex subunits A, B and C.
The RcsA-RcsB-RcsC (YlbF-YmcA-YaaT) complex was previously reported to control Spo0A activity and to do so by accelerating the phosphorylation of Spo0A. Our work shows that the complex has a function independent of the phosphorelay. Specifically, we have shown that mutants of the complex are not impaired for Spo0A activity under biofilm-inducing conditions. Strictly speaking, we cannot rule out the possibility that the complex both stimulates flux through the phosphorelay and independently causes degradation of mRNAs for SinR and other proteins (such as proteins involved in growth in rich medium). However, a more parsimonious explanation for the results reported by Carabetta et al. (2013) is that under certain conditions or in certain genetic backgrounds rcsA (ylbF), rcsB (ymcA), and rcsC (yaaT), mutations raise the levels of mRNAs for metabolic enzymes that influence the activity of the histidine kinases that feed phosphoryl groups into the relay or, conceivably, of negative regulators of the phosphorelay, such as Spo0E, MecA, or the Rap phosphatases.
Our work showing that RcsA, RcsB, and RcsC function via an interaction with Rny raises two important questions. One is whether the complex influences the specificity of RNase Y. RNase Y is known to play a general role in mRNA turnover (Durand et al., 2012; Shahbabian et al., 2009, (Lehnik-Habrink et al., 2011b)) but also preferentially destabilizes certain mRNAs such as that for sinR (Lehnik-Habrink et al., 2011b). It will therefore be interesting to carry out RNA-seq experiments with mutants of each of the complex proteins as well as with an mutants lacking RNase Y to identify additional candidates for substrates and to investigate the possibility that a subset of RNase Y targets depend on only one or more of the proteins.
A second question raised by our work is whether the activity of the complex is influenced by physiological or environmental conditions. For example, might the capacity of the complex to degrade sinR mRNA be dependent on growth and/or medium conditions? That is, is sinR mRNA less stable under biofilm-inducing conditions than during exponential phase growth or in medium that does not support biofilm formation? If so, then the RNase Y-RcsA-RcsB-RcsC complex might serve as an environmental or metabolic sensor that operates at the level of mRNA stability.
SinR is emerging as a hub for at least three inputs in the decision to form a biofilm, all operating at a post-transcriptional level. First, SinR is subject to antagonism by the anti-repressor SinI, whose synthesis is under the control of Spo0A~P via the phosphorelay (Kearns et al., 2005). Second, sinR mRNA is subject to a serine sensing mechanism mediated by serine-sensitive codons, which respond to the metabolic state of the cell (Subramaniam et al., 2013). Third, as we have now seen, sinR mRNA is subject to degradation by a multi-protein complex and conceivably, as we hypothesize above, this complex is itself responding to environmental conditions. In this view the formation of a multicellular community is a pivotal decision for B. subtilis that is based on the integration of multiple environmental and metabolic inputs that are channeled into the synthesis or activity of SinR.
Materials and methods
Bacterial strains and media
In general, Bacillus subtilis strains PY79, 3610, and their derivatives as well as Escherichia coli strain DH5α were grown in Luria-Bertani (LB) medium (10 g tryptone, 5 g yeast extract, and 5 g NaCl per liter) in liquid shaking culture or on agar plates (1.5% Bacto-agar) at 37°C. When necessary antibiotics were added to the media, for B. subtilis strains: spectinomycin (100 µg ml−1), kanamycin (10 µg ml−1), chloremphinicol (5 µg ml−1), and mls (erythromycin 0.5 µg/ml and lincomycin 2.5 µg/ml), and for E. coli: ampicillin (100 µg ml−1), chloramphenicol (20 µg ml−1), and kanamycin (10 µg ml−1). Biofilm formation was induced by growth on the minimal medium MSgg: 5 mM potassium phosphate (pH7.0), 100 mM MOPS (pH 7.0), 2 mM MgCl2, 700 µM CaCl2, 50 µM MnCl2, 50 µM FeCl3, 1 µM ZnCl2, 2 µM thiamine, 0.5% glycerol, 0.5% glutamic acid, 50 µg ml−1 tryptophan, 50 µg ml−1 threonine, and 50 µg ml−1 phenylalanine. MSgg was supplemented with 1.5% Bacto-agar for solid plates.
