Abstract
Collagen is a unique, triple helical molecule which forms the major part of extracellular matrix. It is the most abundant protein in the human body, representing 30% of its dry weight. It is the fibrous structural protein that makes up the white fibers (collagen fibers) of skin, tendons, bones, cartilage and all other connective tissues. Collagens are not only essential for the mechanical resistance and resilience of multicellular organisms, but are also signaling molecules defining cellular shape and behavior. The human body has at least 16 types of collagen, but the most prominent types are I, II and III. Collagens are produced by several cell types and are distinguishable by their molecular compositions, morphologic characteristics, distribution, functions and pathogenesis. This is the major fibrous glycoprotein present in the extracellular matrix and in connective tissue and helps in maintaining the structural integrity of these tissues. It has a triple helical structure. Various studies have proved that mutations that modify folding of the triple helix result in identifiable genetic disorders. Collagen diseases share certain similarities with autoimmune diseases, because autoantibodies specific to each collagen disease are produced. Therefore, this review highlights the role of collagen in normal health and also the disorders associated with structural and functional defects in collagen.
Key Words: Autoimmune diseases, collagen, triple helical structure
INTRODUCTION
The word collagen is derived from Greek origin: Kolla (glue) and gene. It is the fibrous structural protein that makes up the white fibers (collagen fibers) of skin, tendons, bones, cartilage and all other connective tissues. It is also found widespread in the gelatinous substances of the body. Collagen is the natural protein that constitutes most of the body's structural support and is the primary substance of connective tissue. Without collagen a human being would be reduced to clump of cells interconnected by few neurons.[1]
HISTORY AND BACKGROUND
The molecular and packing structures of collagen have eluded scientists over decades of research. The first evidence that it possesses a regular structure at the molecular level was presented in the mid-1930s. Since that time many prominent scholars, including Nobel laureates Crick, Pauling, Rich and Yonath and others including Brodsky, Berman and Ramachandran, concentrated on the conformation of the collagen monomer.[2,3,4] so far the triple-helical “Madras” model provided an essentially correct model of the molecule's quaternary structure although this model still requires some refinement.[3,5] The packing structure of collagen has not been defined to the same degree outside of the fibrillar collagen types, although it has been long known to be hexagonal or quasi-hexagonal. As with its monomeric structure, several conflicting models alleged that either the packing arrangement of collagen molecules is “sheet-like” or microfibrillar.[3,6,7]
LIGHT MICROSCOPIC STRUCTURE
Under light microscope collagen fibers are seen in bundles which are 1–12 microns and which branch and anastomose with adjacent bundles, but the individual fibers do not branch.[8]
ELECTRON MICROSCOPIC STRUCTURE
Under electron microscope, collagen fibrils show characteristic cross striations (dark and light bands) which are due to regular arrangement of collagen molecules within the collagen fibrils. These molecules of collagen are about 300 nm in length and about 1.5 nm in thickness. These are also known as tropocollagen molecules, which are made up of three polypeptide chains called alpha chains that are arranged in the form of triple helix.[8]
The α chains are left-handed helices which wrap around each other into a right-handed, ropelike triple helical rod. Depending on the type of collagen, the molecule may be made up of either three identical α chains or two or three different α chain. The triple helix may be a continuous stretch, or it may be interrupted by non-collagenous segments. Within the triple helical domain, glycine occupies every third position in the repetitive amino acid sequence Gly-X-Y, where X and Y are amino acid other than glycine. Glycine is essential for triple helical conformation because larger amino acid will not fit in the center of the triple helix. Proline frequently occupies the X and Y positions.[9,10,11]
Collagen contains two unique amino acids, hydroxyproline and hydroxylysine (Hyl). In vertebrate's collagens, these amino acids are present in the Y position. The collagen molecule is stabilized through the formation of number of lysine derived intra- and inter-molecular cross–links[12,13] [Figure 1].
Figure 1.
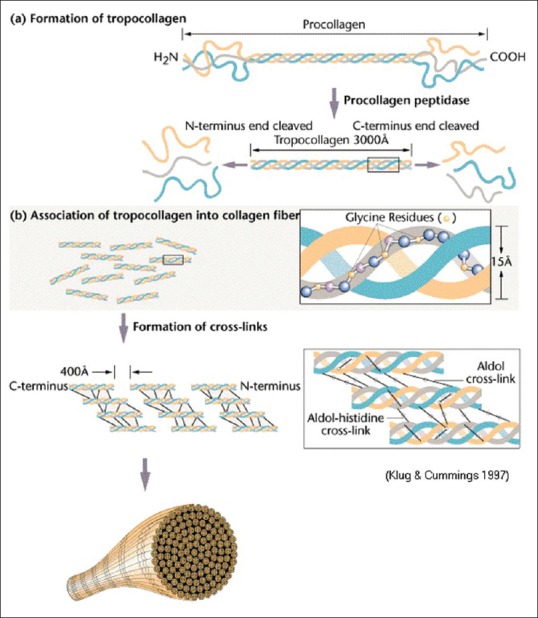
Molecular structure of collagen. (Courtesy: Klug W.S, Cummings M.R. Concepts of Genetics. 5th edition 1997; p. 49-55)
BIOSYNTHESIS OF COLLAGENS
Collagen is synthesized mainly by the Mesenchymal cells and their derivatives such as fibroblasts, chondrocytes, osteoblasts, odontoblasts and cementoblasts and other cells such as epithelial cells, endothelial cells, muscle cells and Schwann cells.[14]
COLLAGEN SYNTHESIS AND ASSEMBLY
Collagen synthesis and assembly into fibers occurs via series of:
Intracellular events
Extracellular events.
