Abstract
Background
Chronic pain conditions are highly prevalent in patients with mild traumatic brain injury. Supraspinal diffuse axonal injury is known to dissociate brain functional connectivity in these patients. The effect of this dissociated state on supraspinal pain network is largely unknown. A functional magnetic resonance imaging study was conducted to compare the supraspinal pain network in patients with mild traumatic brain injury to the gender and age-matched healthy controls with the hypothesis that the functional connectivities of the medial prefrontal cortices, a supraspinal pain modulatory region to other pain-related sensory discriminatory and affective regions in the mild traumatic brain injury subjects are significantly reduced in comparison to healthy controls.
Results
The mild traumatic brain injury group (N = 15) demonstrated significantly (P < 0.01, cluster threshold > 150 voxels) less activities in the thalamus, pons, anterior cingulate cortex, insula, dorsolateral prefrontal cortex, and medial prefrontal cortices than the healthy control group (N = 15). Granger Causality Analyses (GCA) indicated while the left medial prefrontal cortices of the healthy control group cast a noticeable degree of outward (to affect) causality inference to multiple pain processing related regions, this outward inference pattern was not observed in the mild traumatic brain injury group. On the other hand, only patients’ bilateral anterior cingulate cortex received multiple inward (to be affected) causality inferences from regions including the primary and secondary somatosensory cortices and the inferior parietal lobe. Resting state functional connectivity analyses indicated that the medial prefrontal cortices of the mild traumatic brain injury group demonstrated a significantly (P < 0.01, F = 3.6, cluster size > 150 voxels) higher degree of functional connectivity to the inferior parietal lobe, premotor and secondary somatosensory cortex than the controls. Conversely, the anterior cingulate cortex of the healthy group demonstrated significantly (P < 0.01, F = 3.84, cluster size > 150 voxels) less degree of functional connectivities to the inferior parietal lobe and secondary somatosensory cortex than their mild traumatic brain injury counterparts.
Conclusions
In short, the current study demonstrates that patients with mild traumatic brain injury and headaches appear to have an altered state of supraspinal modulatory and affective functions related to pain perception.
Keywords: Traumatic brain injury, chronic posttraumatic headaches, pain, functional magnetic resonance imaging, supraspinal pain processing, mild traumatic brain injury, resting state functional connectivity
Introduction
Chronic pain conditions such as persistent headache are highly prevalent in patients with mild traumatic brain injury (MTBI). This patient population was known to have a state of microscopic diffuse central axonal injury affecting supraspinal functional connectivities.1,2 To what extent this impaired functional connectivity may affect supraspinal pain processing both at evoked and resting states is largely unknown. As in other neurological diseases, understanding the underlying neurological functional changes can facilitate treatment development. Based on previous studies, the supraspinal pain processing network is known to involve: (1) the thalamus (TH) and pons, which relate sensory afferent signals to other supraspinal regions; (2) the sensory discriminatory regions including the primary and secondary somatosensory cortices (SSC1 and SSC2), and the inferior parietal lobe (IPL); (3) the affective regions such as the anterior cingulate cortex (ACC) and insula (IN); and(4) the modulatory regions involving the dorsolateral prefrontal cortex (DLPFC), and various regions of the prefrontal cortices (PFCs).3,4 The IN is also implicated in assessing the magnitude of pain.3,5,6 Furthermore, the IPL is also known to be involved in spatial discriminatory functions of pain perception.7–9
Chronic pain conditions can occur as a result of maladaptation in the supraspinal pain processing and functional connectivity.3,10 In the case of MTBI, a recent study with cranial pressure pain threshold assessments suggested that the occurrence of chronic pain in MTBI could be attributed to an elevated supraspinal affective pain state and/or a lack of supraspinal modulatory functions in this patient population.11 However, further confirmatory studies are required to support this assertion and provide guidance for treatment development.
Here, the authors hypothesize that patients with MTBI suffer from a state of altered supraspinal modulatory and affective response to pain. To assess this hypothesis, a study with functional magnetic resonance imaging (fMRI) was conducted to compare the supraspinal resting state functional connectivity and response to evoked heat pain (HP) in patients with MTBI-related headache with gender and age-matched healthy controls.
Methods
With institutional human subject committee approval, subjects (all Veterans) who attended the traumatic brain injury (TBI) clinic were consented, screened, and enrolled based on the following inclusion criteria: male or female age between 18 and 60; history of MTBI and established diagnosis of posttraumatic headache based on the ICHD-212,13 diagnostic criteria including:
Headache, no typical characteristics known, fulfilling criteria C and D
- Head trauma that includes the following:
- either no loss of consciousness or loss of consciousness of <30 min duration
- Glasgow Coma Scale (GCS) ≥13
- symptoms and/or signs diagnostic of concussion as discussed in the below diagnostic criteria of MTBI
Headache occurs within seven days after head trauma
Headache persists for >three months after head trauma
Additional headache inclusion criteria consisted of: (1) an average chronic persistent daily (24/7) headache intensity greater than 30 on the 0–100 mechanical visual analog scale (M-VAS) at the screening visit (Visit 1);14 and (2) an average intensity of this chronic persistent headache greater than 3/10 on a numerical rating scale reported in the headache diary filled out daily by the patients between Visit 1 and scanning visit (Visit 2). MTBI diagnosis was based on the published criteria from the 1993 American Congress of Rehabilitation Medicine and recent recommendation from the Department of Defense.15 Specifically, the diagnostic criteria state that a traumatically induced physiological disruption of brain function, as manifested by at least one of the following categories: (1) any loss of consciousness ≤30 min, (2) posttraumatic amnesia ≤24 h, and (3) an initial GCS score was ≥13, 30 min after the injury. Exclusion criteria included: history of pacemaker implant; pregnancy; ferromagnetic material such as shrapnel, bullet fragments, or implanted devices in the brain or body that would not be compatible with MRI; history of life threatening diseases, dementia, or major psychiatric illnesses; documented diagnosis of posttraumatic stress disorder (PTSD) or Mississippi Scale for PTSD score ≥130; documented Major Depression or Hamilton Rating Scale for Depression score ≥19; presence of any other chronic neuropathic pain states or neurological diseases such as seizure; involvement of litigation; inability to understand the study instruction and to communicate in English; history of chronic headache diagnoses such as migraine, tension, or cluster headaches prior to the incidence of MTBI. However, subjects with occasional preinjury tension (less than once every three months and lasting no more than 24 h) or migraines (less than once every four months and lasting no more than 6 h) headaches were not excluded from the study. Patients’ records were verified for the diagnoses MTBI and posttraumatic headache, the mechanisms of injury and the duration of the headache. Although some subjects might have previous or subsequent incidences of MTBI, the MTBI mechanisms listed in Table 1 were confirmed as the direct causes leading to the onset of intractable persistent headache based on patient record review and interviews at the screening visit. Healthy controls were recruited from a healthy subject list consisting of students, and healthcare workers. For the current study, they were screened based on the healthy subject enrollment criteria detailed in previously published fMRI-related studies.16,17
Table 1.
