Abstract
Background:
The initiation of oral anticoagulation therapy after valve replacement surgery requires strict monitoring because these patients are at high risk for the development of thrombotic complications and present an increased risk of bleeding.
Objectives:
The aim of this study was to examine the total healthcare costs of oral anticoagulant treatment with vitamin K antagonists in patients with metallic prosthetic valves in the mitral position.
Methods:
Data from clinical records were used in the study including international normalized ratio results, number of medical visits, type of anticoagulant, use of rescue medication and hospital admissions from related complications. The drug cost was calculated based on the official Spanish Ministry of Health price list. Monitoring expenses were included in the cost of the medical supplies used in the procedures. Hospitalization costs were calculated using the diagnosis-related group price for each case.
Results:
We collected data from 151 patients receiving oral anticoagulation therapy with vitamin K antagonist who were diagnosed with mitral prosthesis (n = 90), mitro-aortic prosthesis (n = 57), and mitral and tricuspid prosthesis (n = 4). The total direct healthcare cost was €15302.59, with a mean total cost per patient per year of €1558.15 (±2774.58) consisting of 44.38 (±42.30) for drug cost, €71.41 (±21.43) for international normalized ratio monitoring, €429.52 (±126.87) for medical visits, €26.31 (±28.38) for rescue medication and €986.53 (±2735.68) for related complications.
Conclusion:
Most direct healthcare costs associated with the sampled patients arose from the specialist-care monitoring required for treatment. Good monitoring is inversely related to direct healthcare costs.
Keywords: Oral anticoagulant treatment, cost, metallic prosthetic valve
Background
Rheumatic mitral valve disease is an acquired valvular disease with an increased risk of thrombotic complications.1 In large databases in United States, mitral valve disease is the most frequent valvular lesion,2 being the prevalence in general population approximately 2.4%.3 The incidence of mitral regurgitation is greater than 10% in patients aged over 55 years; its prevalence is approximately 1.7% (1.5%–1.9%) in general population and reaches 9.3% in subjects aged over 75 years.4,5 In contrast to other countries, the main etiology in Spain is rheumatic disease.6 Thromboembolic complications are a major cause of morbidity and mortality in patients with mitral valve prostheses with a frequency of 0.6%–2.3% events per patient per year.7,8 Thromboembolic complications can be prevented with adequate oral anticoagulation therapy (OAT).9,10 OAT after valve replacement surgery requires strict monitoring because of high risk for the development of thrombotic complications and an increased postoperative sensitivity to vitamin K antagonist (VKA) (higher risk of bleeding).7,11 Usually, these patients require close monitoring of OAT-VKA and entail an expense in resources that is not well quantified.
Objective
The main objective of this study is to discern whether there is a positive correlation between maintaining the international normalized ratio (INR) in the therapeutic range (TR) and the direct healthcare costs of OAT-VKA.
Methods
The study used an observational approach and a retrospective analysis of records of patients who visited the Hematology Service of the University Hospital Fundación Jiménez Díaz (UH-FJD), Madrid, Spain, between 1 January 2008 and 30 December 2012.
Patient selection
Patient data were extracted from anonymous clinical records. Patients with a metallic prosthetic valve in the mitral position, mitro-aortic position or mitral and tricuspid position, who received OAT-VKA (warfarin sodium or acenocoumarol) were eligible for inclusion. There was no gender or age discrimination. Only data from patients who visited the hospital for a minimum period of 5 months and 1 day were included.
Data collection
The following data were collected for each patient: number of medical visits, devices required for INR monitoring (test strip, pipette and lancet), INR value of each visit, VKA dose and regimen (measured in mean weekly dose per patient and type of drug), rescue medication (measured in mean weekly dose per patient) and hospital admissions due to related complications resulting from INR values outside the TR.
