Abstract
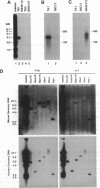
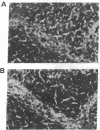
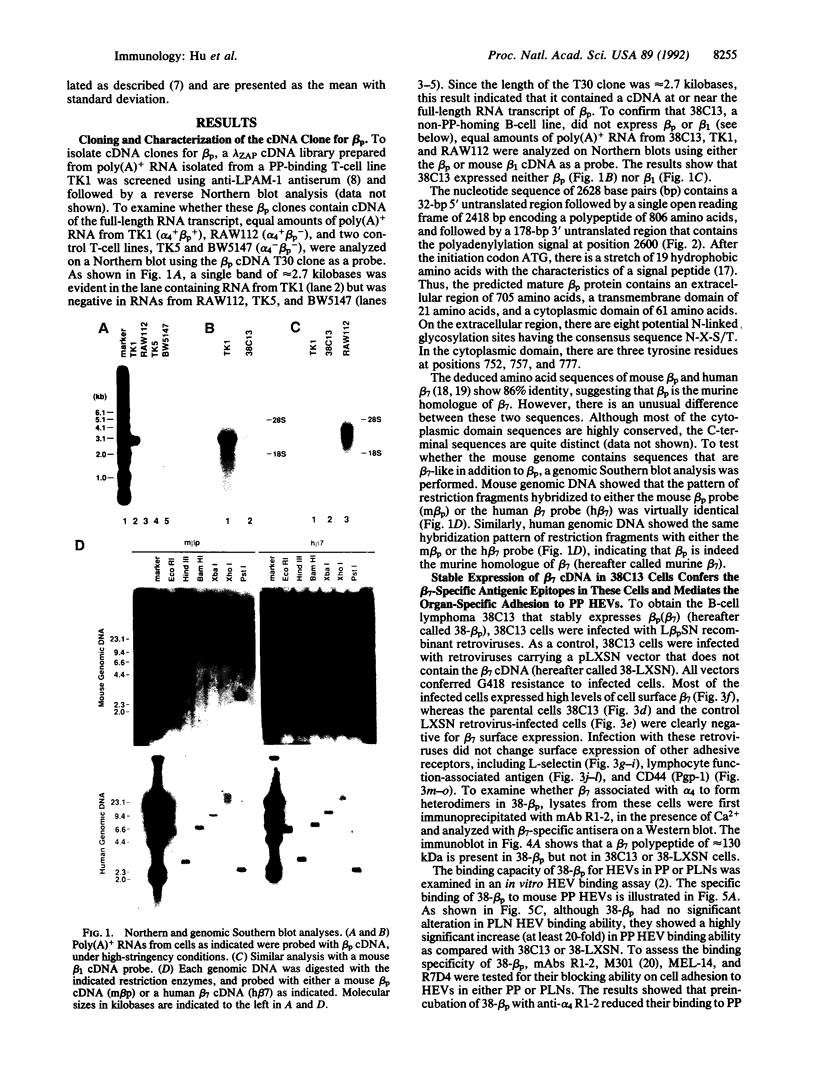
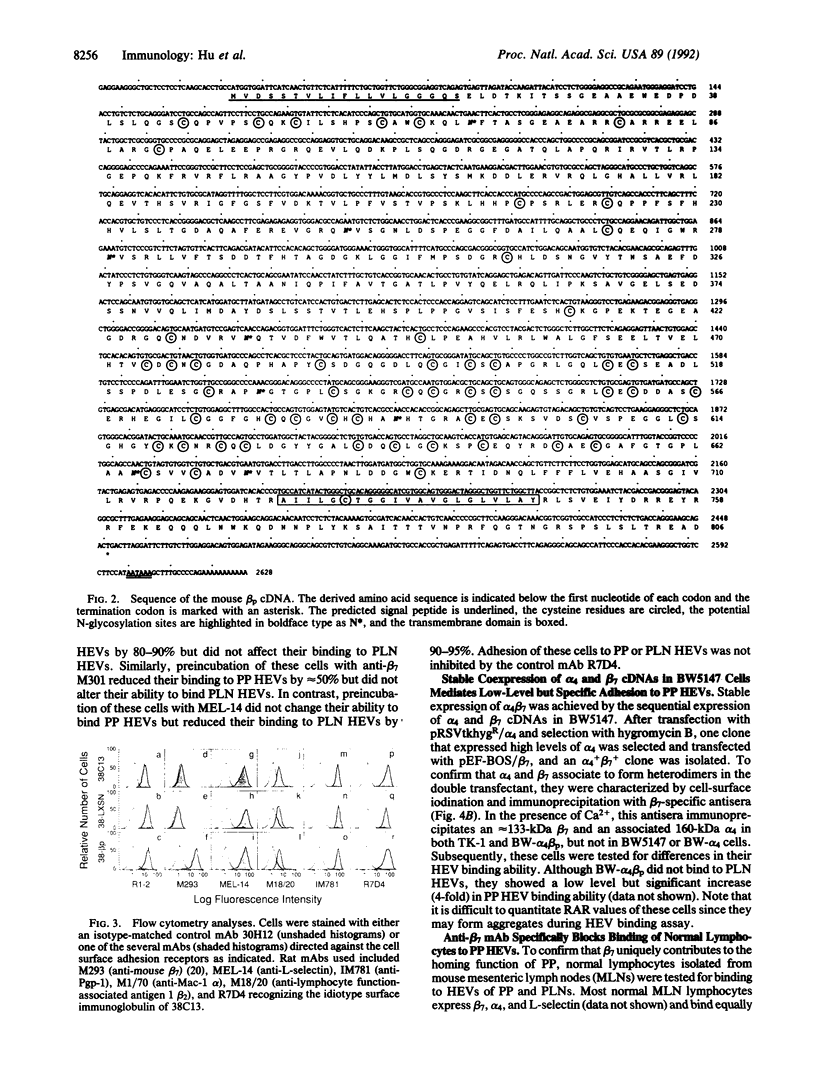
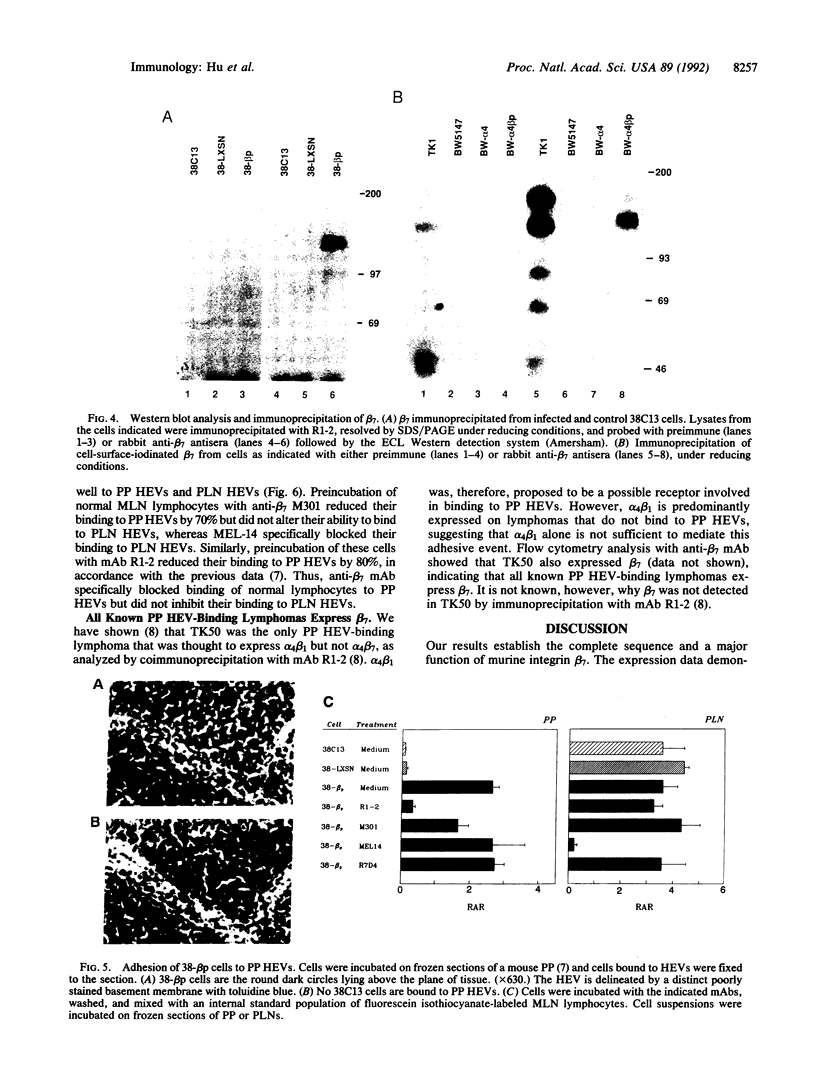
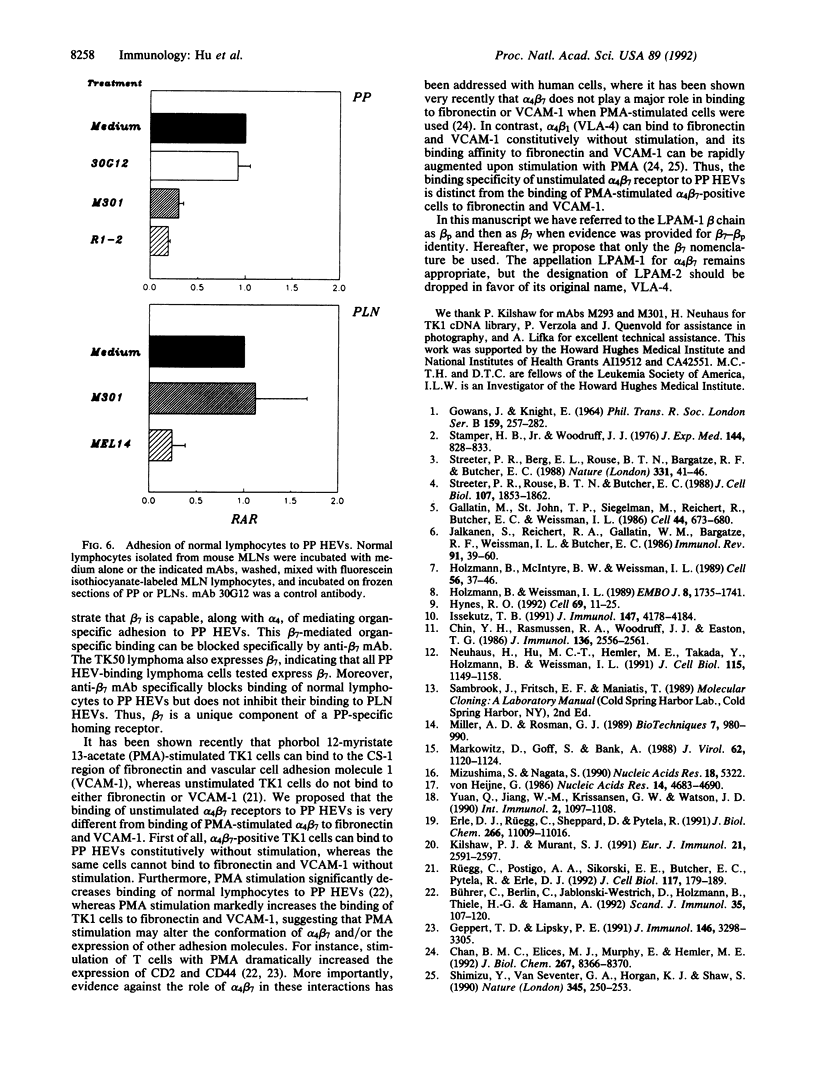
Lymphocytes express integrin receptors, termed lymphocyte Peyer's patch high endothelial venule (HEV) adhesion molecules (LPAMs), that mediate their organ-specific adhesion to specialized HEVs found in mucosal lymphoid organs (Peyer's patches). LPAM-1 consists of a murine integrin alpha 4 noncovalently associated with integrin beta p. Here, we describe the cloning and expression of a mouse cDNA encoding beta p, which is an 806-amino acid transmembrane glycoprotein. The genomic Southern blot analysis indicates that beta p is the murine homologue of human beta 7. The function of alpha 4 beta 7 as a Peyer's patch-specific adhesion molecule was tested directly by expression of the murine beta 7 cDNA in an alpha 4+ beta 7-B-cell line or coexpression of the alpha 4 and beta 7 cDNAs in an alpha 4-beta 7-T-cell line. The transfected cells exhibited a new Peyer's patch-specific adhesive phenotype that could be specifically blocked by monoclonal antibodies against alpha 4 and beta 7. Moreover, an anti-beta 7 monoclonal antibody specifically blocked binding of normal lymphocytes to Peyer's patch HEV but did not inhibit their binding to peripheral lymph node HEVs, indicating that beta 7 is a unique component of the Peyer's patch-specific homing receptor.