Intracellular events
Pre-procollagen synthesis occurs at rough endoplasmic reticulum (RER) and is directed by mRNAs that encode different types of α chain. Hydroxylation of specific proline and lysine residues of the forming polypeptide chain occur within the RER which is catalyzed by specific hyrdroxylases that require Vitamin C as a cofactor. Attachment of sugar (glycosylation) to specific Hyl residue also occurs within RER. Procollagen triple – helix formation takes places in the RER and is regulated by propeptides. The three α – chains align and coil into triple helix. The addition of carbohydrate occurs in the Golgi complex and the oligosaccharides chains are completed. Secretion of procollagen occurs by exocytosis.[15]
Extracellular events
Cleavage of procollagen is catalyzed by procollagen peptidases, which remove most of the propeptide sequences at the ends of each α-chain, yielding tropocollagen.
Self-assembly of tropocollagen occurs as insoluble tropocollagen molecules aggregate near the cell surface. Fibrils characteristic of Types I, II, III, V, VII collagen are produced which have a transverse banding periodicity of 67 nm in Types I, II, III collagen. The periodicity varies with different types of collagen. Cross-linking occurs between adjacent tropocollagen molecules and involves the formation of lysine and Hyl which imparts tensile strength to collagen fibrils[15] [Table 1 and Figure 2].
Table 1.
Types of collagen
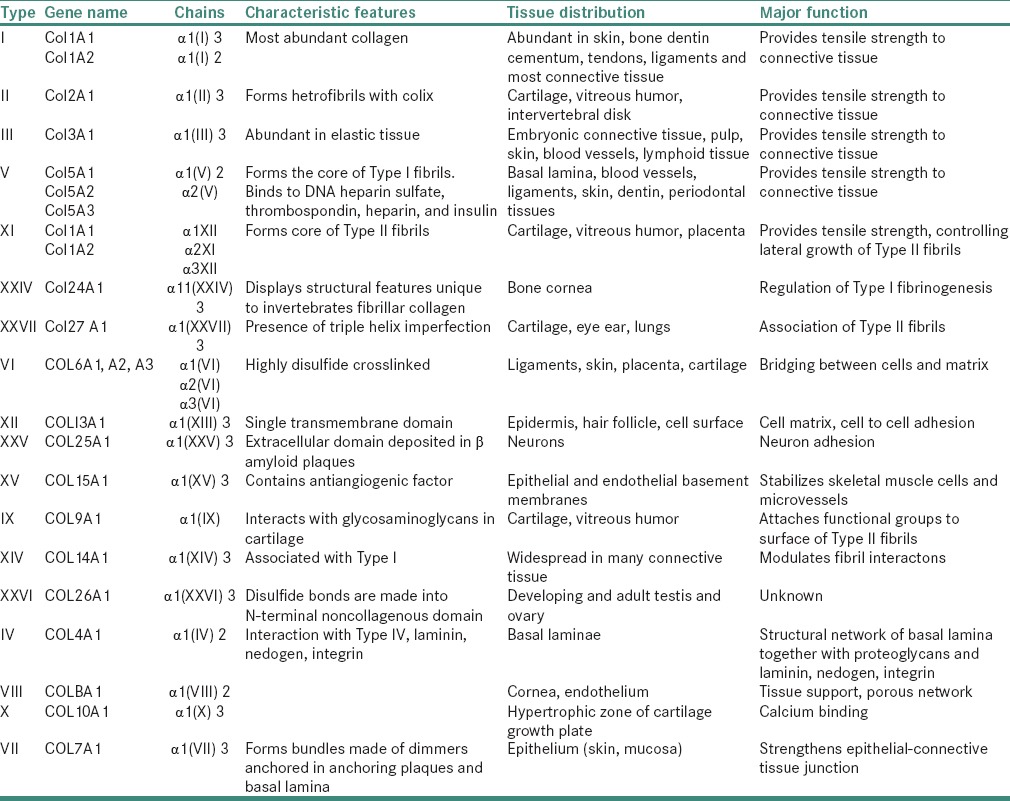
Figure 2.
