Abstract
Context:
Myofibroblasts are fibroblasts with smooth muscle-like features characterized by the presence of a contractile apparatus and found in the connective tissue stroma of normal tissues such as blood vessels and lymph nodes. They are now thought to play a role in the synthesis and reorganization of extracellular matrix, which could contribute to the aggressive biologic behavior of the lesions.
Aims:
To compare the mean number of stromal myofibroblasts in dentigerous cysts (DCs), keratocystic odontogenic tumor (KCOT) and ameloblastoma; and to derive a correlation between the stromal myofibroblasts and the known biologic behavior of the lesions.
Settings and Design:
A cross-sectional immunohistochemical analysis of cases of DC, KCOT and ameloblastoma.
Materials and Methods:
Twenty paraffin-embedded tissue blocks each of DC, KCOT and multicystic ameloblastoma were selected for the study and diagnosis confirmed through hematoxylin and eosin staining. Tissue sections were analyzed for the number of myofibroblasts using alpha smooth muscle actin (α-SMA) immunostaining.
Statistical Analysis:
Differences in the mean number of α-SMA positive cells in each group were analyzed using one-way ANOVA test. Intergroup comparisons of mean values of α-SMA positive cells were performed using Mann-Whitney U-test.
Results:
Ameloblastoma showed the highest number of myofibroblasts, whereas DC showed the lowest. Among the groups, there were significant differences between the myofibroblast counts among DC and KCOT and between DC and ameloblastoma, whereas the difference in counts was not statistically significant between KCOT and ameloblastoma. A positive correlation was observed between the myofibroblast count and the known biologic behavior of the lesions.
Conclusion:
Myofibroblasts may act in close association with the epithelial cells to bring about changes in stromal microenvironment, favorable to the growth and progression of the lesion. They may be of great value in predicting the biologic behavior and growth potential of such lesions.
Key Words: Ameloblastoma, dentigerous cyst, keratocystic odontogenic tumor, myofibroblasts, smooth muscle actin
INTRODUCTION
Myofibroblasts are fibroblasts with smooth muscle-like features characterized by the presence of a contractile apparatus and found in the connective tissue stroma. They are found in normal tissues such as blood vessels, lymph nodes and bone marrow. Basically, they are thought to play a role in the synthesis of extracellular matrix (ECM) and its reorganization, as in wound healing.[1,2,3] Myofibroblasts have also been shown to be present in various invasive and metastatic malignant tumors. Earlier, it was believed that their presence at the invasive front of the tumor was a part of the host defense mechanism against the tumor, but more recent studies suggest that the presence of these cells at the tumor front actually promotes the growth and invasion of the tumor.[4,5]
Changes in the stromal microenvironment are thought to play a key role in various pathologies including oral cancer. Stromal events such as activities of resident fibroblasts, myofibroblast differentiation and presence of specific stromal proteins such as fibronectin and proteolytic enzymes have been reported as key features in these lesions. Modification of ECM, thought to be mediated by myofibroblasts, has been reported to create a favorable microenvironment for tumor growth, invasion and metastasis.[6,7]
Several odontogenic lesions including keratocystic odontogenic tumors (KCOT) and ameloblastomas although defined as benign are known to demonstrate locally aggressive clinical behavior. Although the role of several epithelium associated factors have been studied in the relative aggressive biologic behavior of odontogenic cysts and tumors, only a few studies have investigated the role of non-epithelial factors, namely stroma and the components including myofibroblasts (MFs) that could contribute to the variable biologic behavior of these lesions. Hence, this study was undertaken to investigate the presence and number of myofibroblasts in 3 odontogenic lesions, namely dentigerous cyst (DC), KCOT and ameloblastoma and to compare the results with the known and reported biologic behavior of these lesions, in a quest to derive a correlation between the number of stromal myofibroblasts and the biologic aggressiveness of the lesions.
