Abstract
Pregnancies in women on chronic dialysis for end-stage renal disease are high risk, but outcomes appear to have improved with increasing experience and advances in dialysis care. This paper reviews the existing data on outcomes in such pregnancies to enable evidence-based preconception counselling and anticipation of antenatal complications.
Keywords: obstetrics, pregnancy outcome, renal dialysis, end-stage renal failure
Pregnancy is uncommon in women with end-stage renal disease as uraemia interferes with the normal functioning of the hypothalamic-pituitary-ovarian axis, resulting in anovulatory cycles, amenorrhoea and impaired fertility. These women are also more likely to be discouraged from conceiving or to decide on termination after being counselled regarding their high obstetric risk.
This pessimistic perception is largely a result of the poor outcomes reported in early studies on pregnancies in dialysis patients – the European Dialysis and Transplant Association study looking at pregnancies between 1967 and 1978 found that only 23% were successful1 while the 1981 Japanese nationwide survey reported an even more dismal 9% live birth rate.2
Since then, however, support for these pregnancies has improved with advances in the management of patients with renal failure, such as the introduction of high-flux dialysis and the use of erythropoietin therapy for anaemia, and progress in care for premature neonates. This appears to have translated into more encouraging survival rates: Souqiyyeh et al. 3 described a 30% live birth rate in Saudi Arabia 1992, Toma et al. 2 reported 66.2% in Japan in 1999, Okundaye et al. 4 found 40.2% in the United States in 1998, and there has also been a growing number of case reports detailing successful singleton and even multiple5,6 pregnancies.
In view of this apparent shift towards improved outcomes, this paper aims to review the existing data on obstetric outcomes in women with end-stage renal failure on chronic dialysis. This information will help clinicians provide evidence-based prenatal counselling for prospective mothers on chronic dialysis.
METHODS
Search strategy
The MEDLINE and EMBASE databases from January 1995 to December 2009 were searched using the search strategy detailed in Figure 1. A hand-search of the bibliographies of retrieved papers was also carried out to obtain additional references. The search was limited to studies written in English and those carried out on human and female subjects.
Figure 1.
Search strategy
Selection criteria
Studies were included if they:
were peer-reviewed publications of observational studies (i.e. cross-sectional, cohort and case-control studies, case series and case reports);
studied pregnancy outcomes of patients with end-stage renal disease who conceived while on either chronic haemodialysis or chronic peritoneal dialysis.
As pregnancies in women on dialysis are scarce, the majority of published studies are either case reports or small case series. While these introduce the potential problems of reporting bias and heterogeneity of management, they were nonetheless included in our review, as, in the absence of an international registry, they remain the main sources of data for evaluating outcomes and complications.
Studies were excluded if they were:
review articles;
studies containing duplicate data;
studies based on registry or survey data, because of the potential for duplication of data;
studies reporting pregnancy outcomes where it was not possible to derive data specific for women who conceived while on chronic dialysis; or
studies focusing exclusively on patients who commenced dialysis only after conceiving, on temporary dialysis for acute renal failure or who ceased dialysis after renal transplantation.
If both patients who started dialysis prior to pregnancy and those who started dialysis only after conception were included in the same study, only data pertaining to the former was extracted.
Study selection and data collection were performed independently using standardized forms by two of the investigators (LY and EWH) and controversies were resolved by discussion.
Data collection
The following data were collected:
Study information: title, author, year and journal;
Demographics: number of women, number of pregnancies, number of conceptions, maternal age, mode of dialysis and duration of dialysis prior to pregnancy;
Pregnancy complications and outcomes: early pregnancy losses (spontaneous miscarriages and therapeutic abortions <20 weeks of gestation), continuing conceptions (conceptions that continued past 20 weeks gestation), fetal abnormalities, antepartum haemorrhage, placental abruption, poly- and oligohydramnios, stillbirths (infants born at ≥20 weeks gestation who did not, at any time after being born, breathe or show any other sign of life), preterm births (live deliveries before 37 weeks of gestation), live births, indications for delivery, mode of delivery and gestational age at delivery;
Maternal complications: hypertensive disease (worsening of pre-existing hypertension, pregnancy-induced hypertension or preeclampsia), gestational diabetes, peritonitis, haemoperitoneum, caesarean sections and maternal deaths; and
Neonatal outcomes: birth weights, Apgar scores, neonatal deaths (death of a live born infant within the first 28 days of life) and perinatal deaths (sum of stillbirths and neonatal deaths).
These outcomes were chosen because of their relevance to obstetric practice and patient counselling.