Cohort of patients with mild traumatic brain injury related headache (MTBI-HA).
| Subject | Age | Gender | Mechanisms of MTBI | MTBI Event | History of HA prior to MTBI Event | Average Daily HA Intensity (M-VAS) | Duration of persistent MTBI-HA (months) | Medication for HA |
|---|---|---|---|---|---|---|---|---|
| 1 | 36 | F | NB | FALL | NO | 45 | 36 | None |
| 2 | 27 | F | NB | BHT | NO | 50 | 84 | None |
| 3 | 29 | F | NB | MCA | NO | 80 | 72 | None |
| 4 | 33 | F | NB | ASSAULT | NO | 50 | 84 | None |
| 5 | 54 | M | NB | BHT | NO | 30 | 84 | Amitriptylinea |
| 6 | 28 | M | B | IED | NO | 60 | 48 | None |
| 7 | 26 | M | B | IED | NO | 60 | 48 | Gabapentina, Tramadolb |
| 8 | 39 | M | B | IED | NO | 60 | 60 | Desipraminea |
| 9 | 25 | M | B | IED | NO | 45 | 84 | None |
| 10 | 40 | M | B | IED | NO | 60 | 108 | Amitriptylinea, Aspirinb |
| 11 | 35 | M | B | IED | NO | 70 | 96 | Duloxetinea |
| 12 | 33 | M | B | IED | NO | 100 | 84 | Nortriptylinea, Sumatriptinb |
| 13 | 40 | M | B | IED | NO | 55 | 72 | Naproxenb |
| 14 | 26 | M | B | IED | NO | 50 | 36 | None |
| 15 | 38 | M | NB | BHT | NO | 50 | 48 | Depsipraminea, Naproxenb |
| AVERAGE | 34 | 58 | 70 |
HA: headache; M-VAS: Mechanical Visual Analog Scale Score; M: male; F: female; B: blast related; NB: non-blast related; FALL: falling accident; BHT: blunt head trauma; MCA: motorcycle accident; IED: improvised explosive device.
Daily headache medication.
As needed headache medication.
Prescanning assessments
Prior to each fMRI session, each subject was asked to rate the average intensity of their persistent daily headaches on a M-VAS.14 An elastic band consisting of 13 increments was used to consistently mark the location of the thermal threshold measurement and HP stimulation. The band was extended from the medial malleolus to the medial tibial plateau and the testing/stimulation location was marked at the medial aspect of the left calf between the sixth and seventh marking of an elastic band. A Thermal Sensory Analyzer (Medoc Advanced Medical Systems, MN) was used to assess the non-noxious and noxious thermal thresholds including cool and warm, cold, and heat pain thresholds via a thermode with a contact area of 30 × 30 mm. This method of peripheral sensory testing has been well established in literature and commonly applied to pain-related studies.18–22 After the initial thermal sensory threshold assessments, a 6-sec HP stimulus at a subject specific threshold was applied at the marked location. The subjects were then asked to rate the intensities of HP on a M-VAS. During the HP-fMRI session, the HP threshold temperature with the intensity score ≥30 was delivered as the HP stimulus to the subject. This method of subject specific HP delivery minimizes any unnecessary distraction to the subjects during the scanning and thus optimizing the correlation of supraspinal responses with the stimulation paradigms.16,17,23
Neuroimaging data acquisition
Patients were asked not to take any headache-related medications on the day of scanning. Head movement during scanning were minimized by instructing the subjects to hold the head still during the scanning, applying padding between the subjects’ head and the head coil, and having subjects wear a cervical collar to minimize both lateral and axial head movements.23,24 A 5-min resting fMRI data were collected via a 1.5T GE scanner with T2*-weighted EPI-sequence (TE = 30 ms, TR = 2.0 s, α = 90°, TH = 4 mm, 32 slices, FOV = 220 × 220 mm2, MA = 64 × 64). Evoked HP fMRI was conducted with the same pulse sequence and intermittent 6 sec of subject threshold specific HP stimuli delivered via a fMRI-compatible Peltier probe at the medial aspect of the left mid-calf with various intervals (20–40 sec) of a baseline temperature at 32℃. This established stimulation paradigm was repeated 20 times to complete the session.16 Anatomical scans were obtained with rapid gradient-echo (MP RAGE) samplings (176 slices T1, 256 × 256 and 1 cm slice thickness).
Data analysis
A simple paired t-test analysis was conducted for the thermal sensory threshold data, and within- and between-group random effect analyses, and GCA were conducted for the fMRI data in the Brain Voyager platform using protocols described by Goebel et al.25 Using Self-organizing group level Independent Component Analysis (SogICA),26,27 between-group (MTBI > healthy) pain-related clusters were adopted as seeded regions for subsequent ANCOVA resting state functional connectivity analyses.
fMRI data processing and analysis
Preprocessing of functional data
First, raw functional data in the Dicom format was converted into Brain Voyager’s internal “FMR” data format. Standard preprocessing steps including head motion correction, slice scan time correction, drift removal, and spatial smoothing with Gaussian filter (FWHM = 5 mm) were performed for all functional data sets.
Preprocessing of anatomical data
The anatomical data of each subject was also converted into Brain Voyager’s “VMR” data format. Intensity inhomogeneities correction was performed, and the data were then transformed into AC-PC Talairach standard space after being resampled to 1-mm resolution. To avoid quality loss due to successive data sampling, the three spatial transformations were combined and applied backward in one step. In addition, to form a single 4 × 4 transformation matrix, the two affine transformations, iso-voxel scaling, and AC–PC transformation were concatenated. Followed by application of the inverted spatial transformation matrix, the coordinates of each voxel in the target (Talairach) space was affined back to the original space so that data points could be sampled with sinc interpolation in the original three dimensional (3D) space.
Brain segmentation
The brain was further segmented from surrounding head tissue using an automatic “brain peeling” tool as described by Goebel et al.25 so that a 3-D image of the brain could be visualized.
Cortex segmentation
For cortical functional data analysis, additional brain segmentation was carried out for the gray/white matter boundary using automatic segmentation routines.28 Applying an analysis of intensity histograms, the white/gray matter border was segmented via a region-growing method. Additional smoothing steps were subsequently applied using a “bridge removal” algorithm in which each segmented hemisphere was smoothed to topologically correct mesh representations.28 Repeated small morphing steps were also conducted until the central sulcus were visible and areas of activities including those in the sulcus could be visualized. The inflated cortical meshes were used to sample the functional data so that subsequent cortex-based data analysis in a mesh time course format could be carried out at each vertex (node) for each run of each subject.
Normalization of functional data
To transform the functional data into Talairach space, the functional time series data on each subject were first coregistered with the corresponding 3D anatomical data set. The coregistered data were then transformed into the Talairach space resulting in normalized 4D volume time course data ready for both within- and between-group analyses.