In addition, other variables were analyzed:
Characteristics of the implanted prosthesis: model, implementation date and thrombotic risk (graded according to the recommendations of the European Guidelines of Cardiology 2012);
Admissions or emergency room visits due to related complications of OAT-VKA;
Hospitalization for bridge therapy for invasive procedures: procedures and the type of bridge used.
Data analysis
A previously developed model was used to measure the time within the therapeutic range (TTR).12 The cardiologist or cardiovascular surgeon usually assigned each patient a TR based on either the prosthesis model or thrombotic and/or bleeding complications related to OAT.1,6 The patients were divided into three groups according to TR: 2.5–3.5, 3–3.5 and 3–4. Visits were classified as “visits within the TR” or “visits outside the TR”:
2.5–3.5: INR > 2.5 or INR ⩽ 3.5
3–3.5: INR > 3 or INR ⩽ 3.5
3–4: INR > 3 or INR ⩽ 4
Visits outside the TR entailed additional costs, so also took into account rescue medication, costs stemming from related complications (emergency visits, hospitalizations and bridge therapy) and more frequent monitoring requirements.
According to the method of Rosendaal et al., to calculate the TTR, we estimated the number of days when each patient exhibited values within the TR and recorded the INR value of each of these days. The INR value recorded on a given visit cannot be taken in isolation. Our clinical experience shows that INR readings fluctuate between visits and those values can fall within the TR at some time points but not others. Several adjustments were made to avoid error. The data were not considered linear when more than 56 days elapsed between any two visits; therefore, these periods were excluded from the calculation as previously recommended.13 INR data collected on the 5 days following a known interruption of treatment were considered void. The patients were divided into two groups:
Group 1: <49% of TTR;
Group 2: ⩾50% of TTR.
Healthcare resources assessment
The total direct healthcare costs were calculated as the sum of the following costs:13 OAT-VKA, INR monitoring, medical visits, rescue medication (heparin/vitamin K) and related complications (hospitalizations, bridge therapy and emergency room visits). Direct healthcare costs are available as Supplementary Material (Table II).
OAT (drug costs)
Drug prices (VKA and rescue medication) were obtained from the Spanish official drug database.14 The annual mean treatment cost per patient was calculated using the following formula
where DD is the daily dose (mg/day), P is the price per milligram and TD is the treatment (days).
INR monitoring
INR monitoring included internal assessments of the healthcare resources required to perform the test (strips, pipettes and lancets) and when necessary the medical equipment needed to confirm the results.
Medical visits (for INR monitoring)
According to the hospital’s analytical accounting, the cost of the medical visit was €17.41/visit.
Rescue medication
Costs of additional medication directly related to OAT-VKA: low molecular weight heparin (LMWH) and vitamin K 2.5 mg ampoule, vial 10 mg vitamin K (Supplementary Table II). Prices were obtained from the Spanish official drug database.
Related complications
Hospital admissions, emergency visits and admissions for bridging therapy were measured according to the diagnosis-related group (DRG) price applied for each particular admission (admissions due to hemorrhage or thrombosis). All costs were estimated in current euros for the year 201015 (Supplementary Table II).
Results
Patients’ characteristics
The study included 151 patients with the following primary diagnoses: mitral prosthesis (n = 90), mitro-aortic prosthesis (n = 57) and mitral and tricuspid prosthesis (n = 4). The mean age was 66.62 (±10.28) years and 77.5% were women. The mean patient follow-up was 36 (±7.43) months (Supplementary Table I).
OAT (drug)
The mean weekly dosage was 14.63 (±6.7) mg. The OAT total cost was €15302.49 with a mean cost per patient of €101.34 (±57.46) during the study period (5 years); the mean cost per patient per year is €44.38 (±42.30) (Table 1).
Table 1.