Strain construction
Strains were constructed using standard genetic cloning protocols for B. subtilis (Sambrook 2001). Antibiotic marked fragments of DNA were introduced into PY79 by transformation (Wilson & Bott, 1968) and into 3610 by SPP1 phage transduction (Yasbin & Young, 1974). The markerless deletion of yaaT was created using the pminimad system as previously described (Arnaud et al., 2004, Patrick & Kearns, 2008, Chen et al., 2009). An insertional deletion of rny was obtained from Byoung Mo Koo and the marker was removed through a cre recombinase system. Synonymous mutants of sinR were generated at the native locus using synthetic DNA fragments followed by allelic exchange as previously described (Subramaniam et al., 2013). The complementation strain of rny was created by integrating rny with its native promoter into the chromosome at amyE. Reporter strains were created by fusing lacZ to the promoter for the gene of interest and integration into the chromosome at amyE by cloning the promoter into the plasmid pDG268 (Antoniewski et al., 1990). The PsinR-lacZ was integrated into the chromosome at an alternative amyE site inserted into bkdB near the terminus (strain AHB290 a gift from A. Camp) also described in (Chai et al., 2011). The promoter for sinR was amplified using the primers PsinRF1 and PsinRR1 and contains 190 bp directly upstream of the sinR open reading frame. Strain genotypes, full construction details, and a list of plasmids used is available in the supplemental materials.
β-galactosidase assays
The following protocol was adapted from a previously established protocol adapted to a plate reader (Kearns et al., 2005). Cultures were grown overnight in LB shaking at 25°C. The cultures were diluted back to an O.D.600 of 0.05 in liquid MSgg. Samples were collected at the indicated times for the analysis of β-galactosidase activity. One milliliter of cells were collected by centrifugation and resuspended in 500 µL of Z buffer (40 mM NaH2PO4, 60 mM Na2HPO4, 1 mM MgSO4, 10 mM KCl, and 38 mM β-mercaptoethanol). To 96 well plates, 50 µL of each resuspension as well as 50 µL of a 5-fold and 25-fold dilution were added to 50 µL of Z buffer containing lysozyme (0.2 mg ml−1 final concentration). Plates were incubated at 37°C for 25 min. Reactions were started by the addition of 20 µL of 4 mg ml−1 2-nitrophenyl β-D-galactopyranoside in Z buffer. The absorbance at 420 nm was read on a plate reader every minute for one hour. The reported activity was calculated according to the following equation: dilution factor × VmaxOD420/OD600.
Western blotting
For western blots, cleared whole cell lysates were run on 12.5% SDS-PAGE nupage gels (Life Technologies) and transferred to a PVDF membrane (Millipore, Billerica, MA) by electro blotting (Bio Rad, Hercules, CA). Bands were compared to the Benchmark Prestained Protein Ladder (Life Technologies). Samples were normalized to total protein concentration determined by Bradford assay (Bio Rad). Rabbit anti-SigA polyclonal antibodies were used at a concentration of 1:100,000. Rabbit anti-SinR polyclonal, affinity purified antibodies were used at a concentration of 1:1500. Rabbit anti-SinI polyclonal antibodies were used at a concentration of 1:5000. They were visualized with goat anti-rabbit antibodies conjugated to Horseradish Peroxidase at a concentration of 1:10,000 (Bio Rad) and were developed with SuperSignal West Dura chemiluminescent substrate (Thermo, Waltham MA). For full details for sample preparation and imaging see previous work (Subramaniam et al., 2013). For Fig 2B the quantification represents the average of biological triplicates and the standard deviation is reported. For each replicate the ylbF, ymcA, and spo0A mutants were normalized to wild type.