Within- and between-group general linear model analysis
First, a protocol file representing the onset and duration of the events for the stimulation conditions was derived. To account for the hemodynamic delay and dispersion, each of the predictors was derived by convoluting an appropriate box-car waveform with a double-gamma hemodynamic response function29 to extract brain regions with both positively (activation) and negatively (deactivation) correlated blood oxygen level dependent responses. In addition, to address the issues of multiple comparisons, a False Discovery Rate correction was applied. For both within- and between-group random effect analyses, any supraspinal regions with significant (P < 0.01 and cluster threshold > 150 voxels) activation and deactivation were recorded. These cluster size and statistical significance cutoffs were used in previous studies using similar HP stimulation paradigms.16,17,23 The spatial coordinates of significant clusters (VOI) were first complied in a text file allowing anatomical naming via Talairach Client (http://www.talairach.org/client.html). The naming of the regions was further confirmed with BV Tutor probabilistic anatomical map. For 3D cortical labeling, Patches of Interest (POI) were first selected using BVQX “POI Analysis Tool.” The POI’s were then converted to VOI’s to extrapolate the details (including spatial coordinates) of the defined region and classify them using the above-mentioned VOI naming methodology.
Granger Causality Analysis
GCA was conducted to explore the causal interaction (inference) of seeded region(s) of interest (SROI) extracted from between-group random effect analysis according to an established analytical procedure.16,17 A SROI was used to estimate effective connectivity among clusters in the group with the Brain Voyager’s GCA plug-in. The result of the analysis was noted as either positive values signifying significant outward (to affect) inference from the SROI to the targeted regions or negative values representing inward (to be affected) inference to the SROI.30 In addition, clusters information including coordinates, sizes, and Brodmann areas were converted by the Talairach Client into a text format after verifying the data with Brain Tutor.31,32 The resulting text was imported to a spreadsheet, and the network of inference was mapped onto a spatial representation of the brain network involved in acute thermal pain processing within each group.
All applied fMRI data processing steps, analytical approaches, and selected cluster threshold were similar to previous published HP-related studies.16,17,23
Resting state functional connectivity
Using Self-organizing group level Independent Component Analysis (SogICA),26,27 significant between-group (MTBI > healthy) pain-related clusters in the medial prefrontal cortices (MPFCs) and ACC were adopted as seeded regions for subsequent ANCOVA resting state functional connectivity analyses.
Results
Patient and healthy subjects demographic data
A total of 15 patients with MTBI and 15 healthy controls were enrolled in the study. The patient cohort consisted of 11 male subjects with an average age (years old ± SD) of 34.6 ± 8.7 and 4 female subjects with an average age of 31.2 ± 4.0. The average ages of the male and female healthy subjects were 33.6 ± 9.2 and 29.5 ± 5.5, respectively. Although three patients (Subject #s 1, 4, and 5 in Table 1) reported an additional head trauma event prior to the headache causing MTBI incidence, the remainder recalled only a single MTBI event leading to the symptom of persistent headache after the injury. In the healthy control group, none of the subjects reported any significant head trauma meeting the diagnostic criteria of either MTBI or posttraumatic chronic headache as stipulated in the study enrollment criteria. In the patient group, the average duration of headache was around 70 months, and the average daily headache intensity on a 0–100 Mechanical Analogue Scale (M-VAS ± SD) was 57.7 ± 16.4 (Table 1). Nine MTBI patients reported blast-related injury due to improvised explosive devices and the remaining six subjects sustained non-blast-related head trauma. The average headache intensities (M-VAS ± SD) for blast-related and non-blast-related injury were 62.2 ± 15.8 and 50.8 ± 16.2, respectively. No statistical significant difference (P = 0.20) in the headache intensity was found between the two injury mechanisms in a two-tail t-test analysis. All anatomical brain MRI showed no gross pathology.
Thermal sensory thresholds analysis
The cool, warm, cold, and heat pain thresholds (℃ ± SD) for the healthy subjects were 24.3 ± 3.0, 39.2 ± 3.9, 3.0 ± 4.5, and 47.6 ± 2.2, respectively, and for the patients were 23.2 ± 2.7, 37.8 ± 2.7, 1.5 ± 1.9, and 46.1 ± 3.0, respectively. No significant difference in thermal sensory thresholds was found between the two groups.
Within-group fMRI data analysis
The within-group random effect analyses indicated with a brief period of HP stimuli, the MTBI group demonstrated a significant (P < 0.01, cluster threshold > 150 voxels) supraspinal activation primarily in the ACC region, whereas the matched healthy group demonstrated significant (P < 0.01, cluster threshold > 150 voxels) deactivations mainly in the sensory discriminatory region (SSC2), and activation in the pain assessment (IN) and modulatory regions including the premotor and motor cortices, and the PFCs (see Tables 2 and 3).
Table 2.
MTBI within-group analysis.
| Hemisphere | Regions of activity | T value | Cluster voxel size | Brodmann area | Peak coordinates X, Y, Z | P value |
|---|---|---|---|---|---|---|
| Affective and emotional | ||||||
| ACC | 3.751666 | 161 | 31 | 11, −35, 44 | 0.002146 | |
ACC: Anterior Cingulate Cortex.
Table 3.
Healthy within-group analysis.
| Hemisphere | Regions of activity | T value | Cluster voxel size | Brodmann area | Peak coordinates X, Y, Z | P value |
|---|---|---|---|---|---|---|
| Left | Sensory/discriminatory | |||||
| SSC2 | −3.927339 | 221 | 7 | −37, −50, 54 | 0.001518 | |
| Affective and emotional | ||||||
| Insula | 4.564844 | 457 | 39 | −31, 25, 0 | 0.000441 | |
| Neuromodulatory response | ||||||
| Prefrontal cortex | 4.107708 | 1235 | 11 | −24, 16, −2 | 0.001066 | |
| Right | Sensory/discriminatory | |||||
| SSC2 | −4.946156 | 182 | 7 | 38, −30, 60 | 0.000215 | |
| Neuromodulatory response | ||||||
| Motor | 4.460837 | 237 | 4 | 20, 1, 57 | 0.000538 | |
SSC: Secondary Somatosensory Cortex.