Total direct costs for anticoagulated patients with metallic prosthetic valves in the mitral position.
| Total cost € (%) | Mean cost per patient over study period |
Mean cost estimated per patient per year |
|||||
|---|---|---|---|---|---|---|---|
| € (%) | SD | Median | € (%) | SD | Median | ||
| OAT (drug) treatment | €15302.49 (2.7%) | €101.34 (2.6%) | €57.46 | €94,76 | €44.38 (2.8%) | €42.30 | €32.412 |
| INR monitoring | €31053.41 (5.4%) | €205.65 (5.4%) | €109.69 | €237,78 | €71.41 (4.6%) | €21.43 | €71.13 |
| Medical visits | €187060.76 (32.4%) | €1238.81 (32.4%) | €659.39 | €1444,69 | €429.52 (27.4%) | €126.87 | €433.95 |
| Rescue medication | €10564.18 (1.8%) | €69.96 (1.8%) | €76.93 | €46.71 | €26.31 (1.7%) | €28.38 | €19.08 |
| Related complications | |||||||
| Emergency visits | €23604.00 | €156.32 | €278.43 | €0.00 | €65.74 | €126.37 | €0.00 |
| Hospitalization | €180366.29 | €1194.48 | €2854.74 | €0.00 | €678.3 | €2443.66 | €0.00 |
| Bridge therapy | €129630.06 | €858.47 | €6263.17 | €0.00 | €242.49 | €1064.37 | €0.00 |
| Total | €333600.35 (57.8%) | €2209.27 (57.7%) | €6263.18 | €0.00 | €986.53 (63.3%) | €2735.68 | €0.00 |
| Total | €577581.18 | €3825.04 | €6483.09 | €2295.34 | €1558.15 | €2774.58 | €659.93 |
OAT: oral anticoagulation therapy; SD: standard deviation; INR: international normalized ratio.
INR monitoring
INR monitoring total cost was €31053.41 with a mean estimated cost per patient for the study period of €205.65 (±109.69) and an estimated cost per patient per year of €71.41 (±21.43) (Table 1).
Medical visits
The patients registered 10,747 total visits with a mean of 71.17 (±37.88) visits per patient throughout the 5-year study period. During the study period, 76 visits were first-time visits for patients with mitral prosthesis and the remainders (10,671) were successive visits. We forecast the mean number of visits per patient per year would be 24.68 (±7.19). The estimated cost per patient per year for medical visits was €429.52 (±126.87) (Table 1).
Rescue medication
The cost of rescue medication was €10564.18, with a mean cost per patient of €69.96 (±76.93) and an estimated cost per patient per year of €26.31 (±28.38) (Table 1).
Related complications
During the study, 31 patients were hospitalized due to complications related to OAT, leading to 603 hospital admission days. Regarding the hemorrhagic complications, 23 patients needed hematoid concentration transfusions; the most frequent reason for hospitalization was rectal bleeding. In regard to thrombotic complications, seven patients suffered an ictus and five stent-related thrombosis events were observed. Furthermore, 16 patients were hospitalized due to bridge therapy with unfractionated heparin (UFH) stemming from invasive procedures or surgery that required interruption of OAT.
Throughout the study, 84 visits to emergency care were itemized, three of which were due to a transitory ischemic attack. The other 81 visits were due to hemorrhagic complications. The total hospital admission cost was €333600.35 for all patients (n = 151) over the study period. The mean cost per patient per year was €986.53 (±2735.68) (Table 1).
Effect of time in TR
We analyzed whether the INR results measured during each visit were within the TR. A total of 4317 (40.16%) out of all visits were classified as INR values “within TR.” Figure 1(a) shows the visits that were below, within or above the TR. The group with the highest percentage of patients within TR had a target INR of 2.5–3.5. In contrast, the group with the lowest percentage of patients within TR had a target INR of 3–3.5.
Figure 1.