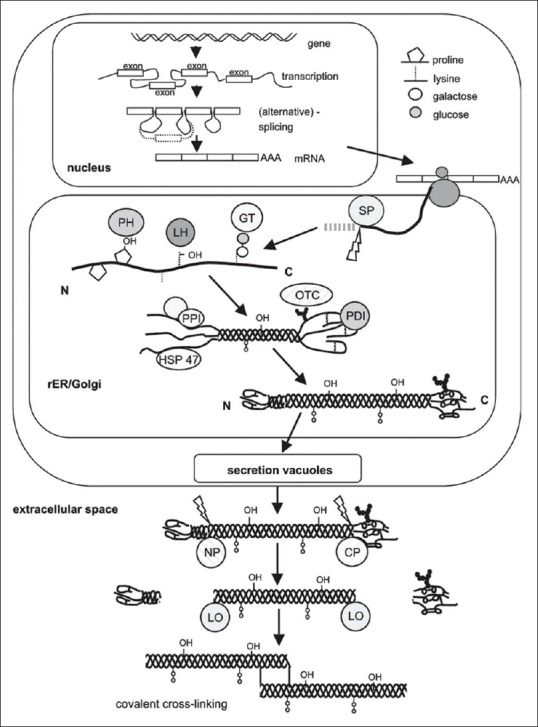
Schematic representation of collagen synthesis starting from the nuclear transcription of the collagen genes, mRNA processing, ribosomal protein synthesis (translation) and posttranslational modifications, secretion and the final steps of fibril formation. (SP: Signal peptidase; GT: Hydroxylysyl galactosyltransferase and galactosylhydroxylysyl glucosyltransferase; LH: Lysyl hydroxylase; PH: Prolylhydroxylase; OTC: Oligosaccharyl transferase complex; PDI: Protein disulphide isomerase; PPI: Peptidyl-prolyl cis-trans-isomerase; NP: Procollagen N-proteinase; CP: Procollagen C-proteinase; LO: Lysyl oxidase; HSP47: Heat shock protein 47, colligin1). (Courtesy K. Gelse et al./Advanced Drug Delivery Reviews 55 (2003) 1531-1546)
REGULATION OF COLLAGEN BIOSYNTHESIS
Regulation of collagen production occurs at gene transcription level, collagen gene expression is regulated in a cell and tissue-specific manner during both normal development and homeostasis. Regulation of transcription rate is mediated primarily through promoters and enhancer element. In the Type I collagen gene, promoter and enhancer sequence are present in the first introns of COL1A2 gene. In Type IV collagen, the pair of genes for COL4A1 and COL4A2 is arranged in a unique head-to-head arrangement separated by a short 130 bp segment, with the binding site for SP1 at its center. The intervening sequence interacts with enhancer and negative regulatory elements located in COL4A1 and COL4A2 genes, respectively, thus regulating the expression of both the genes. The magnitude of collagen synthesis is dependent on the levels of the mRNA for its alpha chains. Collagen synthesis is regulated post transtionally by the extent of prolyl hydroxylation, and it is also influenced by a variety of growth factors, hormones, cytokines and lymphokines. Transforming growth factor-beta (TGF-β) have a positive influence and TGF-α have a negative influence on production of collagen.[16,17]
DEGRADATION AND REMODELLING OF COLLAGEN
It is primarily mediated by collagenases and several other enzymes which belong to a family of enzymes called matrix metalloproteinases (MMPs). Based on their substrate specificity Woessner et al. 1991, Mignatti et al. 1996 have classified them into the following types:
Collagenases
Gelatinases
Stromelysins.
COLLAGENASE 1/MATRIX METALLOPROTEINASES-1/FIBROBLAST TYPE COLLAGENASE
It is produced by a variety of human epithelial and mesenchymal cells including keratinocytes, fibroblasts and macrophages. It can hydrolyze Type I, II, III, VI, VIII and X collagen and gelatine (Birkedal-Hansen et al. 1993). It hydrolyzes Type III collagen molecules faster when compared to Type I.
COLLAGENASE 2/POLYMORPHONUCLEAR LEUKOCYTE COLLAGENASE MATRIX METALLOPROTEINASES-8
It hydrolyzes both Type I and Type III collagen. It degrades Type I faster than Type III collagen. It is found only in the specific granules of polymorphonuclear (PMN) neutrophil cells.
GELATINASE GROUP OF MATRIX METALLOPROTEINASES
They include gelatinase A or MMP-2, gelatinase B or MMP-9 both have a high affinity for gelatin but can also cleave Types IV, VII, X and XI collagens and elastin. Gelatinase B is produced by eosinophils, macrophages, keratinocytes and is also stored in the PMN neutrophil granules.
STROMELYSINS
They have the ability to degrade proteoglycans, basement membrane, laminin and fibronectin in addition to the collagens. Types identified are stromelysin-1 (MMP-3), stromelysin-2 (MMP-10) and stromelysin-3 (MMP-11).