Northern blotting
Samples for northern blot analysis were prepared from cells grown in liquid shaking cultures of MSgg at an OD600 of 2.0–2.5. Preparations of total RNA samples from Bacillus subtilis were created using a Trizol extraction as previously described (Cheung et al., 1994). Samples were run on 1.5% agarose gels containing 20 mM guanidine thiocyanate (Goda & Minton, 1995). For a loading control, rRNA was visualized by ethidium bromide staining. RNA was transferred to Hybond-N+ membranes (Amersham Biosciences, Piscataway NJ) using a capillary transfer. Transcripts were detected using Digoxigenin (DIG) probes (Roche Diagnostics, Indianapolis IN). A probe against SinR was created by in vitro transcription with T7 RNA polymerase (Roche) using PCR generated DNA fragments as a template. The specific primers are detailed in the supplemental information. Hybridization and signal detection were carried out according to the manufacturer’s instructions (DIG RNA labeling and detection kit, Roche Diagnostics) with the exception that the sinR probe hybridization was carried out at 74°C. For cggR and gapA the probe was hybridized at 70°C. Bands were compared to the RNA Molecular Weight Marker III (Roche). For figure 3B quantification was carried out by measuring the densitometry of the sinR only transcript in ylbF and wild type. The average of 3 biological replicates is reported with the standard deviation. As loading controls rRNA was visualized either by ethidium bromide staining with UV imaging or methylene blue (0.25% methylene blue, 0.3 M sodium acetate pH 5.2) staining and imaging with a Sony digital camera as indicated in figure legends.
Image Quantification and Statistics
Densitometry analysis was performed using ImageJ software (http://rsbweb.nih.gov.ij) Rectangles were drawn around distinct bands and the average pixel intensity was measured. The same sized of the rectangle is used to quantify bands that are being compared, and the density of the background of the same size is subtracted from the measurement. For statistics the sample sizes are indicated in the legends. The error bars represent the standard deviation. Two-tailed P-values were determined based on unpaired t-tests. In images statistical significance is indicated as follows; “*” for P≤0.05, “**” for P≤0.01, and “***” for P≤0.001.
Bacterial two-hybrid
Each “Bait” protein was cloned with a N-terminal fusion of λCI repressor (residues 1–239 of λCI). The “bait” protein was cloned into the NotI and BamhI sites of pAC-CI using isothermal assembly. For each bait the entire ORF was used except for RNase Y, which 25 amino acid truncation of the N-terminus was used to remove a transmembrane domain. The “Prey” protein was cloned with a N-terminal fusion to the N-terminal domain of αRNAP (αNTD residues 1–248). The prey was cloned into the BamHI and NotI sites of pBRα using isothermal assembly. For a list of plasmids and primers see supporting information. Bait and Prey fusions to be tested were cotransformed into the reporter strain FW102 OL2–62, which contains a lacZ reporter. To test for an interaction, the reporter strain was grown in liquid shaking LB with appropriate antibiotics overnight at 37°. The next morning the cultures were back diluted 1:100 into LB (kan20, amp100, cm20) supplemented with 20 nM IPTG. One ml of each culture was collected after 3 hrs of shaking at 37° and the OD600 was measured. The pellet was resuspended in 1 ml of buffer Z (40 mM NaH2PO4, 60 mM Na2HPO4, 1 mM MgSO4, 10 mM KCl, and 38 mM β-mercaptoethanol). To this 100 µl of SDS (2.5%) and 50 µl of chloroform were added to lyse cells. The samples were briefly vortexed. To each sample, 200 µl of ONPG (4 mg ml−1) was added for 5 minutes. The reaction was stopped by the addition of sodium bicarbonate (500 µl of 1M). The samples were centrifuged on a benchtop centrifuge at 13K rpm for 5 minutes. The absorbance at 420 nm was determined for each of the supernatants. The miller unites were calculated as follows: (OD420/(OD600*1 ml*5 min))*1,000. For each pair tested two biological replicates. The standard deviation is reported.
Supplementary Material
Acknowledgments
We thank C. Lee, Alan Grossman, and Ole Skovgaard who helped us eliminate a model we considered prior to the one presented here. We thank Gene Wei-Li for insightful discussion. This work was funded by the National Institutes of Health grant GM18568.