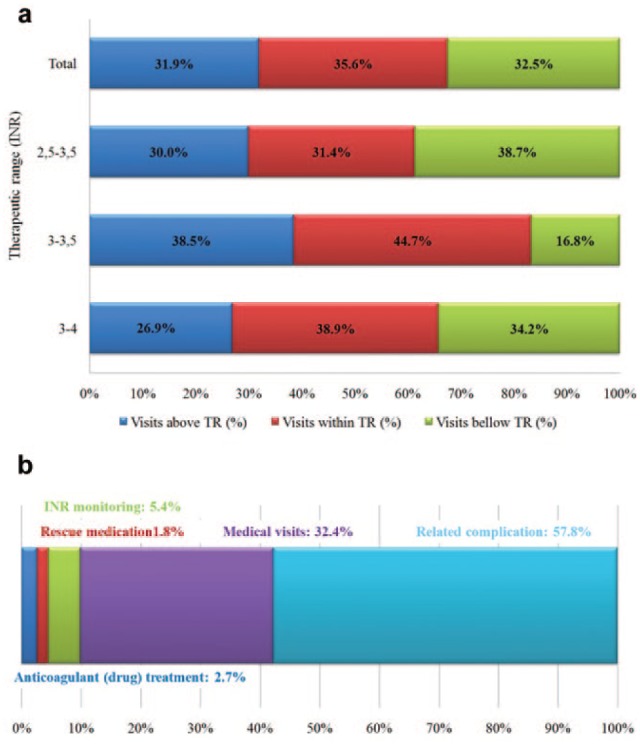
(a) Percentage of visits within TR. (b) Percentage of total direct treatment costs for anticoagulated patients with metallic prosthetic valves in the mitral position.
TR: therapeutic range; INR: international normalized ratio.
The mean cost per patient per year for subjects with a TTR < 49% was €1620.96 (±1717.34), and for patients with a TTR ⩾ 50% was €1499.37 (±3497.27) (Table 2). This difference is due to the costs associated with related complications and rescue medication.
Table 2.
Mean direct costs of oral anticoagulation therapy stratified by time in therapeutic range (TTR).
| OAT | INR monitoring | Medical visits | Rescue medication | Related complications | Total | |
|---|---|---|---|---|---|---|
| TTR < 49% (n = 73) | ||||||
| Mean | €45.07 | €72.26 | €428.56 | €31.15 | €1049.08 | €1620.96 |
| SD | €42.48 | €22.31 | €130.57 | €28.80 | €1695.68 | €1717.34 |
| TTR ⩾ 50% (n = 78) | ||||||
| Mean | €43.74 | €70.62 | €430.41 | €21.78 | €928.00 | €1499.37 |
| SD | €42.38 | €20.71 | €124.15 | €27.43 | €3447.26 | €3497.27 |
| All (n = 151) | ||||||
| Mean | €44.38 | €71.41 | €429.52 | €26.31 | €986.53 | €1558.15 |
| SD | €42.30 | €21.43 | €126.87 | €28.38 | €2735.68 | €2774.58 |
OAT: oral anticoagulation treatment with vitamin K antagonists; INR: international normalized ratio; SD: standard deviation.
Effect of target therapeutic INR
The mean cost is higher in the group of patients with a target INR of 3–3.5. Significant differences were observed for the cost of INR monitoring between the group with TR = 2.5–3.5 (€69.55 ± 22.48) and the group with TR = 3–3.5 (€78.42 ± 17.96) (p = 0.084). Significant differences were also observed in the cost of rescue medication from the group with TR = 2.5–3.5 (€24.99 ± 26.92) and the group with TR = 3–4 (€45.97 ± 45.39) (p = 0.014). Mean direct costs of OAT by TR are shown in Table 3.
Table 3.