MATERIALS AND METHODS
This was a cross-sectional immunohistochemical study of selected cases of previously diagnosed DC, KCOT and multicystic ameloblastoma from our departmental archives. This study obtained the ethical clearance from the Institutional Thesis Protocol Review Committee. The archives in the Department of Oral Pathology and Microbiology were scanned to retrieve paraffin – embedded blocks of previously diagnosed cases of DC, KCOT and multicystic ameloblastomas. Twenty cases each was selected to comprise the study group with a total sample size of sixty cases. Three micrometer thick sections were taken from each block and stained with hematoxylin and eosin stain to confirm the diagnosis under light microscopy. As a previous study[8] had reported an inversely proportional relation between the number of inflammatory cells and the number of myofibroblasts, those cases which showed the presence of inflammatory infiltrate in the connective tissue were excluded from the study group, and only those cases devoid of inflammation were selected to make up the required number in the study group.
Three micrometer thick sections were then obtained from each of the selected cases for immunohistochemical staining with alpha-smooth muscle actin (α-SMA). The slides were deparaffinized by passing them through two changes of xylene for 5 min each. They were hydrated in two changes of 100% ethanol for 1 min each. The slides were then transferred to citrate buffer and autoclaved for antigen retrieval at 15 lbs pressure for 15 min. After allowing to cool, they were washed in phosphate buffer solution. The slides were then treated with protein block reagent for 10 min. Immunohistochemical staining was then performed using DAKO primary antibody (mouse antihuman antibody α-SMA) as per the manufacturer's instructions. The slides were then mounted in DPX and observed under light microscope for the results.
Ten representative fields were selected for counting in each of the α-SMA stained slides. For cystic lesions, counting was done from the field immediately beneath the lining epithelium. For solid tumors, it was done from the field immediately surrounding the epithelial tumor islands or strands. Counting was performed using “Labomed” binocular microscope with a 10x eyepiece fitted with a 1 cm2 graticule and x40 objective. The area encompassed by the graticule was taken as one microscopic field. The α-SMA positive cells (MFs) were identified in each of the ten fields chosen. The α-SMA positive cells in the blood vessel wall served as the control [Figure 1]. Those cells immediately surrounding the blood vessels were not counted. All other positively stained cells in each field were counted and their numbers were recorded. The total number of MFs in all the ten fields counted for a slide was calculated. The mean number of MFs was also calculated.
Figure 1.
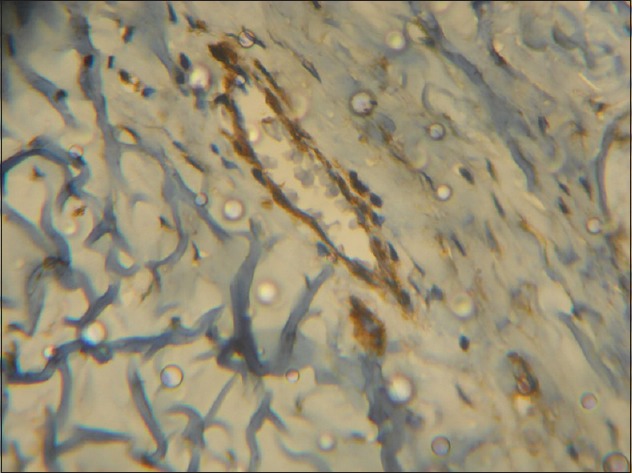
Photomicrograph showing alpha-smooth muscle actin positive cells in the blood-vessel wall that served as the control (IHC stain, ×400)
The percentage of α-SMA positive cells in each group was also calculated by noting the number of positive and negative cases separately for each of the 3 groups.
The distribution of MFs in positive cases from each group was also observed, i.e. whether the MFs were closer to the epithelial lining and epithelial tumor islands or if they were in the deeper part of the connective tissue stroma.
Statistical analysis of the data obtained was performed using IBM-SPSS 20.0 (Chicago, USA) software. Differences in the mean number of α-SMA positive cells in each group were analyzed using one-way ANOVA test. A P < 0.05 was considered for statistical significance. Intergroup comparison of the mean value of MFs was performed using the Mann-Whitney U test.
