Oral squamous cell carcinoma (OSCC) is the most common malignant neoplasm of oral cavity, which is usually preceded by potentially malignant disorder.[1,2] Unpredictable behavior such as tumor cell cannibalism,[3,4,5] microbial interactions with signaling pathways,[6] and psycho-cellular interactions[7] baffle us in understanding the tumor biology of OSCC. There are so many such unique aspects associated with OSCC that have a direct or indirect relationship with biological behavior of the tumor. Hence, we should accept and respect the uniqueness of OSCC.[8,9] One of such behaviors of cancer cell is efferocytosis, which is not yet reported and explored in OSCC till date.
Efferocytosis word is derived from efferre, Latin for “to take to the grave” or “to bury.” In cell biology, it is the process by which dying/dead cells (e.g., apoptotic or necrotic cells) are eliminated by phagocytic cells. It can be regarded as a phenomenon of “burying of dead cells.”[10] As phagocytosis of apoptotic cells shows distinctive morphologic features and unique downstream consequences, Henson[10] and Gardai et al.[11] coined the term efferocytosis, specific for the aforementioned phenomenon.
During efferocytosis, the cell membrane of phagocytic cells alters and engulfs the apoptotic cell, forming a large fluid-filled vesicle containing the dead cell. This ingested vesicle is called an efferosome (in analogy to the term phagosome). This process is similar to the process of macropinocytosis. In apoptosis, efferocytosis plays the role of removing the dead cells before their membrane integrity is breached and their contents seep into the surrounding tissue. This prevents the exposure of tissues to the toxic and caustic enzymes, oxidants, and other intracellular components such as proteases and caspases which would otherwise affect the integrity of the tissue.[12]
Efferocytosis is executed not only by the ‘professional’ phagocytic cells such as macrophages or dendritic cells but also by many other cell types such as epithelial cells and fibroblasts. To distinguish themselves from the living cells, apoptotic cells carry specific ‘devour me’ signals such as the presence of phosphatidylserine (PS) (resulting from phospholipid flip-flop) or calreticulin on the outer layer of the cell membrane.[11]
Efferocytosis triggers specific downstream intracellular signal transduction pathways that result in anti-inflammatory, anti-protease, and growth-promoting effects. Conversely, impaired efferocytosis has been linked to autoimmune diseases and tissue damage. The process of efferocytosis causes the host cell to produce mediators such as hepatocyte and vascular endothelial growth factors, which are believed to promote replacement of the dead cells.[12]
Defective efferocytosis has been demonstrated in diseases such as cystic fibrosis, bronchiectasis, chronic obstructive pulmonary disease, asthma, idiopathic pulmonary fibrosis, rheumatoid arthritis, systemic lupus erythematosus, glomerulonephritis, and atherosclerosis.[12]
EFFEROCYTOSIS IN ORAL CANCER
Apoptosis in OSCC is a very common event that can easily be recognized on routine histopathological examination. Hence, efferocytosis in OSCC is quite a conceivable phenomenon. It is well elucidated in the literature that epithelial cells can also perform efferocytosis. With this view in mind, we searched OSCC slides for histopathological evidence of efferocytosis by tumor (epithelial) cells. On careful histopathological examination, we observed tumor cells of OSCC engulfing the apoptotic cancer cells. The internalized apoptotic cells showed classical signs of programmed cell death including densely condensed eosinophilic cytoplasm, pyknotic nuclei (condensation of chromatin) and loss of intercellular bridges [Figure 1a and b]. These internalized apoptotic cells gave the impression of being surrounded by a clear halo representing efferosome formation by the engulfing or host tumor cell. The nuclei of these host cells appeared to be pushed towards periphery giving it a semilunar or crescent shape. There was no evidence of intercellular bridges between internalized apoptotic cell and the host tumor cell [Figure 1a and b]. Both partial and complete engulfment were reported in OSCC. Moreover, cannibalism could also be spotted along with efferocytosis in a single slide [Figure 2].
Figure 1.
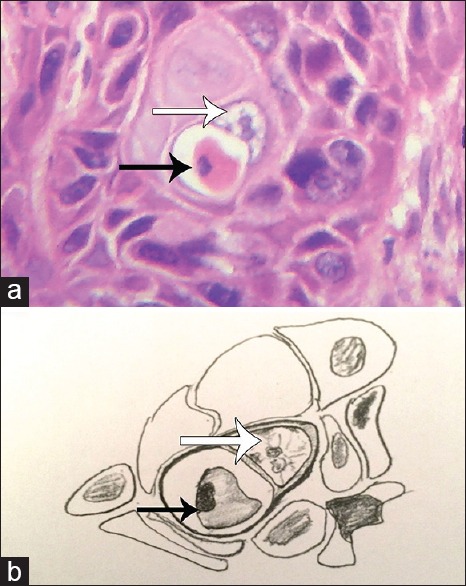
(a) Photomicrograph showing complete engulfment of apoptotic cell (black arrow) by oral squamous cell carcinoma tumor cell (white arrow) (H&E stain, ×400), (b) Hand-drawn illustration of the photomicrograph of 1a showing host cell (white arrow) and apoptotic cell (black arrow)
Figure 2.