Mean direct costs of oral anticoagulation therapy stratified by target therapeutic range (INR).
| Total cost € (%) | Mean cost per patient over study period |
Mean cost per patient per year |
|||||
|---|---|---|---|---|---|---|---|
| € | SD | Median | € | SD | Median | ||
| 2.5–3.5 (n = 102) | |||||||
| OAT | 9995.53(2.8) | € 98.00 | €52.05 | €95.18 | € 43.26 | € 37.72 | € 31.25 |
| INR monitoring | € 19853.19 (5.5) | € 194.64 | €108.90 | €203.62 | € 69.55 | € 22.48 | € 68.53 |
| Medical visits | € 120309.29 (33.4) | € 1179.50 | €658.80 | €1201.0 | € 421.07 | € 134.80 | 417.28 |
| Rescue medication | € 6246.26 (1.7) | € 61.24 | €63.02 | €37.95 | € 24.99 | € 26.92 | 16.24 |
| Related complications | € 203929.07 (56.6) | € 1999.30 | €7039.36 | €0.00 | € 838.06 | € 2293.90 | € 0.00 |
| Total | € 360333.34 | € 3532.68 | €7298.29 | €2102.87 | € 1396.93 | € 2334.78 | € 630.14 |
| 3–3.5 (n = 34) | |||||||
| OAT | € 3464.93 (2.1) | € 101.91 | €60.33 | €95.58 | € 42.08 | € 54.29 | € 32.52 |
| INR monitoring | € 8259.41(4.9) | € 242.92 | €100.01 | €269.21 | € 78.42 | € 17.96 | € 76.81 |
| Medical visits | € 49484.85 (29.3) | € 1455.44 | €598.08 | €1636.15 | € 469.04 | € 103.16 | € 465.01 |
| Rescue medication | € 2475.74 (1.5) | € 72.82 | €71.93 | €60.37 | € 21.63 | € 19.07 | € 20.47 |
| Related complications | € 105184.16 (62.3) | € 3093.65 | €4816.33 | €0.00 | € 1537.52 | € 4124.82 | € 0.00 |
| Total | € 168869.09 | € 4966.74 | €4864.84 | €2583.81 | € 2148.68 | € 4171.60 | € 751.78 |
| 3–4 (n = 15) | |||||||
| OAT | € 1842.03 (3.8) | € 122.80 | €81.73 | €93.70 | € 57.23 | € 41.93 | € 41.74 |
| INR monitoring | € 2940.81(6.1) | € 196.05 | €124.16 | €267.84 | € 68.13 | € 18.93 | € 66.55 |
| Medical visits | € 17266.61(35.7) | € 1151.11 | €732.46 | € 1514.31 | € 397.37 | € 104.16 | € 393.87 |
| Rescue medication | € 1842.18 (3.8) | € 122.81 | €137.86 | €90.97 | € 45.97 | € 45.39 | € 24.42 |
| Related complications | € 24487.12 (50.6) | € 1632.47 | €2356.13 | €281.00 | € 747.22 | € 1076.79 | € 122.83 |
| Total | € 48378.75 | € 3225.25 | € 2469.29 | € 2.550.79 | € 1315.91 | € 1018.29 | € 781.73 |
OAT: oral anticoagulation therapy with vitamin K antagonists; INR: international normalized ratio; SD: standard deviation.
Total costs
The total cost for the study period was €577581.18; the mean cost per patient over the study period was €3825.04 (±6483.09) and the estimated mean cost per patient per year was €1558.15 (±2774.58) (Table 1). Related complications (57.76%) and medical visits (32.39%) comprised the largest proportion of the costs over the study period (Figure 1(b)).
Discussion
Patients with prosthetic heart valves in the mitral position are a special group among the subjects receiving indefinite OAT, because they have a higher thrombotic risk and in general are patients with more comorbidity. VKA are the only anticoagulant drugs available for the chronic treatment of these patients. This group of drugs has a peculiar management strategy due to its pharmacokinetic characteristics, the need for close monitoring of its anticoagulant effect and dose adjustment in situations of infra- or supradosing. Control of OAT-VKA generates a series of non-negligible costs although the drug acquisition costs are very low. The majority of spending is generated due to the costs of monitoring visits and INR control.