References
- Aguilar C, Vlamakis H, Losick R, Kolter R. Thinking about Bacillus subtilis as a multicellular organism. Current Opinion in Microbiology. 2007;10:638–643. doi: 10.1016/j.mib.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniewski C, Savelli B, Stragier P. The spoIIJ gene, which regulates early developmental steps in Bacillus subtilis, belongs to a class of environmentally responsive genes. Journal of bacteriology. 1990;172:86–93. doi: 10.1128/jb.172.1.86-93.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnaud M, Chastanet A, Debarbouille M. New vector for efficient allelic replacement in naturally nontransformable, low-GC-content, gram-positive bacteria. Applied and environmental microbiology. 2004;70:6887–6891. doi: 10.1128/AEM.70.11.6887-6891.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai U, Mandic-Mulec I, Smith I. SinI modulates the activity of SinR, a developmental switch protein of Bacillus subtilis, by protein-protein interaction. Genes Dev. 1993;7:139–148. doi: 10.1101/gad.7.1.139. [DOI] [PubMed] [Google Scholar]
- Banse AV, Chastanet A, Rahn-Lee L, Hobbs EC, Losick R. Parallel pathways of repression and antirepression governing the transition to stationary phase in Bacillus subtilis. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:15547–15552. doi: 10.1073/pnas.0805203105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branda SS, González-Pastor JE, Ben-Yehuda S, Losick R, Kolter R. Fruiting body formation by Bacillus subtilis. Proceedings of the National Academy of Sciences. 2001;98:11621–11626. doi: 10.1073/pnas.191384198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branda SS, Gonzalez-Pastor JE, Dervyn E, Ehrlich SD, Losick R, Kolter R. Genes involved in formation of structured multicellular communities by Bacillus subtilis. Journal of bacteriology. 2004;186:3970–3979. doi: 10.1128/JB.186.12.3970-3979.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmann F, Sawant P, Bramkamp M. Identification of interaction partners of the dynamin-like protein DynA from Bacillus subtilis. Communicative & integrative biology. 2012;5:362–369. doi: 10.4161/cib.20215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carabetta VJ, Tanner AW, Greco TM, Defrancesco M, Cristea IM, Dubnau D. A complex of YlbF, YmcA and YaaT regulates sporulation, competence and biofilm formation by accelerating the phosphorylation of Spo0A. Mol Microbiol. 2013;88:283–300. doi: 10.1111/mmi.12186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai Y, Chu F, Kolter R, Losick R. Bistability and biofilm formation in Bacillus subtilis. Molecular Microbiology. 2008;67:254–263. doi: 10.1111/j.1365-2958.2007.06040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai Y, Norman T, Kolter R, Losick R. An epigenetic switch governing daughter cell separation in Bacillus subtilis. Genes & Development. 2010;24:754–765. doi: 10.1101/gad.1915010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai Y, Norman T, Kolter R, Losick R. Evidence that metabolism and chromosome copy number control mutually exclusive cell fates in Bacillus subtilis. 2011:1402–1413. doi: 10.1038/emboj.2011.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Guttenplan SB, Blair KM, Kearns DB. Role of the sigmaD-dependent autolysins in Bacillus subtilis population heterogeneity. Journal of bacteriology. 2009;191:5775–5784. doi: 10.1128/JB.00521-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung AL, Eberhardt KJ, Fischetti VA. A method to isolate RNA from gram-positive bacteria and mycobacteria. Analytical biochemistry. 1994;222:511–514. doi: 10.1006/abio.1994.1528. [DOI] [PubMed] [Google Scholar]