RESULTS
A total number of sixty cases were taken for the study, twenty from each group and the mean count of α-SMA positive cells (MFs) was 12.3 for DC, 22.9 for KCOT and 24.8 for ameloblastoma, with a P = 0.001, which was statistically significant. The percentage of a-SMA positive cases in each group was 60% for DC, 90% for KCOT and 95% for ameloblastoma [Table 1]. Among the three groups studied, ameloblastoma showed the highest mean number of MFs [Figure 2], whereas DC showed the lowest number [Figure 3]. KCOT showed a mean number of MFs, which was less than but very close to that of ameloblastoma, but much higher than that of DC [Figure 4].
Table 1.
Positivity to alpha-smooth muscle actin staining and mean myofibroblast count in dentigerous cyst, keratocystic odontogenic tumor and ameloblastoma

Figure 2.
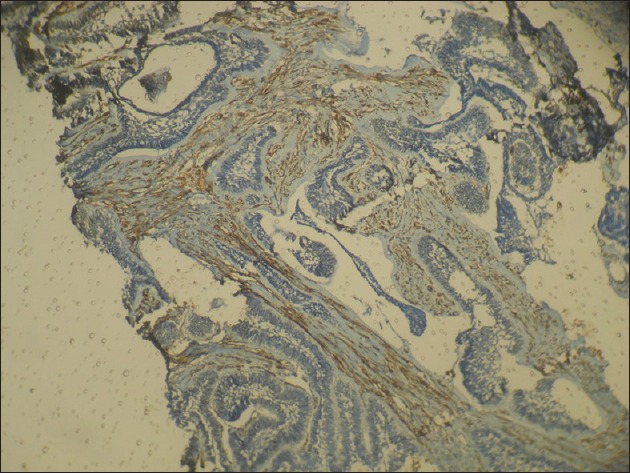
Photomicrograph showing numerous alpha-smooth muscle actin positive cells in the connective tissue stroma in ameloblastoma (IHC stain, ×40)
Figure 3.
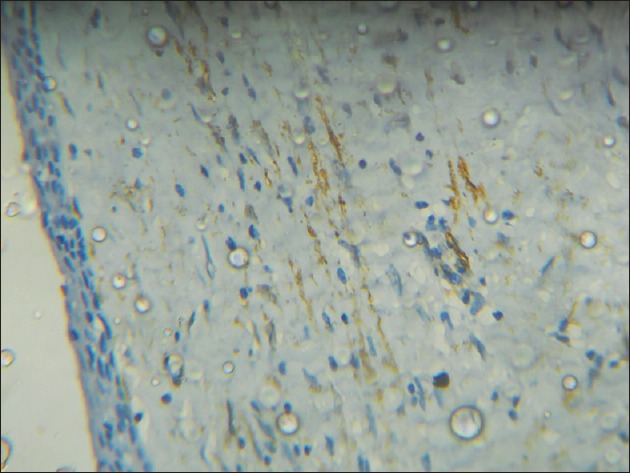
Photomicrograph showing less number of alpha-smooth muscle actin positive cells in the fibrous wall in dentigerous cyst (IHC stain, ×100)
Figure 4.
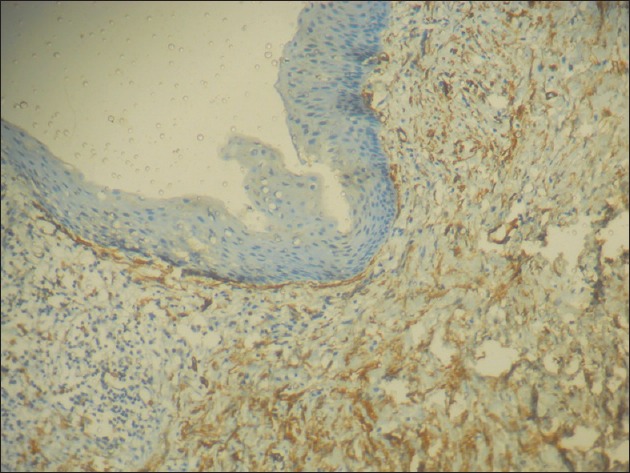
Photomicrograph showing many alpha-smooth muscle actin positive cells in the fibrous wall in keratocystic odontogenic tumor (IHC stain, ×100)
Between the groups, the mean number of MFs was compared between DC and KCOT, and the difference was found to be statistically significant (P = 0.001). Between DC and ameloblastoma too, the difference in MFs was found to be statistically significant (P = 0.001). When these counts were compared between KCOT and ameloblastoma, the difference was not statistically significant.