We considered the VKA consumption, INR monitoring, medical visits and complications related to the OAT that resulted in hospitalization, and estimated the cost of treatment with VKA to be €44.38 (±€42.30) per patient per year. This cost was double that reported by Navarro et al.,16 which was €22 per patient per year; however, only 19% of these patients had prostheses.
The cost of additional rescue medication due to poor INR control was €69.96 (±76.93) per patient during the 5-year period (mean €26.31 per patient per year). We found that patients with higher TR required an increased amount of therapy with LMWH. The cost of rescue medication was doubled at €122.81 (±137.86) over the study period (mean €45.97 per patient per year) for patients with a TR from 3 to 4 compared to patients with a TR from 2.5 to 3.5 (€61.24 (±63.02) over the study period; mean €24.99 per patient per year).
The cost of INR monitoring was €205.65 per patient over the study period which corresponds to a mean cost of €71.41 per patient per year and approximately 25 visits per year. This cost depends on the frequency of the visits, resulting in a direct proportionality between them. In the study by Navarro et al.,16 this cost included the cost of material and the cost of the medical staff, whereas in our study this cost strictly referred to the consumable materials required for the INR determination.
Certainly, one of the most important items in the cost is the human resources and infrastructure consumed, which represents a high cost. Analytical accounting methods were used to calculate the price of a medical visit by computing the costs associated with the healthcare staff, cleanliness, electricity, and water and amortizing the materials used, resulting in a cost of €17.41 per visit. When analyzing the costs of visits, we observed what group of patients generated a higher cost (TR = 2.5–3.5).
The costs of visits in this study were higher than those calculated in the work of Navarro et al.,16 which were approximately €191 per patient per year. This discrepancy may be partially due to the different invoicing model used in this study. The costs were also higher than those generated by patients with non-valvular atrial fibrillation (NVAF) in the study by Hidalgo-Vega et al.17 These results are consistent because patients with mitral prostheses are a more complex group that requires more accurate monitoring and develops worse complications related to OAT.
The mean cost of complications related to OAT-VKA was estimated to be €2209.27 per patient over the study period (mean €986.53 per patient per year). This cost was higher in the group of patients with TR = 3–3.5.
The mean cost per patient per year was €1558.15. As discussed above, to date no published cost study has been performed solely in patients with mechanical mitral prosthesis on a national or international level; thus, we cannot compare our results with other studies. However, recently, studies investigating the costs of OAT-VKA have been published, especially in patients with NVAF, which is a much more common disorder with several therapeutic alternatives to classic VKA.
Regarding the direct costs of OAT-VKA, a study of a group of patients with NVAF in the UH-FJD estimated that the mean cost (including drug costs, INR monitoring, rescue medication and medical visits: hospital admissions for thrombotic or hemorrhagic complications) was between €392 and €1341 depending on the scenario of valuation applied.17 Moreover, positive and significant correlations between costs and patients with poor INR control have been described. An analysis of the direct healthcare costs must reference one work that involved the major hospitals of the Spanish national health system in 2008 by Navarro et al.,16 because it is the only study that collected the cost of OAT-VKA in a disaggregated manner and also included patients with mitral prostheses.
The direct cost of patients with mitral prosthesis is higher than patients with NVAF. Patients with prosthetic heart valves in the mitral position had a cost of €3825.04 per 5 years in our study (€1558.15 per year), which was much higher than the costs recorded by Hidalgo et al. of €392 per year17 and the €441 per year reported by Navarro et al.16 Therefore, these patients generate a higher cost to the National Health System than patients with NVAF because their management is complex and the risk of complications is higher. The decisive factor in the cost is monitoring visits that patients require as a result of their pathology, because the costs of medication and consumables are similar in both groups of patients.