Between-group fMRI data analysis
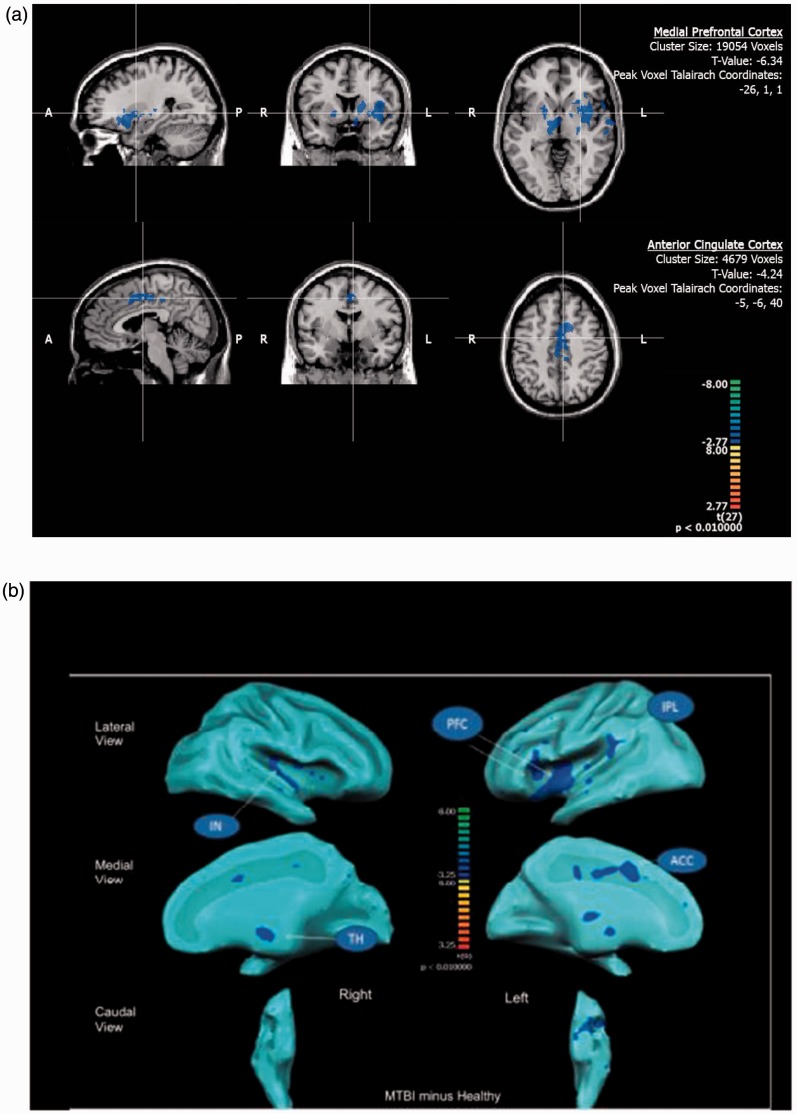
The between-group random effect analysis (MTBI group > Healthy group) indicated the MTBI group demonstrated significantly (P < 0.01, cluster threshold > 150 voxels) less activity in the thalamus, pons, insula, DLPFC, MPFCs, and ACC (Figure 1(a)) than the healthy subject group when both groups were exposed to a similar intensity and duration of HP stimuli (see Table 4 and Figure 1(b)).
Figure 1.
(a) Between-group brain activity differences in response to heat pain stimulation at the seeded regions (medial prefrontal cortex and anterior cingulate cortex) marked with crosshair showing significantly (P < 0.01, cluster threshold > 150 voxels) less activities (blue color in the reference Z-scale) in the mild traumatic brain injury patients in comparison to their healthy counterparts. A: Anterior: P: Posterior; R: Right; L: Left. (b) An overall cortical projection of between-group (mild traumatic brain injury patients minus healthy controls) differences in response to heat pain stimulation with brain areas showing significantly (P < 0.01, cluster threshold > 150 voxels) less activities (blue color in the Z-scale reference). PFCs: Medial Prefrontal Cortices; IPL: inferior Parietal Lobe; ACC: Anterior Cingulate Cortex; TH: Thalamus; IN: Insula.
Table 4.
Between-group analysis (MTBI > healthy).
| Hemisphere | Region of activity | T value | P value | BA | Cluster voxel size | X | Y (Peak) | Z |
|---|---|---|---|---|---|---|---|---|
| Left | Affective and emotional | |||||||
| Insula | −3.560392 | 0.001398 | 13 | 406 | −25 | −23 | 18 | |
| ACC (B) | −4.23592 | 0.000236 | 24–31 | 4679 | 2 | −23 | 42 | |
| Sensory/discriminatory | ||||||||
| Thalamus | −4.045769 | 0.000392 | n/a | 764 | −13 | −20 | −3 | |
| SSC2 | −4.007399 | 0.000434 | 7 | 402 | −13 | −56 | 57 | |
| Neuromodulatory response | ||||||||
| PFCs | −6.337683 | 0.000001 | 11 | 19054 | −31 | 13 | −12 | |
| Right | Affective and emotional | |||||||
| Insula | −4.309925 | 0.000194 | 13 | 3415 | 41 | −8 | 0 | |
| ACC (B) | −4.23592 | 0.000236 | 24–31 | 4679 | 2 | −23 | 42 | |
| Sensory/discriminatory | ||||||||
| SSC2 | −3.572439 | 0.001355 | 5 | 783 | 5 | −50 | 57 | |
| Neuromodulatory response | ||||||||
| DLPFC | −3.665129 | 0.001066 | 46 | 836 | 26 | 16 | 6 | |
| Pons | −4.198683 | 0.000261 | n/a | 401 | −1 | −17 | −24 | |
ACC(B): bilateral anterior cingulate cortices; SSC2: secondary somatosensory cortex; DLPFC: dorsolateral prefrontal cortex; PFCs: prefrontal cortices; BA: brodmann area; n/a: non-applicable.
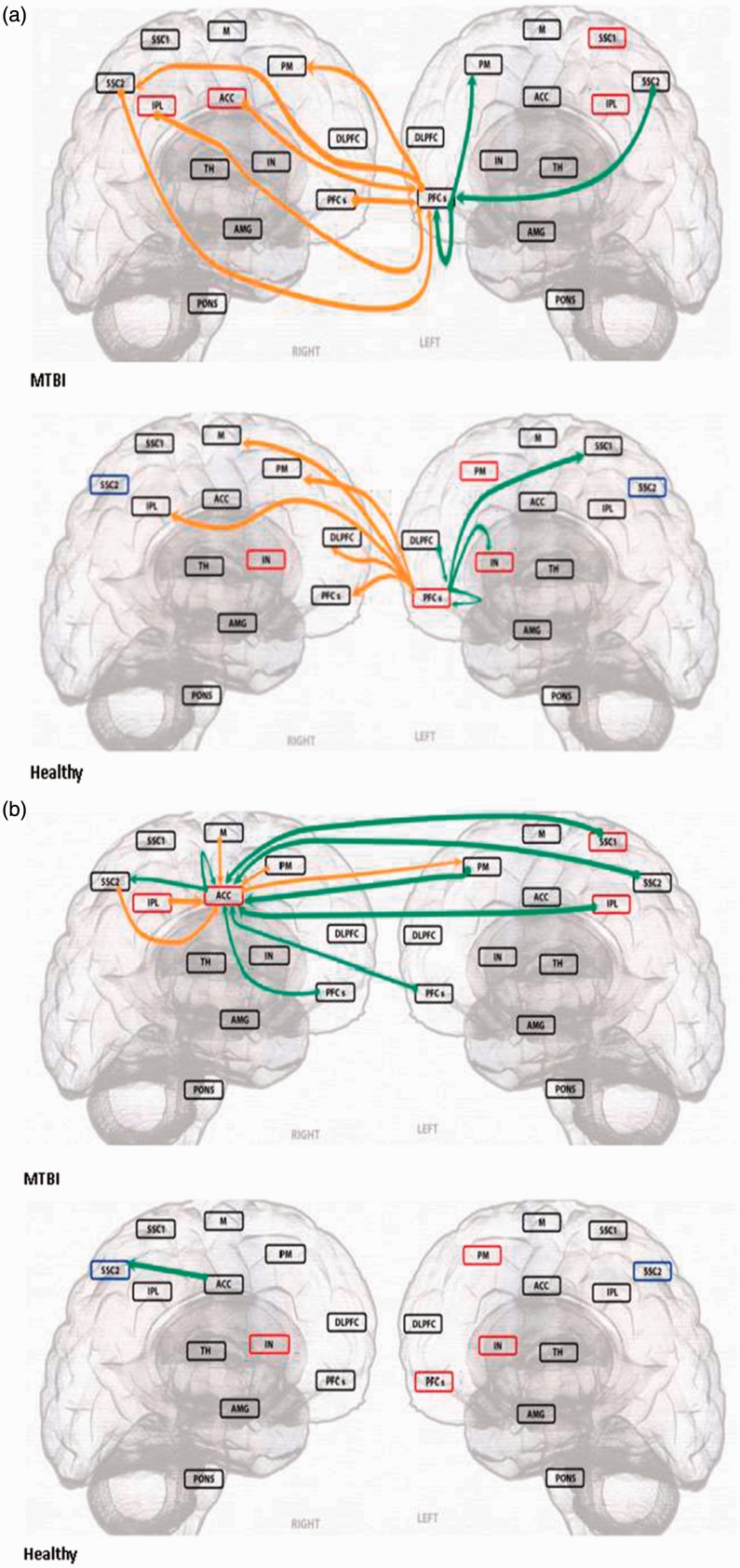
Granger Causality Analysis