The distribution of MFs was also observed in the stained sections of each group. In DC, out of the 12 cases which showed α-SMA positivity, the MFs were dispersed in the deeper portion of the fibrous capsule, away from the cystic lining. In the 18 cases of KCOT which were α-SMA positive, a high density of these cells was observed in the fibrous capsule immediately subjacent to the cyst lining [Figure 5]. Among the 19 α-SMA positive cases of ameloblastoma, the positive cells were largely observed in the connective tissue adjacent to the tumor islands [Figure 6].
Figure 5.
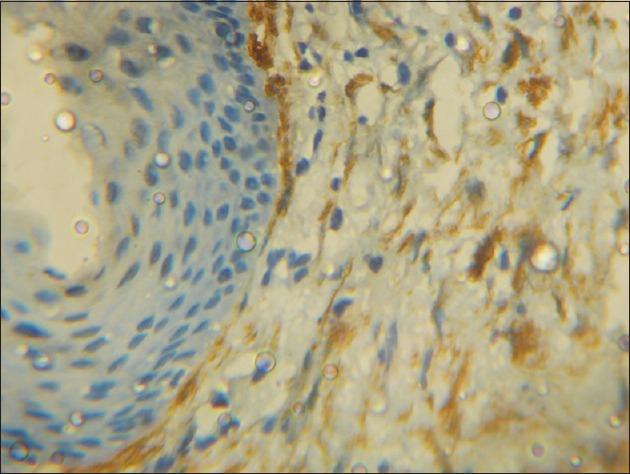
Photomicrograph showing numerous alpha-smooth muscle actin positive cells in the fibrous wall immediately subjacent to the epithelial lining in keratocystic odontogenic tumor (H&E stain, ×400)
Figure 6.
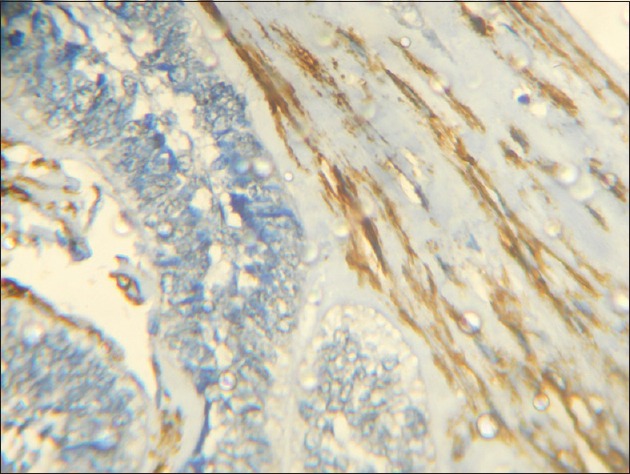
Photomicrograph showing many alpha-smooth muscle actin positive cells in the connective tissue stroma surrounding the tumor islands in ameloblastoma (IHC stain, ×400)
DISCUSSION
Gabbiani et al. in 1971[9,10] on the basis of their experiments on the granulation tissue using different pharmacological agents, observed that granulation tissue at the wound site contained a group of contractile cells which promoted wound contraction and healing. They termed these cells as “myofibroblasts”. Subsequent studies have highlighted the importance of these cells in different physiologic and pathologic processes of the body. It is now known that MFs are differentiated fibroblasts that express α-SMA and have characteristics intermediate between the conventional fibroblasts and the smooth muscle cells.[3] Since their discovery, MFs have been reported to be found in giant cell fibromas,[11] peripheral giant cell granulomas,[12] sarcomas[13] and gingival hyperplasias.[14] There have also been reports that pulpal fibroblasts may acquire the phenotype of MFs through transforming growth factor beta 1 TGF-β1), secreted by the stromal cells which in turn upregulate their expression of α-SMA.[15] MFs have also been shown to be present in the stroma of various malignant and metastatic neoplasms, in which, they were thought to be part of the host tissue reaction to prevent the infiltration/invasion of the neoplastic cells. The finding of numerous MFs at the invasive front of the tumor in these studies strengthened this thought.[16]
However, research over the past 10 years has given abundant evidence on the fact that the presence of MFs at the invasive front of the tumor was not part of the host defense against tumor invasion, but on the other hand, their presence actually promoted invasion. Stromal microenvironment is thought to be extremely crucial for the maintenance of tissue integrity. The presence of cancer cells is followed by some changes that occur within the epithelium due to which the normal stroma is transformed into a reactive one. The neoplastic cells secrete TGF-β1 cytokine which is thought to promote the differentiation of fibroblasts into MFs.[4,5,17] These in turn secrete cytokines and matrix metalloproteases which contribute to the destruction of the ECM facilitating tumor growth.[18] Thus, due to their ability to modify ECM, MFs are thought to actively participate in both tumor invasion and metastasis. Referred to as stromagenesis, this process of ECM modification, orchestrated by the stromal MFs facilitates a favorable microenvironment in the stroma for the growth, invasion and metastasis of the tumor.[17,19]