MATRILYSIN (MATRIX METALLOPROTEINASES-7) AND METALLOELASTASE (MATRIX METALLOPROTEINASES-12)
They are also known to possess significant collagenolytic activity.[5,16,18,19,20]
STAINING REACTIONS OF COLLAGEN
Commonly used stains are hematoxylin and eosin, which stains collagen pink in color,[21,22] Masson trichrome which stains collagen blue,[21] Van Gieson which stains collagen red[21,23] and Picrosirius red stain. Staining reaction for collagen in Picrosirius red stain, changes from greenish-yellow to orange-red as degree of hyalinization increases.[24]
THERAPEUTIC APPLICATIONS
Collagen is a safe and effective biomaterial because of its high tensile strength, biocompatibility and absorbability in living tissue. It is used to repair tissues such as bone, tendons, ligaments, skin and also used as sealants, implant coating, adhesion barrier and tissue engineering devices.
Collagen-based localized drug delivery systems
Collatamp G-Collatamp G is an implantable Type I collagen sponge impregnated with 2.0 mg/cm2 of gentamicin sulfate which is indicated for surgical treatment and postsurgical prevention of infection in the bone and soft tissue.[25,26]
Collagen membranes used in guided tissue regeneration
Intrinsic collagen participates in soft tissue and bone healing whereas exogenous collagen exhibits hemostatic activity and can attract and activate neutrophils and fibroblasts which interact with various cells, during tissue remodeling and wound healing. These biological activities, along with low immunogenicity, make collagen an attractive biomaterial.[26]
Surgical hemostats
Collagen is a natural hemostat and a wide variety of collagen-based products are used in surgery and dentistry to control excessive bleeding or hemorrhage.[27]
Collagen stentsand vascular graft coatings
Expandable, intra-arterial stents are widely used for treating coronary artery diseases. In addition to mechanical dilation, biopolymer-coated stents may provide supplementary functions such as local drug delivery, gene transfer, reduction of operative blood loss and facilitation of endothelial cell in-growth.[28]
SPECIAL STAINS FOR DETECTION OF COLLAGEN
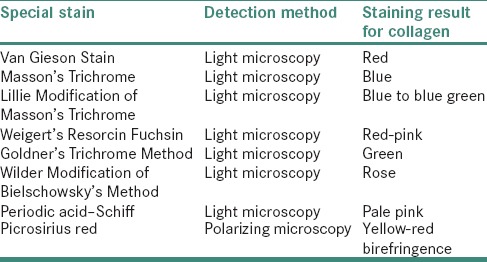
Trichrome stains are employed which utilizes a number of techniques for the selective demonstration of collagen fibers in various connective tissues [Table 2].
Table 2.
Special stains for collagen detection
EFFECTS OF DRUGS ON COLLAGEN
Phenytoin sodium
It is believed to stimulate the high-affinity phenotype of fibroblast. Exposure of the gingiva fibroblast to phenytoin increases the level of translatable collagen mRNA; thus, there is an increased steady state level of collagen mRNA and not a decrease in collagen degradation.[29]
Cyclosporin
Bartold has reported a stimulatory effect on DNA synthesis which increases the synthesis of collagen. It also negates the inhibitory effect of lipopolysaccharides indicating a possible observed relationship between areas of prominent gingival overgrowth and dental plaque.[30]
Calcium channel blocker
Lucas et al. have shown that nifedipine induced hyperplasia is due to an increase in the ground substance. Gingival overgrowth may also be related to calcium-dependent inhibitory effect on T-cells and subsequent immunosuppression. Blockade of intracellular calcium uptake by the fibroblast alters both their secretory properties as well as the synthesis of collagenases.[31]
COLLAGEN DISORDERS
Three types of alterations can affect collagen and lead to tissue changes in these disorders: A defect in the structure of collagen, molecular defect in processing enzymes, mechanisms affecting the expression of collagen gene as in acquired defects [Table 3].
Table 3.