To assess causal interactions of the brain regions involved in pain-related affective and modulatory functions, two (left MPFCs and ACC) large (>4000 voxels) seeded region of interest (SROIs) extracted from the between-group analysis were subjected to the within-group GCA. The healthy subjects’ within-group GCA of the left MPFCs indicated the regions cast significant outward (to affect) causality inference to multiple regions involving the SSC1, IPL, M, PM, IN, DLPFC, and PFCs, whereas a similar within-group analysis of the MTBI subjects demonstrated the same SROI mainly received inward (to be affected) causality inferences from multiple regions including the SSC2, IPL, ACC, and contralateral PFCs and cast very few outward inferences to the other pain-related supraspinal regions except the bilateral PM (Figure 2(a)). In addition, the healthy subjects’ ACC (extending both hemispheres and only shown as in the right hemisphere) cast an outward causality inference only to the SSC2, whereas the same SROI in the MTBI subjects received inward causality inferences from multiple regions (SSC1, SSC2, and IPL) primarily involving in the sensory discriminatory function (Figure 2(b)).
Figure 2.
| Green arrow | Orange arrow | |
|---|---|---|
| SROI casts outward inference | [RT]>>>>LT | [RT]>>>>RT |
| [LT]>>>>LT | [LT]>>>>RT | |
| SROI receive inward inference | LT>>>>[RT] | RT>>>>[RT] |
| LT>>>>[LT] | RT>>>>[LT] |
Resting state functional connectivity
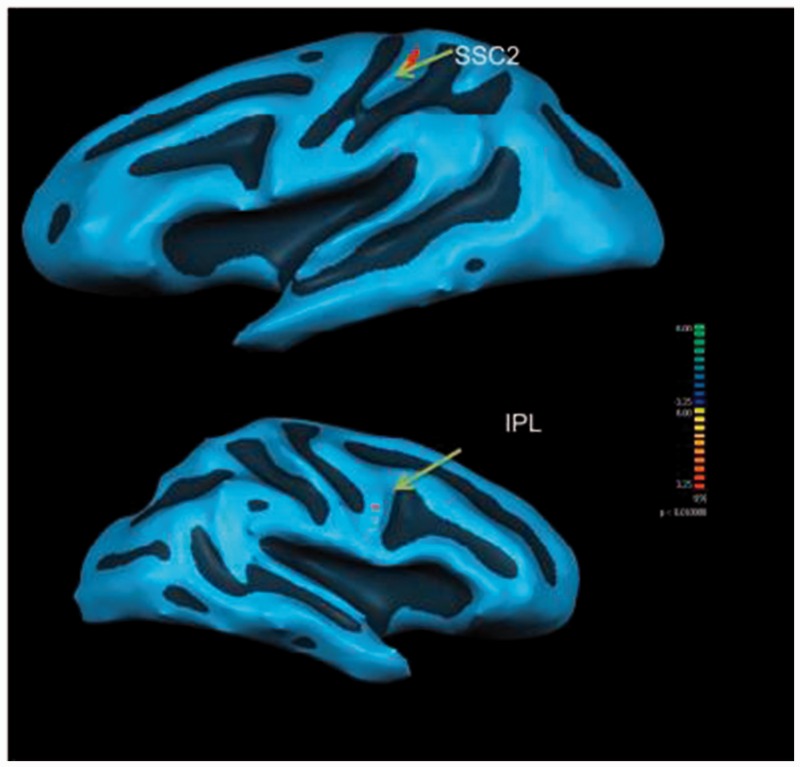
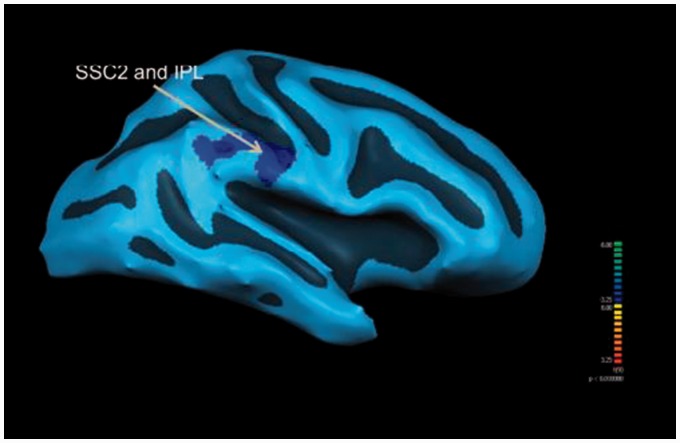
With the left MPFCs as the seeded region, the MTBI group demonstrated significantly (P < 0.01, F = 3.6, cluster size > 150 voxels) higher functional connectivities to the IPL, premotor, and secondary somatosensory cortex (SSC2). On the other hand, the ACC of the healthy group demonstrated significantly (P < 0.01, F = 3.84, cluster size > 150 voxels) less functional connectivities to the IPL and SSC2 than their MTBI counterparts at the resting state (see Figures 3 and 4).
Figure 3.
Resting state functional connectivity difference with the left medial prefrontal cortex (seeded region) of the Healthy Controls (N = 15) demonstrating more significant (P < 0.01) functional connectivity to the left secondary somatosensory cortex (SSC2) and right inferior parietal lobe (IPL) than patients with mild traumatic brain injury (N = 15).
Figure 4.
Resting state functional connectivity difference with the right anterior cingulate cortex (seeded region) of the healthy controls (N = 15) demonstrating less significant (P < 0.01) functional connectivities to the right secondary somatosensory cortex (SSC2) and inferior parietal lobe (IPL) than patients with mild traumatic brain injury (N = 15).