Although many studies were carried out to investigate the role of MFs in malignant neoplasms including oral squamous cell carcinomas (SCCs),[17,20,21] only a very few studies have investigated their presence and role in odontogenic lesions. Two of the earliest studies in this regard have researched on the presence of MFs in ameloblastomas,[22,23] whereas two recent studies have investigated their possible role in both odontogenic cysts and tumors.[8,24] With this background, the present study was undertaken to investigate the presence, number and distribution of MFs in three odontogenic lesions, namely DC, KCOT and ameloblastoma, and to correlate the same with the known reported biologic aggressiveness of the lesions.
It has been strongly suggested in the recent times that the presence of MFs at the invasive front is not a part of the host defense mechanism against tumor invasion, but it is a process that actually promotes tumor invasion. Several odontogenic lesions, especially KCOTs and multicystic ameloblastomas, although defined as benign are known to demonstrate locally aggressive behavior. However, only a few studies have investigated the role of nonepithelial factors (such as collagen and MF) that could contribute to the variable biologic behavior of these lesions. Hence, it warrants the need to evaluate the presence and distribution of MFs in odontogenic cysts and tumors and correlate the same with the known and reported biological behavior of these lesions.
The mean number of α-SMA positive cells (MFs) per high power field was calculated for each group, and this was found to be highest in ameloblastoma (24.8 ± 3.87) and lowest in DC (12.3 ± 4.05). KCOT showed a value close to but slightly lesser than that of ameloblastoma (22.9 ± 6.51). A previous study by Vered et al. in 2005[24] states that DC showed the lowest number of MF positivity among the odontogenic cysts studied, whereas KCOT [previously odontogenic keratocyst (OKC)] showed the highest MF positivity. Two other studies[8,25] have reported that MF positivity was at least marginally higher in KCOT than in ameloblastoma, but without any statistically significant difference. In the present study, it was found that ameloblastoma showed a marginally increased count of MF when compared to KCOT, but this difference too was not significant. DC, on the other hand, showed significantly low MF positivity than both KCOT and ameloblastoma.
The percentage positivity of α-SMA staining in each of the three groups was also noted. DC gave the lowest percentage of positive MF staining (60%), ameloblastoma showed the highest percentage of positive MFs (95%), whereas KCOT showed 90% positivity for MFs. A previous study in this regard[25] has reported a marginally increased positive staining for KCOT (OKC –92%) when compared to ameloblastoma (88%). However, there was no significant difference in the MF counts among both these groups.