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bührer C., Berlin C., Jablonski-Westrich D., Holzmann B., Thiele H. G., Hamann A. Lymphocyte activation and regulation of three adhesion molecules with supposed function in homing: LECAM-1 (MEL-14 antigen), LPAM-1/2 (alpha 4-integrin) and CD44 (Pgp-1). Scand J Immunol. 1992 Jan;35(1):107–120. doi: 10.1111/j.1365-3083.1992.tb02839.x. [DOI] [PubMed] [Google Scholar]
- Chan B. M., Elices M. J., Murphy E., Hemler M. E. Adhesion to vascular cell adhesion molecule 1 and fibronectin. Comparison of alpha 4 beta 1 (VLA-4) and alpha 4 beta 7 on the human B cell line JY. J Biol Chem. 1992 Apr 25;267(12):8366–8370. [PubMed] [Google Scholar]
- Chin Y. H., Rasmussen R. A., Woodruff J. J., Easton T. G. A monoclonal anti-HEBFPP antibody with specificity for lymphocyte surface molecules mediating adhesion to Peyer's patch high endothelium of the rat. J Immunol. 1986 Apr 1;136(7):2556–2561. [PubMed] [Google Scholar]
- Erle D. J., Rüegg C., Sheppard D., Pytela R. Complete amino acid sequence of an integrin beta subunit (beta 7) identified in leukocytes. J Biol Chem. 1991 Jun 15;266(17):11009–11016. [PubMed] [Google Scholar]
- GOWANS J. L., KNIGHT E. J. THE ROUTE OF RE-CIRCULATION OF LYMPHOCYTES IN THE RAT. Proc R Soc Lond B Biol Sci. 1964 Jan 14;159:257–282. doi: 10.1098/rspb.1964.0001. [DOI] [PubMed] [Google Scholar]