Discussion
Several observations from the current study are worthy of discussion. First, in the within-group analysis, the MTBI patients demonstrated supraspinal activation mainly in regions associated with pain affective response (ACC), but no corresponding response from the modulatory regions when they were exposed to a short period of HP stimuli. On the other hand, the healthy subjects demonstrated robust responses from the supraspinal modulatory regions including the motor and PFCs when they were subjected to the same intensity and duration of noxious stimuli. Further within-group functional causal connectivity assessments of the modulatory response in the two groups indicated the MTBI subjects’ MPFCs cast a much lower number of outward causality inferences to other pain-related supraspinal regions than the healthy subjects. On the other hand, the ACC of the MTBI subjects received a much higher degree of inward causality inference from the sensory and discriminatory pain processing regions in comparison to the healthy subjects. This contrast in functional causality connectivities between the MTBI and the healthy subjects suggests that the supraspinal modulatory functions of MTBI subjects on the affective response to pain are diminished in comparison to the healthy controls. This observation is in line with previous studies in human experimental pain and patients with chronic pain states. In both cases, pain can occur when there is a supraspinal imbalance/dissociation between modulatory and affective responses to pain.16,33,34 Second, in the between-group comparison, the MTBI patients were found to be less reactive in the supraspinal pain modulatory response involving the MPFCs in comparison to the age and gender matched healthy controls. These findings highly correlate with the results of a previous human study demonstrating deficits in prefrontal cortical activities required for cognitive task35 in the MTBI patients, and other animal studies demonstrating functional deficits in PFCs after TBI resulting in pain regulatory, mood and cognitive dysfunctions.36,37,38 Thus, these current findings further support an emerging assertion that chronic headache is a form of central pain conditions due to damage in brain functions involved in supraspinal pain processing.39 In addition, increasing evidence suggests that either peripherally or centrally originated pain can become centralized through maladaptive responses within the supraspinal pain network, causing pain perception and mood alterations.40 Therefore, chronic pain conditions such as intractable persistent headache should be considered as a brain disease. Given that the sample size in the current study is similar to previous published functional imaging studies involving HP, the authors believe these statistically significant findings are highly relevant to the supraspinal impairments caused by the traumatic injury incidences.3
Second, no significant differences were found between the two groups in their sensory threshold assessments, suggesting that the ascending pain pathway in the patient group was not significantly affected as most of the axonal injury occurs in the supraspinal region. With diffuse axonal injury in the major cortical white matter tracts, MTBI patients are known to have functional deficit in fine motor skill, attention, mood, and memory.41–46 Correlating with these functional deficits is elevated motor evoked potential thresholds found in this patient population.47 These abnormal electrophysiological findings in the MTBI patients suggest a deficiency in the cortical excitability and conductivity in brain areas associated with pain modulation/adaptation. This recently acquired understanding in MTBI symptom-related neuronal morphological and functional changes provides a feasible direction for treatment development. Already, recent studies have demonstrated that non-invasively stimulating supraspinal regions known to have pain modulatory functions may minimize the headache symptoms in patients with MTBI and other associated post-concussive symptoms.48,49 Thus, applicable non-invasive neuromodulatory or functionally restoring treatment options such as transcranial magnetic stimulation should be considered in this patient population.50,51
While both PTSD and depression are common comorbid conditions in MTBI, the impact of these conditions on pain perception has not been thoroughly defined. A previous study demonstrated that MTBI patients with major depression showed significantly more activities in bilateral amygdalae, thalamus, and prefrontal cortical activities during face matching of fearful stimuli than MTBI patients without depression. Interestingly, while some studies suggested MTBI subjects with major depression demonstrated similar supraspinal functions as those with depression independent of MTBI52 or PTSD without MTBI,53 other studies have demonstrated a positive correlation among white matter deficits, default mode network connectivity deficiency with the occurrence of PTSD, and depressive symptoms in the MTBI patients.54,55 To minimize these confounding issues, the current study excluded patients with significant depression and PTSD symptoms meeting the clinical diagnostic criteria. Thus, to fully understand the impact of these comorbid conditions in pain, future studies correlating the severity of these comorbid symptoms to the severities of supraspinal pain processing alteration should be conducted.
The current study consists of several limitations worthy of further discussion. First, the heterogeneity in the mechanisms of injury may minimize the generalization of the observed results to any particular mechanisms of injury. Although the mechanisms of injury may differ, recent studies suggest both blast and non-blast MTBI patients suffer from similar neuropsychological impairments,56 and there are no significant differences between the two main injury mechanisms in various neuropsychological impairments.57,58 Other studies also suggest TBI itself, independent of injury mechanisms and combat exposure intensity, is a primary driver of adverse outcomes59 and symptoms such as PTSD and depression are correlated with MTBI independent of the mechanisms of injury.60 On the other hand, the severity of headache appears to correlate with the severity of depression and PTSD.61–65 Thus, future studies correlating the severity of headache or pain to supraspinal prefrontal cortical activities and psychological symptoms such as depression and PTSD can further enhance our current understanding in their relationship. Second, the chronicity of the headache problem in the studied patient population was quite extensive with the average duration of headache at 70 months and a duration range of 36 to 108 months. This rather wide range of headache durations and an overall high chronicity of the condition precluded the generalization of the result to any acute/sub-acute injury settings. Thus, aside from using the seven-day onset International Classification of Headache Disorder (ICHD-2) diagnostic criteria, future studies may consider assessing the effect of specific increments of chronicity on supraspinal pain processing and modulation. Furthermore, memory dysfunction is a common occurrence in MTBI patient population. Thus, although the investigators conducted due diligence in verifying the patients’ reported date of injury and the onset of headache by carefully reviewing their medical records, minor discrepancies could not be completely excluded. In addition, while the current study demonstrates patients with MTBI appear to suffer from a state of supraspinal pain modulatory functional deficiency, it does not definitively establish the causal effect of the altered state in the development of their headache. Nevertheless, as a first step in characterizing the supraspinal functional deficit associated with MTBI, the current study results do demonstrate with MTBI, supraspinal pain processing and modulation are being affected. Further studies comparing military personnel with non-traumatic headache and/or trauma without headaches are required to further verify the results of these initial mechanistic findings.
Conclusions
In short, patients with MTBI appear to suffer from an altered state of supraspinal modulatory and affective functions related to pain perception. Further assessments are required to correlate the specificity of the current findings to the development of chronic persistent headaches.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The authors thank the funding support of VA Rehabilitation and Research Development Award.