Categorization of collagen disorders
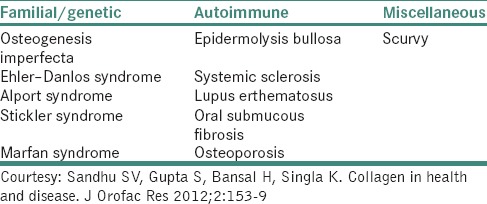
Osteogenesis imperfecta
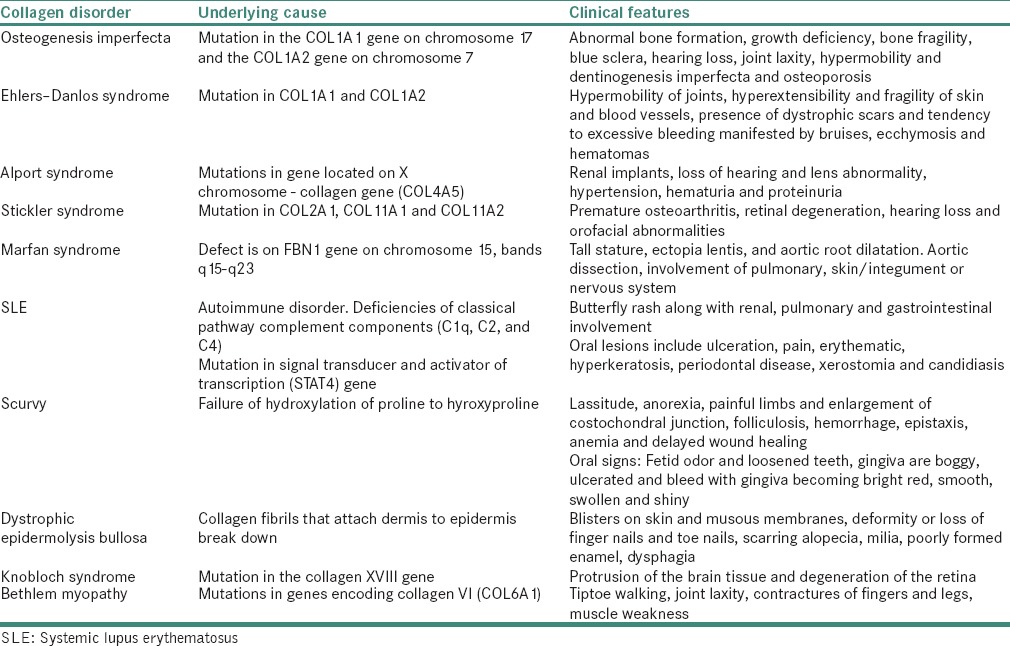
It comprises a heterogeneous group of heritable disorders characterized by impairment of collagen maturation. Except on rare occasions, the disorder arises from heterozygosity for mutation in one of the two genes that guide the formation of Type I collagen: The COL1A1 gene on chromosome 17 and the COL1A2 gene on chromosome 7. The clinical features observed are abnormal bone formation, growth deficiency, bone fragility, blue sclera, hearing loss, joint laxity, hypermobility and dentinogenesis imperfecta and osteoporosis. On fracture, healing occurs with exuberant callus formation.[32,33,34,35,36]
Ehler –Danlos syndrome
It is a name given to a group of more than ten different inherited disorders all involving a genetic defect in collagen and connective tissue synthesis and structures. This syndrome is clinically heterogeneous, the underlying collagen abnormality is different for each type. In some forms of Ehler–Danlos syndrome (EDS), a mutation in COL1A1 and COL1A2 genes is reported which results in interferences with the conversion of procollagen to collagen. This leads to defective crosslinking and consequent reduction in tensile strength of tendons. EDS is characterized by hypermobility of joints, hyperextensibility and fragility of skin and blood vessels, the presence of dystrophic scars and tendency to bleed excessively, manifested by bruises, ecchymosis and hematomas. Oral manifestation includes fragile gingiva, periodontitis, premature loss of deciduous and permanent teeth. Hypoplasia of enamel, recurrent subluxation of temporomandibular joint has also been reported.[32,37,38,39,40]
Alport syndrome
It is a generalized inherited disorder of basement membranes. The mutations occur in gene located on X chromosome. Classical X-linked Alport syndrome affects the α-5 chain of collagen Type IV collagen gene (COL4A5). While the α-3 and α-4 chain of collagen Type IV collagen (COL4 A3 and COL4 A4) are responsible for less frequent recessive forms. It is characterized by renal implants, loss of hearing and lens abnormality, hypertension, hematuria and proteinuria.[32]
Stickler syndrome
It is an autosomal dominant syndrome of premature osteoarthritis, retinal degeneration, hearing loss and orofacial abnormalities. It is caused by a mutation in COL2A1, COL11A1 and COL11A2 procollagen genes of Type 2 and 11 collagen.[32,39]
Marfan syndrome
It includes a spectrum of disorders caused by a heritable genetic defect of connective tissue that has an autosomal dominant mode of transmission. The defect is on FBN1 gene on chromosome 15, bands q15-q23, which codes for the connective tissue protein, fibrillin. In general, patients present with tall stature, ectopia lentis and aortic root dilatation. The diagnosis is made when a patient presents with complications of the syndrome, such as aortic dissection or with the involvement of pulmonary, skin/integument or nervous system.[32,39]
AUTOIMMUNE COLLAGEN DISORDERS
Systemic lupus erythematosus
Systemic lupus erythematosus is a prototypical human autoimmune, collagen vascular or connective tissue disease mediated by pathogenic immune complexes. Generalized findings include fever, weight loss, arthritis, fatigue and malaise. It is also characterized by butterfly rash along with renal, pulmonary and gastrointestinal involvement. Oral lesions include ulceration, pain, erythematic, hyperkeratosis, periodontal disease, xerostomia and candidiasis.[34,41]
Systemic sclerosis