- Gallatin M., St John T. P., Siegelman M., Reichert R., Butcher E. C., Weissman I. L. Lymphocyte homing receptors. Cell. 1986 Mar 14;44(5):673–680. doi: 10.1016/0092-8674(86)90832-9. [DOI] [PubMed] [Google Scholar]
- Geppert T. D., Lipsky P. E. Association of various T cell-surface molecules with the cytoskeleton. Effect of cross-linking and activation. J Immunol. 1991 May 15;146(10):3298–3305. [PubMed] [Google Scholar]
- Holzmann B., McIntyre B. W., Weissman I. L. Identification of a murine Peyer's patch--specific lymphocyte homing receptor as an integrin molecule with an alpha chain homologous to human VLA-4 alpha. Cell. 1989 Jan 13;56(1):37–46. doi: 10.1016/0092-8674(89)90981-1. [DOI] [PubMed] [Google Scholar]
- Holzmann B., Weissman I. L. Peyer's patch-specific lymphocyte homing receptors consist of a VLA-4-like alpha chain associated with either of two integrin beta chains, one of which is novel. EMBO J. 1989 Jun;8(6):1735–1741. doi: 10.1002/j.1460-2075.1989.tb03566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992 Apr 3;69(1):11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Issekutz T. B. Inhibition of in vivo lymphocyte migration to inflammation and homing to lymphoid tissues by the TA-2 monoclonal antibody. A likely role for VLA-4 in vivo. J Immunol. 1991 Dec 15;147(12):4178–4184. [PubMed] [Google Scholar]
- Jalkanen S., Reichert R. A., Gallatin W. M., Bargatze R. F., Weissman I. L., Butcher E. C. Homing receptors and the control of lymphocyte migration. Immunol Rev. 1986 Jun;91:39–60. doi: 10.1111/j.1600-065x.1986.tb01483.x. [DOI] [PubMed] [Google Scholar]