- Chu F, Kearns DB, Branda SS, Kolter R, Losick R. Targets of the master regulator of biofilm formation in Bacillus subtilis. Molecular Microbiology. 2006;59:1216–1228. doi: 10.1111/j.1365-2958.2005.05019.x. [DOI] [PubMed] [Google Scholar]
- Chu F, Kearns DB, McLoon A, Chai Y, Kolter R, Losick R. A novel regulatory protein governing biofilm formation in Bacillus subtilis. Mol Microbiol. 2008;68:1117–1127. doi: 10.1111/j.1365-2958.2008.06201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commichau FM, Rothe FM, Herzberg C, Wagner E, Hellwig D, Lehnik-Habrink M, Hammer E, Volker U, Stulke J. Novel activities of glycolytic enzymes in Bacillus subtilis: interactions with essential proteins involved in mRNA processing. Molecular & cellular proteomics : MCP. 2009;8:1350–1360. doi: 10.1074/mcp.M800546-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deaconescu AM, Chambers AL, Smith AJ, Nickels BE, Hochschild A, Savery NJ, Darst SA. Structural basis for bacterial transcription-coupled DNA repair. Cell. 2006;124:507–520. doi: 10.1016/j.cell.2005.11.045. [DOI] [PubMed] [Google Scholar]
- Deighan P, Diez CM, Leibman M, Hochschild A, Nickels BE. The bacteriophage lambda Q antiterminator protein contacts the beta-flap domain of RNA polymerase. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:15305–15310. doi: 10.1073/pnas.0805757105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand S, Gilet L, Bessieres P, Nicolas P, Condon C. Three essential ribonucleases-RNase Y, J1, and III-control the abundance of a majority of Bacillus subtilis mRNAs. PLoS genetics. 2012;8:e1002520. doi: 10.1371/journal.pgen.1002520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figaro S, Durand S, Gilet L, Cayet N, Sachse M, Condon C. Bacillus subtilis mutants with knockouts of the genes encoding ribonucleases RNase Y and RNase J1 are viable, with major defects in cell morphology, sporulation, and competence. Journal of bacteriology. 2013;195:2340–2348. doi: 10.1128/JB.00164-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goda SK, Minton NP. A simple procedure for gel electrophoresis and northern blotting of RNA. Nucleic acids research. 1995;23:3357–3358. doi: 10.1093/nar/23.16.3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamon MA, Lazazzera BA. The sporulation transcription factor Spo0A is required for biofilm development in Bacillus subtilis. Mol Microbiol. 2001;42:1199–1209. doi: 10.1046/j.1365-2958.2001.02709.x. [DOI] [PubMed] [Google Scholar]
- Hamon MA, Stanley NR, Britton RA, Grossman AD, Lazazzera BA. Identification of AbrB-regulated genes involved in biofilm formation by Bacillus subtilis. Mol Microbiol. 2004;52:847–860. doi: 10.1111/j.1365-2958.2004.04023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoya S, Asai K, Ogasawara N, Takeuchi M, Sato T. Mutation in yaaT leads to significant inhibition of phosphorelay during sporulation in Bacillus subtilis. Journal of bacteriology. 2002;184:5545–5553. doi: 10.1128/JB.184.20.5545-5553.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M, Shao W, Perego M, Hoch JA. Multiple histidine kinases regulate entry into stationary phase and sporulation in Bacillus subtilis. Mol Microbiol. 2000;38:535–542. doi: 10.1046/j.1365-2958.2000.02148.x. [DOI] [PubMed] [Google Scholar]
- Kearns DB, Chu F, Branda SS, Kolter R, Losick R. A master regulator for biofilm formation by Bacillus subtilis. Mol Microbiol. 2005;55:739–749. doi: 10.1111/j.1365-2958.2004.04440.x. [DOI] [PubMed] [Google Scholar]
- Kolter R, Greenberg EP. Microbial sciences: The superficial life of microbes. Nature. 2006;441:300–302. doi: 10.1038/441300a. [DOI] [PubMed] [Google Scholar]
- Kuznedelov K, Minakhin L, Niedziela-Majka A, Dove SL, Rogulja D, Nickels BE, Hochschild A, Heyduk T, Severinov K. A role for interaction of the RNA polymerase flap domain with the sigma subunit in promoter recognition. Science (New York, N.Y.) 2002;295:855–857. doi: 10.1126/science.1066303. [DOI] [PubMed] [Google Scholar]