It is a chronic disease characterized by diffuse sclerosis of skin, gastrointestinal tract, heart muscle, lungs and kidney. The pathological findings state those fibroblasts are activated to form an excessive amount of collagen and other components of the cellular matrix. The clinical findings include thickening of the skin, starting with pitting edema and over several months pitting edema is replaced by tightening and hardening of skin because of its firm fixation to deep connective tissue. This contracture of skin gives a mask like appearance to the face. The mouth aperture is constricted and radial furrows appear, giving a pursed look. The vermilion border is reduced and the lips become immovable making entry to the oral cavity difficult. The oral mucosa is pale and is rigid on palpation. When the tongue is affected, it loses its mobility and papillary pattern and becomes shrunken in later stages. Another characteristic change is a flattening of the palatal rugae. Widening of the periodontal ligament is also seen in this disease.[34,42,43,44]
Oral submucous fibrosis
Oral submucous fibrosis (OSMF) is a chronic, progressive, scarring disease; that predominantly affects people of Southeast Asian origin. This condition was described first by Schwartz (1952). The presence of various autoantibodies in varying titers in several studies have confirm the autoimmune basis of the disease.[32] Genetic susceptibility is associated with OSMF because of raised frequency of HLA – A10, -B7 and – DR3 are found in OSMF patients compared to normal subjects.[33] The disease is considered as a consequence of disturbances in homeostatic equilibrium between synthesis and degradation of extracellular matrix, wherein collagen forms a major component, that can be recognized as a collagen-metabolic disorder. It is characterized by a juxta-epithelial inflammatory reaction followed by a fibroelastic change in lamina propria and associated epithelial atrophy. This leads to restricted mouth opening, resulting in trismus leading to restriction of food consumption, difficulty in maintaining oral health, as well as impairs the ability to speak. The fibroelastic changes are almost entirely due to the abnormal accumulation of collagen in subepithelial layers, resulting in dense bands in the mouth.[32,44,45]
Scurvy
Prolong deficiency of Vitamin C results in scurvy. There is defective formation of collagen in connective tissues because of failure of hydroxylation of proline to hyroxyproline which is a characteristic amino acid of collagen. There is also increased permeability of capillaries (hemorrhage), anemia due to erythropoiesis and defective collagen formation. Clinical features include lassitude, anorexia, painful limbs and enlargement of the costochondral junction, folliculosis, hemorrhage, epistaxis, anemia and delayed wound healing. Oral signs may be cardinal: Fetid odor and loosened teeth, gingiva are boggy, ulcerated and bleed with the interdental and marginal gingiva becoming bright red, smooth, swollen and shiny.[32]
A list of collagen disorders and manifestations is summarized in Table 4.
Table 4.
Collagen disorders and manifestations
In addition to the above-mentioned disorders, excessive deposition of collagen occurs in Scleroderma.
CONCLUSION
Collagen is the most common protein in the animal world. It is found in the interstitial tissue of virtually all parenchymal organs, where they contribute to the stability of tissues and organs and maintain their structural integrity. In spite of the increasing knowledge about the structure, synthesis and genetic bases of collagen, collagen disorders are considered as incurable. Thus, the field of collagen research still remains to be further explored.
Financial support and sponsorship
Nil
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Hulmes DJ. The collagen superfamily – Diverse structures and assemblies. Essays Biochem. 1992;27:49–67. [PubMed] [Google Scholar]
- 2.Wyckoff RW, Corey RB, Biscoe J. X-ray reflections of long spacing from tendon. Science. 1935;82:175–6. doi: 10.1126/science.82.2121.175. [DOI] [PubMed] [Google Scholar]
- 3.Clark G, Parker E, Schaad J, Warren WJ. New measurements of previously unknown large interplanar spacings in natural materials. J Am Chem Soc. 1935;7:1509. [Google Scholar]