Since cell population and turnover are controlled by a balance between cell proliferation and programed cell death, cell proliferation index and apoptosis index are considered important determinants in assessing the biologic aggressiveness of the lesions. Solid multicystic ameloblastomas show a locally invasive and infiltrative behavior with reported recurrences. KCOT (previously OKC) has been seen to possess intrinsic growth potential, leading to its reclassification as an odontogenic tumor by the WHO. Most of the earlier studies have reported a marginally higher proliferative activity in ameloblastic epithelium when compared to the epithelial lining of KCOT but without statistically significant differences between them.[25,26] In another study,[27] it was shown that the mean silver stained nucleolar organizing regions (AgNORs) count was highest in ameloblastoma, followed closely by KCOT (OKC) and it was lowest in DC. AgNOR, a count of the proliferating capacity of the cell, was a measure of the clinical behavior of the lesions and those with higher scores would be expected to exhibit a more aggressive biologic behavior.
In the present study, the mean count of MFs in each group was correlated with the known and reported biologic behavior of the three lesions. Since it has already been reported that the MFs play a vital role in modifying the stroma which further led to the growth and invasion of tumor cells,[17] the stromal MFs are considered to be the determinants of the aggressive nature of a lesion. Lombardi and Morgan, 1995[28] were among the first to confirm the presence of the MFs in the wall of odontogenic cysts. They suggested that MFs may be part of a homeostatic response to the distension of the cyst wall caused by the cyst enlargement. Thus, increased number of MFs in the stroma of KCOT may be considered as directly proportional to its reported aggressive behavior, as compared to the DC, which showed lesser number of MFs in this study, and which reportedly has a lower growth potential when compared to KCOT and ameloblastoma.
The distribution pattern of MFs was also studied in the three groups. It was observed that in KCOT and ameloblastoma, the MF density was more immediately subjacent to the epithelial lining in KCOT and immediately surrounding the tumor islands in ameloblastoma, as compared to the presence of MFs in the deeper portion of stroma away from the lining epithelium in DC. This finding too is in agreement with some of the previous studies[24,29] which have reported increased MF staining in the stroma immediately surrounding the tumor islands in ameloblastoma and very close to the lining of KCOT.
The presence of MFs in the stroma, concentrated at the tumor invasive margins in SCCs has been linked to the role of these cells in the growth and progression of the lesion, by their ability to alter the ECM. A high density of stromal MFs in highly invasive oral SCCs has been interpreted as the involvement of MFs with the creation of a permissive microenvironment in the stroma for tumor growth, progression and invasion.[3] A similar role of MFs could be proposed in the biologically aggressive lesions such as KCOT and ameloblastomas, which show a greater potential for growth when compared to other odontogenic lesions such as DCs. The high density of MFs in the stroma just beneath the lining epithelium and surrounding the tumor islands in KCOT and ameloblastoma, as seen in our study, may be a pointer to the role of these cells in the growth and further progression of these lesions. A positive link could thus be suggested that when more number of MFs were present in the stroma, a more aggressive behavior of the lesion could be anticipated. This could be because the odontogenic epithelium, especially in KCOT and ameloblastoma could act and modulate the stromal MF differentiation in a manner similar to that in SCC.