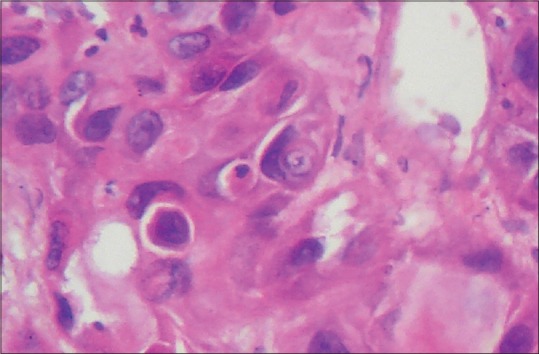
Photomicrograph showing efferocytosis and cellular cannibalism together. The nuclei of engulfing cells showed the characteristic semilunar-shape (H&E stain, ×400)
We also observed internalized apoptotic cells exhibiting various stages of cell death. A small eosinophilic round blob was present inside the cytoplasm of the host cell (epithelial cell) representing the end stage of efferocytosis. This blob was surrounded by a clear halo, suggesting its presence inside the efferosome vacuole [Figure 3]. The nucleus of the host cell showed small concavity on its surface so as to accommodate the internalized cell.
Figure 3.
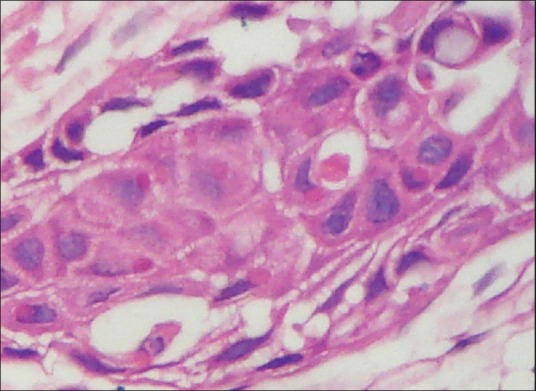
Photomicrograph showing small round eosinophilic blob in the cytoplasm of tumor cell (host cell) of oral squamous cell carcinoma suggesting degradation of apoptotic cells (H&E stain, ×400)
MECHANISM OF EFFEROCYTOSIS
Efferocytosis is cautiously regulated at several levels. At first, cells undergoing apoptosis secrete soluble factors that attract the host cell (macrophages, epithelial cells, or fibroblasts) to the site of death, referred to as ‘find me’ signals, which include the chemokines CXCL1, CXCL14, CCL2, CCL6–8, and CCL11.[13] The apoptotic cell also marks its outer leaflet with the ‘devour me’ signal, allowing the host cells to recognize and bind to the apoptotic cell. PS exposed on the outer leaflet of the plasma membrane of an apoptotic cell is a hallmark of programmed cell death and is the most widely recognized ‘devour me’ signal. Healthy cells actively retain PS on the inner leaflet of the plasma membrane. In contrast, at the onset of apoptosis, PS accumulates on the outer leaflet, marking the apoptotic cell for engulfment.[14] In response to the ‘find me’ signals, the engulfing host cells upregulate cell surface receptors and bridging molecules. These bridging molecules simultaneously bind to PS and host cell surface receptors. Host cells use several cell surface receptors to directly recognize PS and many other receptors that bind to bridging molecules to indirectly bind PS on the apoptotic cell to the host cell.