- Kilshaw P. J., Murant S. J. Expression and regulation of beta 7(beta p) integrins on mouse lymphocytes: relevance to the mucosal immune system. Eur J Immunol. 1991 Oct;21(10):2591–2597. doi: 10.1002/eji.1830211041. [DOI] [PubMed] [Google Scholar]
- Markowitz D., Goff S., Bank A. A safe packaging line for gene transfer: separating viral genes on two different plasmids. J Virol. 1988 Apr;62(4):1120–1124. doi: 10.1128/jvi.62.4.1120-1124.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. D., Rosman G. J. Improved retroviral vectors for gene transfer and expression. Biotechniques. 1989 Oct;7(9):980-2, 984-6, 989-90. [PMC free article] [PubMed] [Google Scholar]
- Mizushima S., Nagata S. pEF-BOS, a powerful mammalian expression vector. Nucleic Acids Res. 1990 Sep 11;18(17):5322–5322. doi: 10.1093/nar/18.17.5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhaus H., Hu M. C., Hemler M. E., Takada Y., Holzmann B., Weissman I. L. Cloning and expression of cDNAs for the alpha subunit of the murine lymphocyte-Peyer's patch adhesion molecule. J Cell Biol. 1991 Nov;115(4):1149–1158. doi: 10.1083/jcb.115.4.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüegg C., Postigo A. A., Sikorski E. E., Butcher E. C., Pytela R., Erle D. J. Role of integrin alpha 4 beta 7/alpha 4 beta P in lymphocyte adherence to fibronectin and VCAM-1 and in homotypic cell clustering. J Cell Biol. 1992 Apr;117(1):179–189. doi: 10.1083/jcb.117.