- 4.Holmes DF, Gilpin CJ, Baldock C, Ziese U, Koster AJ, Kadler KE. Corneal collagen fibril structure in three dimensions: Structural insights into fibril assembly, mechanical properties, and tissue organization. Proc Natl Acad Sci U S A. 2001;98:7307–12. doi: 10.1073/pnas.111150598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hulmes DJ. Building collagen molecules, fibrils, and suprafibrillar structures. J Struct Biol. 2002;137:2–10. doi: 10.1006/jsbi.2002.4450. [DOI] [PubMed] [Google Scholar]
- 6.Hulmes DJ, Miller A. Quasi-hexagonal molecular packing in collagen fibrils. Nature. 1979;282:878–80. doi: 10.1038/282878a0. [DOI] [PubMed] [Google Scholar]
- 7.Jésior JC, Miller A, Berthet-Colominas C. Crystalline three-dimensional packing is a general characteristic of type I collagen fibrils. FEBS Lett. 1980;113:238–40. doi: 10.1016/0014-5793(80)80600-4. [DOI] [PubMed] [Google Scholar]
- 8.Singh I. Inderbir Singh's Textbook of Human Histology with Color Atlas. 4th ed. NewDelhi: Jaypee; 2004. pp. 58–9. Ch. 4. [Google Scholar]
- 9.Perumal S, Antipova O, Orgel JP. Collagen fibril architecture, domain organization, and triple-helical conformation govern its proteolysis. Proc Natl Acad Sci U S A. 2008;105:2824–9. doi: 10.1073/pnas.0710588105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fraser RD, MacRae TP, Suzuki E. Chain conformation in the collagen molecule. J Mol Biol. 1979;129:463–81. doi: 10.1016/0022-2836(79)90507-2. [DOI] [PubMed] [Google Scholar]
- 11.Fraser RD, MacRae TP, Miller A. Molecular packing in type I collagen fibrils. J Mol Biol. 1987;193:115–25. doi: 10.1016/0022-2836(87)90631-0. [DOI] [PubMed] [Google Scholar]
- 12.Wess TJ, Hammersley AP, Wess L, Miller A. Molecular packing of type I collagen in tendon. J Mol Biol. 1998;275:255–67. doi: 10.1006/jmbi.1997.1449. [DOI] [PubMed] [Google Scholar]
- 13.Traub W, Yonath A, Segal DM. On the molecular structure of collagen. Nature. 1969;221:914–7. doi: 10.1038/221914a0. [DOI] [PubMed] [Google Scholar]
- 14.Nanci A. Ten Cate's Textbook of Oral Histology, Development, Structure & Function. 7th ed. NewDelhi: Elsevier; 2008. pp. 66–8. Ch. 4. [Google Scholar]
- 15.Gartner LP, Hiatt JL, Judy M. Textbook of Cell Biology and Histology. 5th ed. Noida: Mosby; 2002. pp. 58–61. Ch. 5. [Google Scholar]
- 16.Traub W, Yonath A, Segal DM. On the molecular structure of collagen. Nature. 1969;221:914–7. doi: 10.1038/221914a0. [DOI] [PubMed] [Google Scholar]
- 17.Okuyama K, Okuyama K, Arnott S, Takayanagi M, Kakudo M. Crystal and molecular structure of a collagen-like polypeptide (Pro-Pro-Gly) 10. J Mol Biol. 1981;152:427–43. doi: 10.1016/0022-2836(81)90252-7. [DOI] [PubMed] [Google Scholar]
- 18.Söderhäll C, Marenholz I, Kerscher T, Rüschendorf F, Esparza-Gordillo J, Worm M, et al. Variants in a novel epidermal collagen gene (COL29A1) are associated with atopic dermatitis. PLoS Biol. 2007;5:e242. doi: 10.1371/journal.pbio.0050242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buehler MJ. Nature designs tough collagen: Explaining the nanostructure of collagen fibrils. Proc Natl Acad Sci U S A. 2006;103:12285–90. doi: 10.1073/pnas.0603216103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bella J, Eaton M, Brodsky B, Berman HM. Synthesis, degradation & remodelling of molecular structure of a collagen-like peptide at 1.9 A resolution. Science. 1994;266:75–81. doi: 10.1126/science.7695699. [DOI] [PubMed] [Google Scholar]
- 21.Bancroft JD, Gamble M. Theory and Practice of Histological Techniques. 5th ed. Edinburgh: Churchill Livingstone; 2002. pp. 139–53. [Google Scholar]
- 22.Solcia E, Capella C, Vassallo G. Lead-haematoxylin as a stain for endocrine cells. Significance of staining and comparison with other selective methods. Histochemie. 1969;20:116–26. doi: 10.1007/BF00268705. [DOI] [PubMed] [Google Scholar]
- 23.Goldfischer S, Coltoff-Schiller B, Schwartz E, Blumenfeld OO. Ultrastructure and staining properties of collagen. J Histochem Cytochem. 1983;31:382–90. doi: 10.1177/31.3.6186732. [DOI] [PubMed] [Google Scholar]
- 24.Ceena DE, Bastian TS, Ashok L, Annigeri RG. Comparative study of clinicofunctional staging of oral submucous fibrosis with qualitative analysis of collagen fibers under polarizing microscopy. Indian J Dent Res. 2009;20:271–6. doi: 10.4103/0970-9290.57356. [DOI] [PubMed] [Google Scholar]
