Abstract
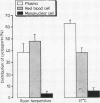
In patients receiving cyclosporin to minimise graft versus host disease after allogeneic bone marrow transplantation, whole blood cyclosporin concentration was roughly twice the serum concentration when blood was separated at 37 degrees C. In turn, blood separation at 37 degrees C resulted in a doubling of serum cyclosporin concentration compared with separation at room temperature. In vitro studies showed that the latter phenomenon was due to a temperature dependent partitioning of cyclosporin between plasma and red cells, such that increased cyclosporin was taken up from the serum into red cells at room temperature. Increasing delay in separation of patient blood (at either temperature) resulted in a gradually increasing cyclosporin serum concentration. Further in vitro studies showed that a distribution equilibrium between blood components was reached within 30 min incubation. Red cell uptake of cyclosporin was saturable at an incubation concentration of greater than 4 microgram/ml, while plasma and mononuclear cells showed a linear uptake to 7 micrograms/ml. The cellular cyclosporin content of a mononuclear cell was roughly 1000 times greater than that of an erythrocyte. For clinical monitoring we recommend the measurement of cyclosporin concentration either in whole blood or in serum separated at 37 degrees C without delay after venepuncture.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrus L., Lafferty K. J. Inhibition of T-cell activity by cyclosporin A. Scand J Immunol. 1981 May;15(5):449–458. doi: 10.1111/j.1365-3083.1982.tb00670.x. [DOI] [PubMed] [Google Scholar]
- Atkinson K., Biggs J. C., Britton K., Short R., Mrongovius R., Concannon A., Dodds A. Oral administration of cyclosporin A for recipients of allogeneic marrow transplants: implications of clinical gut dysfunction. Br J Haematol. 1984 Feb;56(2):223–231. doi: 10.1111/j.1365-2141.1984.tb03950.x. [DOI] [PubMed] [Google Scholar]
- Atkinson K., Biggs J. C., Hayes J., Ralston M., Dodds A. J., Concannon A. J., Naidoo D. Cyclosporin A associated nephrotoxicity in the first 100 days after allogeneic bone marrow transplantation: three distinct syndromes. Br J Haematol. 1983 May;54(1):59–67. doi: 10.1111/j.1365-2141.1983.tb02067.x. [DOI] [PubMed] [Google Scholar]
- Barrett A. J., Kendra J. R., Lucas C. F., Joss D. V., Joshi R., Pendharkar P., Hugh-Jones K. Cyclosporin A as prophylaxis against graft-versus-host disease in 36 patients. Br Med J (Clin Res Ed) 1982 Jul 17;285(6336):162–166. doi: 10.1136/bmj.285.6336.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggs J., Atkinson K., Concannon A., Dodds A., Harkness J., Yuile P., Causer P., Bashir H., Penny R., Nicholls M. Bone marrow transplantation in 33 patients with malignant blood diseases and severe aplastic anaemia. Med J Aust. 1983 Aug 6;2(3):120–125. doi: 10.5694/j.1326-5377.1983.tb122359.x. [DOI] [PubMed] [Google Scholar]
- Bunjes D., Hardt C., Röllinghoff M., Wagner H. Cyclosporin A mediates immunosuppression of primary cytotoxic T cell responses by impairing the release of interleukin 1 and interleukin 2. Eur J Immunol. 1981 Aug;11(8):657–661. doi: 10.1002/eji.1830110812. [DOI] [PubMed] [Google Scholar]
- Donatsch P., Abisch E., Homberger M., Traber R., Trapp M., Voges R. A radioimmunoassay to measure cyclosporin A in plasma and serum samples. J Immunoassay. 1981;2(1):19–32. doi: 10.1080/01971528108062989. [DOI] [PubMed] [Google Scholar]
- Hows J. M., Chipping P. M., Fairhead S., Smith J., Baughan A., Gordon-Smith E. C. Nephrotoxicity in bone marrow transplant recipients treated with cyclosporin A. Br J Haematol. 1983 May;54(1):69–78. doi: 10.1111/j.1365-2141.1983.tb02068.x. [DOI] [PubMed] [Google Scholar]
- Hows J. M., Palmer S., Gordon-Smith E. C. Use of cyclosporin A in allogeneic bone marrow transplantation for severe aplastic anemia. Transplantation. 1982 Apr;33(4):382–386. doi: 10.1097/00007890-198204000-00008. [DOI] [PubMed] [Google Scholar]
- Hows J. M., Smith J. M. In vitro stability of cyclosporin A. J Clin Pathol. 1983 Jun;36(6):720–721. doi: 10.1136/jcp.36.6.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klintmalm G. B., Iwatsuki S., Starzl T. E. Cyclosporin A hepatotoxicity in 66 renal allograft recipients. Transplantation. 1981 Dec;32(6):488–489. doi: 10.1097/00007890-198112000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitz B. A., Wallwork J. L., Hunt S. A., Pennock J. L., Billingham M. E., Oyer P. E., Stinson E. B., Shumway N. E. Heart-lung transplantation: successful therapy for patients with pulmonary vascular disease. N Engl J Med. 1982 Mar 11;306(10):557–564. doi: 10.1056/NEJM198203113061001. [DOI] [PubMed] [Google Scholar]
- Shulman H., Striker G., Deeg H. J., Kennedy M., Storb R., Thomas E. D. Nephrotoxicity of cyclosporin A after allogeneic marrow transplantation: glomerular thromboses and tubular injury. N Engl J Med. 1981 Dec 3;305(23):1392–1395. doi: 10.1056/NEJM198112033052306. [DOI] [PubMed] [Google Scholar]
- Smith J. M., Hows J. M., Gordon-Smith E. C. Stability of cyclosporin A in human serum. J Clin Pathol. 1983 Jan;36(1):41–43. doi: 10.1136/jcp.36.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]