References
- 1.Lewine JD, Davis JT, Bigler ED, et al. Objective documentation of traumatic brain injury subsequent to mild head trauma: multimodal brain imaging with MEG, SPECT, and MRI. J Head Trauma Rehabil 2007; 22: 141–155. [DOI] [PubMed] [Google Scholar]
- 2.Huang MX, Theilmann RJ, Robb A, et al. Integrated imaging approach with MEG and DTI to detect mild traumatic brain injury in military and civilian patients. J Neurotrauma 2009; 26: 1213–1226. [DOI] [PubMed] [Google Scholar]
- 3.Apkarian AV, Bushnell MC, Treede RD, et al. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain 2005; 9: 463–484. [DOI] [PubMed] [Google Scholar]
- 4.Tracey I. Nociceptive processing in the human brain. Curr Opin Neurobiol 2005; 15: 478–487. [DOI] [PubMed] [Google Scholar]
- 5.Neugebauer V, Galhardo V, Maione S, et al. Forebrain pain mechanisms. Brain Res Rev 2009; 60: 226–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tracey I. Neuroimaging of pain mechanisms. Curr Opin Support Palliat Care 2007; 1: 109–116. [DOI] [PubMed] [Google Scholar]
- 7.Oshiro Y, Quevedo AS, McHaffie JG, et al. Brain mechanisms supporting spatial discrimination of pain. J Neurosci 2007; 27: 3388–3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seifert F, Fuchs O, Nickel FT, et al. A functional magnetic resonance imaging navigated repetitive transcranial magnetic stimulation study of the posterior parietal cortex in normal pain and hyperalgesia. Neuroscience 2010; 170: 670–677. [DOI] [PubMed] [Google Scholar]
- 9.Moulton EA, Pendse G, Becerra LR, et al. BOLD responses in somatosensory cortices better reflect heat sensation than pain. J Neurosci 2012; 32: 6024–6031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bouwense SA, Olesen SS, Drewes AM, et al. Is altered central pain processing related to disease stage in chronic pancreatitis patients with pain? An exploratory study. PLoS One 2013; 8: e55460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Defrin R, Riabinin M, Feingold Y, et al. Deficient pain modulatory systems in patients with mild traumatic brain and chronic post-traumatic headache: implications for its mechanism. J Neurotrauma 2015; 32: 28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joubert J. Diagnosing headache. Aust Fam Physician 2005; 34: 621–625. [PubMed] [Google Scholar]
- 13.Headache Classification Subcommittee of the International Headache S. The international classification of headache disorders: 2nd edition. Cephalalgia 2004; 24(Suppl 1): 9–160. [DOI] [PubMed] [Google Scholar]
- 14.Price DD, Bush FM, Long S, et al. A comparison of pain measurement characteristics of mechanical visual analogue and simple numerical rating scales. Pain 1994; 56: 217–226. [DOI] [PubMed] [Google Scholar]
- 15.Ruff RM, Iverson GL, Barth JT, et al. Recommendations for diagnosing a mild traumatic brain injury: a national academy of neuropsychology education paper. Arch Clin Neuropsychol 2009; 24: 3–10. [DOI] [PubMed] [Google Scholar]
- 16.Leung A, Shukla S, Li E, et al. Supraspinal characterization of the thermal grill illusion with fMRI. Mol Pain 2014; 10: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leung A, Zhao Y, Shukla S. The effect of acupuncture needle combination on central pain processing – an fMRI study. Mol Pain 2014; 10: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leung A, Khadivi B, Duann JR, et al. The effect of Ting point (tendinomuscular meridians) electroacupuncture on thermal pain: a model for studying the neuronal mechanism of acupuncture analgesia. J Altern Complement Med 2005; 11: 653–661. [DOI] [PubMed] [Google Scholar]
- 19.Leung A, Li E, Fallah A, et al. The effect of needle combination on the analgesic efficacy of the tendinomuscular meridians(TMM) systems. Med Acupuncture 2007; 19: 191–199. [Google Scholar]
- 20.Leung AY, Kim SJ, Schulteis G, et al. The effect of acupuncture duration on analgesia and peripheral sensory thresholds. BMC Complement Altern Med 2008; 8: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leung A, Wallace MS, Ridgeway B, et al. Concentration-effect relationship of intravenous alfentanil and ketamine on peripheral neurosensory thresholds, allodynia and hyperalgesia of neuropathic pain. Pain 2001; 91: 177–187. [DOI] [PubMed] [Google Scholar]
- 22.Leung AY, Wallace MS, Schulteis G, et al. Qualitative and quantitative characterization of the thermal grill. Pain 2005; 116: 26–32. [DOI] [PubMed] [Google Scholar]
- 23.Shukla S, Torossian A, Duann JR, et al. The analgesic effect of electroacupuncture on acute thermal pain perception – a central neural correlate study with fMRI. Mol Pain 2011; 7: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leung A, Duann JR, Davis M, et al. Facial-Cervical Collar Restraint (FCCR) device in reducing head motion during a noxious stimulus study. Neuroimage 2006; 31: 504. [Google Scholar]
- 25.Goebel R, Esposito F, Formisano E. Analysis of functional image analysis contest (FIAC) data with brainvoyager QX: from single-subject to cortically aligned group general linear model analysis and self-organizing group independent component analysis. Hum Brain Mapp 2006; 27: 392–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Esposito F, Scarabino T, Hyvarinen A, et al. Independent component analysis of fMRI group studies by self-organizing clustering. Neuroimage 2005; 25: 193–205. [DOI] [PubMed] [Google Scholar]
- 27.Himberg J, Hyvarinen A, Esposito F. Validating the independent components of neuroimaging time series via clustering and visualization. Neuroimage 2004; 22: 1214–1222. [DOI] [PubMed] [Google Scholar]
- 28.Kriegeskorte N, Goebel R. An efficient algorithm for topologically correct segmentation of the cortical sheet in anatomical mr volumes. Neuroimage 2001; 14: 329–346. [DOI] [PubMed] [Google Scholar]
- 29.Friston KJ, Fletcher P, Josephs O, et al. Event-related fMRI: characterizing differential responses. Neuroimage 1998; 7: 30–40. [DOI] [PubMed] [Google Scholar]
- 30.Abler B, Roebroeck A, Goebel R, et al. Investigating directed influences between activated brain areas in a motor-response task using fMRI. Magn Reson Imaging 2006; 24: 181–185. [DOI] [PubMed] [Google Scholar]
- 31.Lancaster JL, Rainey LH, Summerlin JL, et al. Automated labeling of the human brain: a preliminary report on the development and evaluation of a forward-transform method. Hum Brain Mapp 1997; 5: 238–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lancaster JL, Woldorff MG, Parsons LM, et al. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp 2000; 10: 120–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmidt-Wilcke T, Ichesco E, Hampson JP, et al. Resting state connectivity correlates with drug and placebo response in fibromyalgia patients. Neuroimage Clin 2014; 6: 252–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmidt-Wilcke T, Kairys A, Ichesco E, et al. Changes in clinical pain in fibromyalgia patients correlate with changes in brain activation in the cingulate cortex in a response inhibition task. Pain Med 2014; 15: 1346–1358. [DOI] [PubMed] [Google Scholar]