CONCLUSION
The previous concept that MFs were part of the host immune response against tumor invasion has quite made way for the hypothesis that MFs may actually be part of the tumor mechanism, promoting its growth. Myofibroblasts may act in close association with the epithelial cells to bring about changes in stromal microenvironment, favorable to the growth and progression of the lesion. Thus, MFs may be of great value in predicting the possible biologic behavior and growth potential of lesions and they have also reaffirmed the role of stromal microenvironment in the growth and progression of the aggressive lesions.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Pallavi DS, Dayanand DS. Myofibroblasts in health and disease. Int J Oral Maxillofac Pathol. 2012;3:23–7. [Google Scholar]
- 2.Angadi PV, Kale AD, Hallikerimath S. Evaluation of myofibroblasts in oral submucous fibrosis: Correlation with disease severity. J Oral Pathol Med. 2011;40:208–13. doi: 10.1111/j.1600-0714.2010.00995.x. [DOI] [PubMed] [Google Scholar]
- 3.de-Assis EM, Pimenta LG, Costa-e-Silva E, Souza PE, Horta MC. Stromal myofibroblasts in oral leukoplakia and oral squamous cell carcinoma. Med Oral Patol Oral Cir Bucal. 2012;17:e733–8. doi: 10.4317/medoral.17834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mareel M, Leroy A. Clinical, cellular, and molecular aspects of cancer invasion. Physiol Rev. 2003;83:337–76. doi: 10.1152/physrev.00024.2002. [DOI] [PubMed] [Google Scholar]
- 5.Liotta LA, Kohn EC. The microenvironment of the tumour-host interface. Nature. 2001;411:375–9. doi: 10.1038/35077241. [DOI] [PubMed] [Google Scholar]
- 6.Hinz B, Pittet P, Smith-Clerc J, Chaponnier C, Meister JJ. Myofibroblast development is characterized by specific cell-cell adherens junctions. Mol Biol Cell. 2004;15:4310–20. doi: 10.1091/mbc.E04-05-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fidler IJ. The pathogenesis of cancer metastasis: The 'seed and soil' hypothesis revisited. Nat Rev Cancer. 2003;3:453–8. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 8.Mashhadiabbas F, Atarbashi MS, Moshref M, Elahi M. Immunohistochemical detection and ultrastructure of myofibroblasts in the stroma of odontogenic cysts and ameloblastoma. Iran Red Crescent Med J. 2010;12:453–7. [Google Scholar]
- 9.Majno G, Gabbiani G, Hirschel BJ, Ryan GB, Statkov PR. Contraction of granulation tissue in vitro: Similarity to smooth muscle. Science. 1971;173:548–50. doi: 10.1126/science.173.3996.548. [DOI] [PubMed] [Google Scholar]
- 10.Gabbiani G, Ryan GB, Majne G. Presence of modified fibroblasts in granulation tissue and their possible role in wound contraction. Experientia. 1971;27:549–50. doi: 10.1007/BF02147594. [DOI] [PubMed] [Google Scholar]
- 11.Weathers DR, Campbell WG. Ultrastructure of the giant-cell fibroma of the oral mucosa. Oral Surg Oral Med Oral Pathol. 1974;38:550–61. doi: 10.1016/0030-4220(74)90086-3. [DOI] [PubMed] [Google Scholar]
- 12.Dayan D, Buchner A, David R. Myofibroblasts in peripheral giant cell granuloma. Light and electron microscopic study. Int J Oral Maxillofac Surg. 1989;18:258–61. doi: 10.1016/s0901-5027(89)80088-8. [DOI] [PubMed] [Google Scholar]
- 13.Fletcher CD. Myofibroblastic tumours: An update. Verh Dtsch Ges Pathol. 1998;82:75–82. [PubMed] [Google Scholar]
- 14.Yamasaki A, Rose GG, Pinero GJ, Mahan CJ. Ultrastructure of fibroblasts in cyclosporin A-induced gingival hyperplasia. J Oral Pathol. 1987;16:129–34. doi: 10.1111/j.1600-0714.1987.tb01479.x. [DOI] [PubMed] [Google Scholar]