- Lehnik-Habrink M, Newman J, Rothe FM, Solovyova AS, Rodrigues C, Herzberg C, Commichau FM, Lewis RJ, Stulke J. RNase Y in Bacillus subtilis: a Natively disordered protein that is the functional equivalent of RNase E from Escherichia coli. Journal of bacteriology. 2011a;193:5431–5441. doi: 10.1128/JB.05500-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehnik-Habrink M, Schaffer M, Mader U, Diethmaier C, Herzberg C, Stulke J. RNA processing in Bacillus subtilis: identification of targets of the essential RNase Y. Mol Microbiol. 2011b;81:1459–1473. doi: 10.1111/j.1365-2958.2011.07777.x. [DOI] [PubMed] [Google Scholar]
- Ludwig H, Homuth G, Schmalisch M, Dyka FM, Hecker M, Stulke J. Transcription of glycolytic genes and operons in Bacillus subtilis: evidence for the presence of multiple levels of control of the gapA operon. Mol Microbiol. 2001;41:409–422. doi: 10.1046/j.1365-2958.2001.02523.x. [DOI] [PubMed] [Google Scholar]
- McLoon AL, Kolodkin-Gal I, Rubinstein SM, Kolter R, Losick R. Spatial Regulation of Histidine Kinases Governing Biofilm Formation in Bacillus subtilis. Journal of bacteriology. 2011;193:679–685. doi: 10.1128/JB.01186-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molle V, Fujita M, Jensen ST, Eichenberger P, Gonzalez-Pastor JE, Liu JS, Losick R. The Spo0A regulon of Bacillus subtilis. Mol Microbiol. 2003;50:1683–1701. doi: 10.1046/j.1365-2958.2003.03818.x. [DOI] [PubMed] [Google Scholar]
- Newman JA, Rodrigues C, Lewis RJ. Molecular basis of the activity of SinR protein, the master regulator of biofilm formation in Bacillus subtilis. The Journal of biological chemistry. 2013;288:10766–10778. doi: 10.1074/jbc.M113.455592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman TM, Lord ND, Paulsson J, Losick R. Memory and modularity in cell-fate decision making. Nature. 2013;503:481–486. doi: 10.1038/nature12804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick JE, Kearns DB. MinJ (YvjD) is a topological determinant of cell division in Bacillus subtilis. Mol Microbiol. 2008;70:1166–1179. doi: 10.1111/j.1365-2958.2008.06469.x. [DOI] [PubMed] [Google Scholar]
- Shahbabian K, Jamalli A, Zig L, Putzer H. RNase Y, a novel endoribonuclease, initiates riboswitch turnover in Bacillus subtilis. The EMBO journal. 2009;28:3523–3533. doi: 10.1038/emboj.2009.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam AR, Deloughery A, Bradshaw N, Chen Y, O'Shea E, Losick R, Chai Y. A serine sensor for multicellularity in a bacterium. eLife. 2013;2:e01501. doi: 10.7554/eLife.01501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortosa P, Albano M, Dubnau D. Characterization of ylbF, a new gene involved in competence development and sporulation in Bacillus subtilis. Mol Microbiol. 2000;35:1110–1119. doi: 10.1046/j.1365-2958.2000.01779.x. [DOI] [PubMed] [Google Scholar]
- Tzeng Y-L, Zhou XZ, Hoch JA. Phosphorylation of the Spo0B Response Regulator Phosphotransferase of the Phosphorelay Initiating Development inBacillus subtilis. Journal of Biological Chemistry. 1998;273:23849–23855. doi: 10.1074/jbc.273.37.23849. [DOI] [PubMed] [Google Scholar]
- Wilson GA, Bott KF. Nutritional factors influencing the development of competence in the Bacillus subtilis transformation system. Journal of bacteriology. 1968;95:1439–1449. doi: 10.1128/jb.95.4.1439-1449.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasbin RE, Young FE. Transduction in Bacillus subtilis by bacteriophage SPP1. Journal of virology. 1974;14:1343–1348. doi: 10.1128/jvi.14.6.1343-1348.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.