- 35.Gosselin N, Chen JK, Bottari C, et al. The influence of pain on cerebral functioning after mild traumatic brain injury. J Neurotrauma 2012; 29: 2625–2634. [DOI] [PubMed] [Google Scholar]
- 36.Cordeiro Matos S, Zhang Z, Seguela P. Peripheral neuropathy induces HCN channel dysfunction in pyramidal neurons of the medial prefrontal cortex. J Neurosci 2015; 35: 13244–13256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meyer DL, Davies DR, Barr JL, et al. Mild traumatic brain injury in the rat alters neuronal number in the limbic system and increases conditioned fear and anxiety-like behaviors. Exp Neurol 2012; 235: 574–587. [DOI] [PubMed] [Google Scholar]
- 38.Hehar H, Yeates K, Kolb B, et al. Impulsivity and concussion in juvenile rats: examining molecular and structural aspects of the frontostriatal pathway. PLoS One 2015; 10: e0139842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Defrin R. Chronic post-traumatic headache: clinical findings and possible mechanisms. J Man Manip Ther 2014; 22: 36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Borsook D. Neurological diseases and pain. Brain 2012; 135: 320–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Caeyenberghs K, Leemans A, Geurts M, et al. Correlations between white matter integrity and motor function in traumatic brain injury patients. Neurorehabil Neural Repair 2011; 25: 492–502. [DOI] [PubMed] [Google Scholar]
- 42.Pal D, Gupta RK, Agarwal S, et al. Diffusion tensor tractography indices in patients with frontal lobe injury and its correlation with neuropsychological tests. Clin Neurol Neurosurg 2012; 114: 564–571. [DOI] [PubMed] [Google Scholar]
- 43.Palacios EM, Fernandez-Espejo D, Junque C, et al. Diffusion tensor imaging differences relate to memory deficits in diffuse traumatic brain injury. BMC Neurol 2011; 11: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yurgelun-Todd DA, Bueler CE, McGlade EC, et al. Neuroimaging correlates of traumatic brain injury and suicidal behavior. J Head Trauma Rehabil 2011; 26: 276–289. [DOI] [PubMed] [Google Scholar]
- 45.Levin HS, Wilde EA, Hanten G, et al. Mental state attributions and diffusion tensor imaging after traumatic brain injury in children. Dev Neuropsychol 2011; 36: 273–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ljungqvist J, Nilsson D, Ljungberg M, et al. Longitudinal study of the diffusion tensor imaging properties of the corpus callosum in acute and chronic diffuse axonal injury. Brain Inj 2011; 25: 370–378. [DOI] [PubMed] [Google Scholar]
- 47.Tallus J, Lioumis P, Hamalainen H, et al. Long-lasting TMS motor threshold elevation in mild traumatic brain injury. Acta Neurol Scand 2012; 126: 178–182. [DOI] [PubMed] [Google Scholar]
- 48.Koski L, Kolivakis T, Yu C, et al. Noninvasive brain stimulation for persistent postconcussion symptoms in mild traumatic brain injury. J Neurotrauma 2015; 32: 38–44. [DOI] [PubMed] [Google Scholar]
- 49.Leung A, Fallah A, Davani A, et al. rTMS in reducing mild TBI related headache – a pilot study. Headache 2014; 54: 90. [Google Scholar]
- 50.Leung A, Shukla S, Fallah A, et al. Repetitive transcranial magnetic stimulation in managing mild traumatic brain injury-related headaches. Neuromodulation 2016; 19: 133–141. [DOI] [PubMed] [Google Scholar]
- 51.George MS, Taylor JJ, Short EB. The expanding evidence base for rTMS treatment of depression. Curr Opin Psychiatry 2013; 26: 13–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McDonald BC, Saykin AJ, McAllister TW. Functional MRI of mild traumatic brain injury (mTBI): progress and perspectives from the first decade of studies. Brain Imaging Behav 2012; 6: 193–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Petrie EC, Cross DJ, Yarnykh VL, et al. Neuroimaging, behavioral, and psychological sequelae of repetitive combined blast/impact mild traumatic brain injury in Iraq and Afghanistan war veterans. J Neurotrauma 2014; 31: 425–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Costanzo ME, Chou YY, Leaman S, et al. Connecting combat-related mild traumatic brain injury with posttraumatic stress disorder symptoms through brain imaging. Neurosci Lett 2014; 577: 11–15. [DOI] [PubMed] [Google Scholar]
- 55.Matthews SC, Strigo IA, Simmons AN, et al. A multimodal imaging study in U.S. veterans of Operations Iraqi and Enduring Freedom with and without major depression after blast-related concussion. Neuroimage 2011; 54(Suppl 1): S69–S75. [DOI] [PubMed] [Google Scholar]
- 56.Lange RT, Pancholi S, Brickell TA, et al. Neuropsychological outcome from blast versus non-blast: mild traumatic brain injury in U.S. military service members. J Int Neuropsychol Soc 2012; 18: 595–605. [DOI] [PubMed] [Google Scholar]
- 57.Belanger HG, Kretzmer T, Vanderploeg RD, et al. Symptom complaints following combat-related traumatic brain injury: relationship to traumatic brain injury severity and posttraumatic stress disorder. J Int Neuropsychol Soc 2010; 16: 194–199. [DOI] [PubMed] [Google Scholar]
- 58.Belanger HG, Kretzmer T, Yoash-Gantz R, et al. Cognitive sequelae of blast-related versus other mechanisms of brain trauma. J Int Neuropsychol Soc 2009; 15: 1–8. [DOI] [PubMed] [Google Scholar]
- 59.Mac Donald CL, Johnson AM, Wierzechowski L, et al. Prospectively assessed clinical outcomes in concussive blast vs nonblast traumatic brain injury among evacuated US military personnel. JAMA Neurol 2014; 71: 994–1002. [DOI] [PubMed] [Google Scholar]
- 60.MacDonald CL, Johnson AM, Nelson EC, et al. Functional status after blast-plus-impact complex concussive traumatic brain injury in evacuated United States military personnel. J Neurotrauma 2014; 31: 889–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang SJ, Rushiti F, Sejdiu X, et al. Survivors of war in northern Kosovo (III): the role of anger and hatred in pain and PTSD and their interactive effects on career outcome, quality of sleep and suicide ideation. Confl Health 2012; 6: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Macgregor AJ, Dougherty AL, Tang JJ, et al. Postconcussive symptom reporting among US combat veterans with mild traumatic brain injury from Operation Iraqi Freedom. J Head Trauma Rehabil 2013; 28: 59–67. [DOI] [PubMed] [Google Scholar]
- 63.Theeler BJ, Flynn FG, Erickson JC. Chronic daily headache in U.S. soldiers after concussion. Headache 2012; 52: 732–738. [DOI] [PubMed] [Google Scholar]
- 64.Wilk JE, Herrell RK, Wynn GH, et al. Mild traumatic brain injury (concussion), posttraumatic stress disorder, and depression in U.S. soldiers involved in combat deployments: association with postdeployment symptoms. Psychosom Med 2012; 74: 249–257. [DOI] [PubMed] [Google Scholar]
- 65.Theeler BJ, Erickson JC. Posttraumatic headache in military personnel and veterans of the Iraq and Afghanistan conflicts. Curr Treat Options Neurol 2012; 14: 36–49. [DOI] [PubMed] [Google Scholar]