Once consumed, the cell is processed by lysosomal degradation.[15] In addition to engulfment and degradation of the apoptotic cell, efferocytosis promotes expression and release of immune suppressive cytokines like interleukin-10 [IL-10], IL-13, IL-4 and transforming growth factor-beta 1 and repression of pro-inflammatory cytokines (IL-12 and interferon gamma). This promotes immune tolerance and tissue repair.[16] Beyond the critical function of efferocytosis in tissue homeostasis, recent studies suggest that efferocytosis may support a more malignant tumor microenvironment, in part because of the cytokine signature associated with the process.[17] In addition, many of the receptors and ligands that facilitate the process of efferocytosis are overexpressed in cancer suggesting a specific role in tumorigenesis.[18]
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Sarode SC, Sarode GS, Karmarkar S, Tupkari JV. A new classification for potentially malignant disorders of the oral cavity. Oral Oncol. 2011;47:920–1. doi: 10.1016/j.oraloncology.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 2.Sarode SC, Sarode GS, Tupkari JV. Oral potentially malignant disorders: Precising the definition. Oral Oncol. 2012;48:759–60. doi: 10.1016/j.oraloncology.2012.02.025. [DOI] [PubMed] [Google Scholar]
- 3.Sarode GS, Sarode SC, Karmarkar S. Complex cannibalism: An unusual finding in oral squamous cell carcinoma. Oral Oncol. 2012;48:e4–6. doi: 10.1016/j.oraloncology.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 4.Sarode SC, Sarode GS. Neutrophil-tumor cell cannibalism in oral squamous cell carcinoma. J Oral Pathol Med. 2014;43:454–8. doi: 10.1111/jop.12157. [DOI] [PubMed] [Google Scholar]
- 5.Sarode SC, Sarode GS. Cellular cannibalism in central and peripheral giant cell granuloma of the oral cavity can predict biological behavior of the lesion. J Oral Pathol Med. 2014;43:459–63. doi: 10.1111/jop.12119. [DOI] [PubMed] [Google Scholar]
- 6.Sarode GS, Sarode SC. E6 oncoprotein interaction with paxillin and FAK. Oral Oncol. 2014;50:e17. doi: 10.1016/j.oraloncology.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 7.Sarode GS, Sarode SC, Anand R, Patil S, Rao R, Augustine D. Psychological intervention in head and neck cancer: From molecular standpoint. World J Dent. 2014;5:249–50. [Google Scholar]
- 8.Sarode GS, Sarode SC, Patil S. Accept and respect the uniqueness of oral cancer. World J Dent. 2014;5:v–vi. [Google Scholar]
- 9.Sarode SC, Sarode GS. Is oral cancer unique in terms of chemotherapeutic and targeted drug metabolism? J Oral Maxillofac Surg. 2015;73:4–6. doi: 10.1016/j.joms.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 10.Henson PM. The final step in programmed cell death: Phagocytes carry apoptotic cells to the grave. Essays Biochem. 2003;39:105–17. doi: 10.1042/bse0390105. [DOI] [PubMed] [Google Scholar]
- 11.Gardai SJ, McPhillips KA, Frasch SC, Janssen WJ, Starefeldt A, Murphy-Ullrich JE, et al. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell. 2005;123:321–34. doi: 10.1016/j.cell.2005.08.032. [DOI] [PubMed] [Google Scholar]
- 12.Vandivier RW, Henson PM, Douglas IS. Burying the dead: The impact of failed apoptotic cell removal (efferocytosis) on chronic inflammatory lung disease. Chest. 2006;129:1673–82. doi: 10.1378/chest.129.6.1673. [DOI] [PubMed] [Google Scholar]
- 13.Cullen SP, Henry CM, Kearney CJ, Logue SE, Feoktistova M, Tynan GA, et al. Fas/CD95-induced chemokines can serve as “find-me” signals for apoptotic cells. Mol Cell. 2013;49:1034–48. doi: 10.1016/j.molcel.2013.01.025. [DOI] [PubMed] [Google Scholar]
- 14.Fadok VA, Voelker DR, Campbell PA, Cohen JJ, Bratton DL, Henson PM. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol. 1992;148:2207–16. [PubMed] [Google Scholar]
- 15.Borisenko GG, Matsura T, Liu SX, Tyurin VA, Jianfei J, Serinkan FB, et al. Macrophage recognition of externalized phosphatidylserine and phagocytosis of apoptotic Jurkat cells – existence of a threshold. Arch Biochem Biophys. 2003;413:41–52. doi: 10.1016/s0003-9861(03)00083-3. [DOI] [PubMed] [Google Scholar]
- 16.Gumienny TL, Brugnera E, Tosello-Trampont AC, Kinchen JM, Haney LB, Nishiwaki K, et al. CED-12/ELMO, a novel member of the CrkII/Dock180/Rac pathway, is required for phagocytosis and cell migration. Cell. 2001;107:27–41. doi: 10.1016/s0092-8674(01)00520-7. [DOI] [PubMed] [Google Scholar]
- 17.Stanford JC, Young C, Hicks D, Owens P, Williams A, Vaught DB, et al. Efferocytosis produces a prometastatic landscape during postpartum mammary gland involution. J Clin Invest. 2014;124:4737–52. doi: 10.1172/JCI76375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nguyen KQ, Tsou WI, Calarese DA, Kimani SG, Singh S, Hsieh S, et al. Overexpression of MERTK receptor tyrosine kinase in epithelial cancer cells drives efferocytosis in a gain-of-function capacity. J Biol Chem. 2014;289:25737–49. doi: 10.1074/jbc.M114.570838. [DOI] [PMC free article] [PubMed] [Google Scholar]