Our study shows that there is an inverse relationship between the direct health costs and good INR control measured by the TTR. When the patients were grouped by TTR < 49% of the time and ⩾50% of time, we observed that the patients with higher TTR decreased the mean total cost per patient. Therefore, any new anticoagulant drug or method for monitoring these patients that leads to an increase in the number of better-controlled patients with a longer TTR will result in a significant reduction in direct health costs.
Self-control may be a useful alternative for patients with mitral prosthesis because this approach can improve the therapeutic control of these patients. This option would offer more perspectives for the young group of patients whose current anticoagulant control limits their way of life. Furthermore, although this approach could mean weekly checks, the costs would be smaller than monitoring in primary or hospital care, because no costs would be incurred by monitoring visits, which as we have seen represent most of the costs generated by patients undergoing OAT-VKA. Additionally, the lower expense related to associated complications is summarized.
Direct oral anticoagulants are an option for some patients (NVAF and venous thromboembolic disease) and may represent a future option for patients with metallic prosthetic valves, because they have clear advantages in terms of management, pharmacokinetics and drug interactions. However, to date, studies conducted in these patients with Dabigatran have not demonstrated an adequate safety profile.18
Despite the limitations of our work, this is the only study of its kind conducted to date. In this regard, there are no papers in the literature to perform an estimation of the direct health costs generated by patients indefinitely anticoagulated due to a metallic mitral prosthesis. The main limitation of our study is the small number of patients analyzed, due to the small number of patients with mechanical prosthesis in tricuspid position, for those probably, the rate of rescue medications needed for mechanical prosthesis-related complications is higher in comparison with these observed in patients with aortic, mitral or mitro-aortic prostheses. One of the limitations of this study is that we only quantified the direct health costs. Thus, the non-health costs have not been calculated, but given the nature of the sample (an elderly population) it seems likely that they would be reduced because the majority of patients are pensioners. However, the lack of direct healthcare costs related to informal care received by this type of patient may be important, and it is to quantify these costs in a future analysis.
Conclusion
Cost studies that take into account as many variables as possible are necessary to make a correct and global estimation of the economic impact of each treatment. Similarly, the availability of studies that determine which patients are at higher risk of developing complications and thus generate greater costs to the National Health System is an essential element of efficient management.
Finally, this work highlights the need to monitor patients who are indefinitely anticoagulated due to a metallic prosthesis valve in the mitral position, including the monitoring of both clinical and economic aspects. Decisions made in the future on the introduction of new drugs for these patients or changes in their clinical management cannot ignore the efficiency aspects of the different alternatives evaluated. Furthermore, our work shows that increasing the number of patients within the TR results in a considerable cost reduction.
Supplementary Material
Acknowledgments
The authors thank Lara Martin, Isaac Aranda and Alexandra Ivanova for their time, knowledge and support in analyzing the data.
Footnotes
Declaration of conflicting interests: G.E. received a non-oriented grant from Fundación Madrileña de Hematología y Hemoterapia that funded parts of this study.
Ethical approval: The databases used did not contain a variable allowing for the individual identification of patients. According to Spanish legislation on observational post-authorization studies for medicinal products for human use (SAS/3470/2009 order (16 December)), this study’s characteristics (observational methodology and retrospective analysis) do not make it necessary to obtain authorization from the hospital Ethics Committee.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the research project ECON2013-48217-C2-2-R “Impacto económico, sanitario y social de las enfermedades y los problemas de salud: información y herramientas para la evaluación de políticas públicas.”
Informed consent: Verbal informed consent was obtained from all subjects before the study. Although data were collected anonymously, all patients were informed by their doctor that the data would be used for the preparation of this work so that each of the subjects gave their consent orally.