- 25.Friess W. Collagen – Biomaterial for drug delivery. Eur J Pharm Biopharm. 1998;45:113–36. doi: 10.1016/s0939-6411(98)00017-4. [DOI] [PubMed] [Google Scholar]
- 26.Stenzel KH, Miyata T, Rubin AL. Collagen as a biomaterial. Annu Rev Biophys Bioeng. 1974;3:231–53. doi: 10.1146/annurev.bb.03.060174.001311. [DOI] [PubMed] [Google Scholar]
- 27.Lazovic G, Colic M, Grubor M, Jovanovic M. The application of collagen sheet in open wound healing. Ann Burns Fire Disasters. 2005;2:131–53. [PMC free article] [PubMed] [Google Scholar]
- 28.Goodwin SC, Yoon HC, Wong GC, Bonilla SM, Vedantham S, Arora LC. Percutaneous delivery of a heparin-impregnated collagen stent-graft in a porcine model of atherosclerotic disease. Invest Radiol. 2000;35:420–5. doi: 10.1097/00004424-200007000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Scheinfeld N. Phenytoin in cutaneous medicine: Its uses, mechanisms and side effects. Dermatol Online J. 2003;9:6. [PubMed] [Google Scholar]
- 30.Calne RY, White DJ, Thiru S, Evans DB, McMaster P, Dunn DC, et al. Cyclosporin A in patients receiving renal allografts from cadaver donors. Lancet. 1978;2:1323–7. doi: 10.1016/s0140-6736(78)91970-0. [DOI] [PubMed] [Google Scholar]
- 31.Wael M, Yousef L, Adel H, Mohamed D. Review: The mechanism of action of calcium channel blockers. Int J Diabetes Metab. 2005;13:76–82. [Google Scholar]
- 32.Sandhu SV, Gupta S, Bansal H, Singla K. Collagen in health and disease. J Orofac Res. 2012;2:153–9. [Google Scholar]
- 33.Shafer WG, Hine MK, Levy BM. A Textbook of Oral Pathology. 4th ed. Noida: Elsevier Saunders Publication; 2009. pp. 109–10. [Google Scholar]
- 34.Neville BW, Damm DD, Allen CM, Bouquot JE. Oral & Maxillofacial Pathology. 2nd ed. Noida: Elsevier Saunders Publication; 2008. pp. 349–50. [Google Scholar]
- 35.Kierzenbaum AL, Abraham L. Histology and Cell Biology – An Introduction to Pathology. St. Louis: Mosby; 2002. pp. 101–3. [Google Scholar]
- 36.Gupte T, Iyer V, Damle SG, Malik N, Halbe A. Osteogenesis imperfecta. J Indian Soc Pedod Prev Dent. 2006;24(Suppl 1):S44–6. [PubMed] [Google Scholar]
- 37.Yen JL, Lin SP, Chen MR, Niu DM. Clinical features of Ehlers-Danlos syndrome. J Formos Med Assoc. 2006;105:475–80. doi: 10.1016/S0929-6646(09)60187-X. [DOI] [PubMed] [Google Scholar]
- 38.Létourneau Y, Pérusse R, Buithieu H. Oral manifestations of Ehlers-Danlos syndrome. J Can Dent Assoc. 2001;67:330–4. [PubMed] [Google Scholar]
- 39.Rose PS, Ahn NU, Levy HP, Magid D, Davis J, Liberfarb RM, et al. The hip in Stickler syndrome. J Pediatr Orthop. 2001;21:657–63. [PubMed] [Google Scholar]
- 40.Rangasetty UC, Karnath BM. Clinical signs of Marfan syndrome. Hosp Physician. 2006;42:33–8. [Google Scholar]
- 41.Lourenço SV, de Carvalho FR, Boggio P, Sotto MN, Vilela MA, Rivitti EA, et al. Lupus erythematosus: Clinical and histopathological study of oral manifestations and immunohistochemical profile of the inflammatory infiltrate. J Cutan Pathol. 2007;34:558–64. doi: 10.1111/j.1600-0560.2006.00652.x. [DOI] [PubMed] [Google Scholar]
- 42.Cazal C, Sobral AP, Neves RF, Freire Filho FW, Cardoso AB, da Silveira MM. Oral complaints in progressive systemic sclerosis: Two cases report. Med Oral Patol Oral Cir Bucal. 2008;13:E114–8. [PubMed] [Google Scholar]
- 43.Barnett AJ, Miller M, Littlejohn GO. The diagnosis and classification of scleroderma (systemic sclerosis) Postgrad Med J. 1988;64:121–5. doi: 10.1136/pgmj.64.748.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reddy V, Wanjari PV, Banda RN, Reddy P. Oral submucous fibrosis: Correlation of clinical grading to various habit factors. Int J Dent Clin. 2011;3:21–4. doi: 10.4103/jispcd.JISPCD_92_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rajalalitha P, Vali S. Molecular pathogenesis of oral submucous fibrosis – A collagen metabolic disorder. J Oral Pathol Med. 2005;34:321–8. doi: 10.1111/j.1600-0714.2005.00325.x. [DOI] [PubMed] [Google Scholar]