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu Y., Van Seventer G. A., Horgan K. J., Shaw S. Regulated expression and binding of three VLA (beta 1) integrin receptors on T cells. Nature. 1990 May 17;345(6272):250–253. doi: 10.1038/345250a0. [DOI] [PubMed] [Google Scholar]
- Stamper H. B., Jr, Woodruff J. J. Lymphocyte homing into lymph nodes: in vitro demonstration of the selective affinity of recirculating lymphocytes for high-endothelial venules. J Exp Med. 1976 Sep 1;144(3):828–833. doi: 10.1084/jem.144.3.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streeter P. R., Berg E. L., Rouse B. T., Bargatze R. F., Butcher E. C. A tissue-specific endothelial cell molecule involved in lymphocyte homing. Nature. 1988 Jan 7;331(6151):41–46. doi: 10.1038/331041a0. [DOI] [PubMed] [Google Scholar]
- Streeter P. R., Rouse B. T., Butcher E. C. Immunohistologic and functional characterization of a vascular addressin involved in lymphocyte homing into peripheral lymph nodes. J Cell Biol. 1988 Nov;107(5):1853–1862. doi: 10.1083/jcb.107.5.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Q. A., Jiang W. M., Krissansen G. W., Watson J. D. Cloning and sequence analysis of a novel beta 2-related integrin transcript from T lymphocytes: homology of integrin cysteine-rich repeats to domain III of laminin B chains. Int Immunol. 1990;2(11):1097–1108. doi: 10.1093/intimm/2.11.1097. [DOI] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]