- 15.Martinez EF, Araújo VC, Sousa SO, Arana-Chavez VE. TGF-beta1 enhances the expression of alpha smooth muscle actin in cultured human pulpal fibroblasts: Immuno chemical and ultrastructural analyses. J Endod. 2007;33:1313–8. doi: 10.1016/j.joen.2007.07.040. [DOI] [PubMed] [Google Scholar]
- 16.Seemayer TA, Schürch W, Lagacé R. The myofibroblast and defense against neoplasia: A hypothesis. Surv Immunol Res. 1982;1:268–73. doi: 10.1007/BF02918468. [DOI] [PubMed] [Google Scholar]
- 17.Lewis MP, Lygoe KA, Nystrom ML, Anderson WP, Speight PM, Marshall JF, et al. Tumour-derived TGF-beta1 modulates myofibroblast differentiation and promotes HGF/SF-dependent invasion of squamous carcinoma cells. Br J Cancer. 2004;90:822–32. doi: 10.1038/sj.bjc.6601611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Wever O, Mareel M. Role of tissue stroma in cancer cell invasion. J Pathol. 2003;200:429–47. doi: 10.1002/path.1398. [DOI] [PubMed] [Google Scholar]
- 19.Giatromanolaki A, Sivridis E, Koukourakis MI. The pathology of tumor stromatogenesis. Cancer Biol Ther. 2007;6:639–45. doi: 10.4161/cbt.6.5.4198. [DOI] [PubMed] [Google Scholar]
- 20.Lewis MP, Lygoe KA, Nystrom ML, Anderson WP, Speight PM, Vered M, et al. Tumor-host histopathologic variables, stromal myofibroblasts and risk score, are significantly associated with recurrent disease in tongue cancer. Cancer Sci. 2010;101:274–80. doi: 10.1111/j.1349-7006.2009.01357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Etemad-Moghadam S, Khalili M, Tirgary F, Alaeddini M. Evaluation of myofibroblasts in oral epithelial dysplasia and squamous cell carcinoma. J Oral Pathol Med. 2009;38:639–43. doi: 10.1111/j.1600-0714.2009.00768.x. [DOI] [PubMed] [Google Scholar]
- 22.Rothouse LS, Majack RA, Fay JT. An ameloblastoma with myofibroblasts and intracellular septate junctions. Cancer. 1980;45:2858–63. doi: 10.1002/1097-0142(19800601)45:11<2858::aid-cncr2820451123>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 23.Smith SM, Bartov SA. Ameloblastoma with myofibroblasts:First report. J Oral Pathol. 1986;15:284–6. doi: 10.1111/j.1600-0714.1986.tb00625.x. [DOI] [PubMed] [Google Scholar]
- 24.Vered M, Shohat I, Buchner A, Dayan D. Myofibroblasts in stroma of odontogenic cysts and tumors can contribute to variations in the biological behavior of lesions. Oral Oncol. 2005;41:1028–33. doi: 10.1016/j.oraloncology.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 25.Joshi P, Bhosale S, Hongal B, Chougule M, Dudanakar M. Comparison of immunoexpression of α-SMA in inflamed and non-inflamed odontogenic keratocyst and ameloblastoma. Int J Appl Dent Sci. 2014;1:4–9. [Google Scholar]
- 26.Takahashi H, Fujita S, Yamabe S, Moriishi T, Okabe H, Tajima Y, et al. Comparison of proliferating cell nuclear antigen expression in odontogenic keratocyst and ameloblastoma: An immunohistochemical study. Annals Cell Pathol. 1998;16:185–92. doi: 10.1155/1998/105193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Allison RT, Spencer S. Nucleolar organiser regions in odontogenic cysts and ameloblastomas. Br J Biomed Sci. 1993;50:309–12. [PubMed] [Google Scholar]
- 28.Lombardi T, Morgan PR. Immunohistochemical characterisation of odontogenic cysts with mesenchymal and myofilament markers. J Oral Pathol Med. 1995;24:170–6. doi: 10.1111/j.1600-0714.1995.tb01160.x. [DOI] [PubMed] [Google Scholar]
- 29.Sherlin HJ, Natesan A, Ram P, Ramani P, Thiruvenkadam C. Immunohistochemical profiling of ameloblastomas using cytokeratin, vimentin, smooth muscle actin, CD34 and S100. Ann Maxillofac Surg. 2013;3:51–7. doi: 10.4103/2231-0746.110084. [DOI] [PMC free article] [PubMed] [Google Scholar]