References
- 1. Whitlock RP, Sun JC, Fremes SE, et al. ; American College of Chest P. Antithrombotic and thrombolytic therapy for valvular disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(2 Suppl.): e576S-e600S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation 2012; 125(1): e2–e220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Freed LA, Levy D, Levine RA, et al. Prevalence and clinical outcome of mitral-valve prolapse. N Engl J Med 1999; 341(1): 1–7. [DOI] [PubMed] [Google Scholar]
- 4. Thourani VH, Weintraub WS, Guyton RA, et al. Outcomes and long-term survival for patients undergoing mitral valve repair versus replacement: effect of age and concomitant coronary artery bypass grafting. Circulation 2003; 108(3): 298–304. [DOI] [PubMed] [Google Scholar]
- 5. Nkomo VT, Gardin JM, Skelton TN, et al. Burden of valvular heart diseases: a population-based study. Lancet 2006; 368(9540): 1005–1011. [DOI] [PubMed] [Google Scholar]
- 6. Joint Task Force on the Management of Valvular Heart Disease of the European Society of C; European Association for Cardio-Thoracic Surgery; Vahanian A, Alfieri O, Andreotti F, et al. Guidelines on the management of valvular heart disease (version 2012). Eur Heart J 2012; 33(19): 2451–2496. [DOI] [PubMed] [Google Scholar]
- 7. Ageno W, Turpie AG. Exaggerated initial response to warfarin following heart valve replacement. Am J Cardiol 1999; 84(8): 905–908. [DOI] [PubMed] [Google Scholar]
- 8. American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions. Society of Thoracic Surgeons; Bonow RO, Carabello BA, Kanu C, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): developed in collaboration with the Society of Cardiovascular Anesthesiologists: endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. Circulation 2006; 114(5): e84–e231. [DOI] [PubMed] [Google Scholar]
- 9. Gersh BJ, Tsang TS, Seward JB. The changing epidemiology and natural history of nonvalvular atrial fibrillation: clinical implications. Trans Am Clin Climatol Assoc 2004; 115: 149–159; discussion159–160. [PMC free article] [PubMed] [Google Scholar]
- 10. Fuster V, Ryden LE, Cannom DS, et al. Guidelines for the management of patients with atrial fibrillation. Executive summary. Rev Esp Cardiol 2006; 59(12): 1329. [PubMed] [Google Scholar]
- 11. Goldhaber SZ. “Bridging” and mechanical heart valves: perils, promises, and predictions. Circulation 2006; 113(4): 470–472. [DOI] [PubMed] [Google Scholar]
- 12. Rosendaal FR, Cannegieter SC, van der Meer FJ, et al. A method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemost 1993; 69(3): 236–239. [PubMed] [Google Scholar]
- 13. Drummond MF, Torrance GW, Stoddart G, et al. Methods for the economic evaluation of health care programmes. Oxford: Oxford University Press, 1997. [Google Scholar]
- 14.Ministerio de Sanidad PSeI: Nomenclator DIGITALIS-INTEGRA, http://www.msssi.gob.es/profesionales/nomenclator.do
- 15.Orden 731/2013, de 6 de septiembre, del Consejero de Sanidad: precios públicos por la prestación de los servicios y actividades de naturaleza sanitaria de la Red de Centros de la Comunidad de Madrid (BOCM nº 215, de 10 de septiembre). Available at: https://www.bocm.es/
- 16. Navarro JL, César JM, Fernández MA, et al. Tratamiento anticoagulante oral. Estudio coste/beneficio. Rev Adm Sanit 2008; 6(3): 525-542 . [Google Scholar]
- 17. Hidalgo-Vega Á, Vidal R, Aranda-Reneo I, et al. Direct vitamin k antagonist anticoagulant treatment health care costs in patients with non-valvular atrial fibrillation. BMC Health Serv Res 2014; 30(14): 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Van de Werf F, Brueckmann M, Connolly SJ, et al. A comparison of dabigatran etexilate with warfarin in patients with mechanical heart valves: THE Randomized, phase II study to evaluate the safety and pharmacokinetics of oral dabigatran etexilate in patients after heart valve replacement (RE-ALIGN). Am Heart J 2012; 163(6): 931.e1–937.e1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