Statistical analysis
Two different denominators – ‘total number of conceptions’ and ‘total number of pregnancies’ – were used in our calculations. The former was larger as it counted individual conceptions from multiple pregnancies.
‘Total number of conceptions’ was used as the denominator for calculations involving poly- and oligohydramnios, spontaneous miscarriage, therapeutic abortions, fetal abnormalities, stillbirths, preterm births and live births, whereas ‘total number of pregnancies’ was taken as the denominator in calculations for maternal hypertensive disease, gestational diabetes, peritonitis and maternal deaths.
RESULTS
Demographics
Twenty-two women representing 24 pregnancies were excluded because they were only started on dialysis after conception. Data from the remaining 208 women representing 222 pregnancies and 225 conceptions, including a pair of twins5 and a set of triplets,6 were analysed. The mean age of the patients was 31.7 ± 10.4 years. All women had end-stage renal failure and were on chronic dialysis prior to conception; mean duration of dialysis at onset of pregnancy was 4.3 ± 8.5 years (Table 1).
Table 1.
Maternal characteristics
| Number of patients | 208 |
| Number of pregnancies | 222 |
| Number of conceptions | 225 |
| Age (mean years ± 2 SD) | 31.7 ± 10.4 |
| Duration of dialysis at onset of pregnancy (mean years ± 2 SD) | 4.3 ± 8.5 |
| Haemodialysis (% of total conceptions) | 211 (93.8%) |
| Peritoneal dialysis (% of total conceptions) | 14 (6.2%) |
Pregnancy complications
Twenty-four (10.7%) conceptions were therapeutically aborted and 13 (5.8%) spontaneously miscarried; the remaining 188 (83.6%) continued past 20 weeks gestation. Of these, 10 (4.5%) ended in stillbirths, while the remaining 178 (79.1%) resulted in live births (Figure 2).2,5–54 The mean gestational age at delivery was 32.9 ± 6.7 weeks, with a total of 160 (71.1%) births occurring before 37 weeks gestation (Table 2).
Figure 2.
Study selection
Table 2.
Outcomes by type of dialysis
| Outcome | Haemodialysis (n = 211)* |
Peritoneal dialysis (n = 14)* |
||
|---|---|---|---|---|
| Live birth | 167 | 79.1% | 11 | 78.6% |
| Miscarriage | 11 | 5.2% | 2 | 14.3% |
| Therapeutic abortion | 24 | 11.4% | 0 | 0% |
| Stillbirth | 9 | 4.3% | 1 | 7.1% |
| Neonatal death | 18 | 8.5% | 1 | 7.1% |
*n = number of conceptions
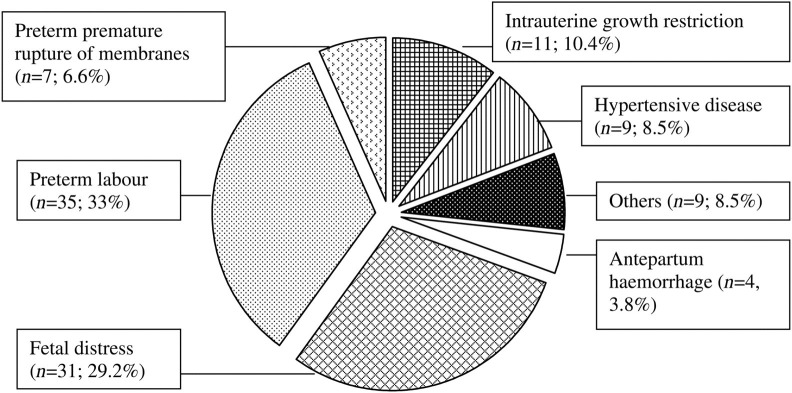
Data for the cause of preterm birth were available for 106 (66.3%) of the preterm deliveries (Figure 3). Forty-two (39.6%) of these were spontaneous preterm births, with 35 women presenting with contractions and seven with preterm premature rupture of membranes, while the remaining 64 (60.4%) resulted from medical intervention. This included 31 who were delivered due to evidence of fetal distress, 11 for intrauterine growth restriction, nine for maternal hypertensive disease, four for antepartum haemorrhage and one for obstetric cholestasis (Figure 4).
Figure 3.
Major pregnancy outcomes
Figure 4.
Aetiology of preterm births (n = 106)
There were three cases of fetal abnormalities,19,39,53 including one case of Apert syndrome39 and another with right-sided pulmonary agenesis.53 Seventy-two (32%) cases of polyhydramnios and six (2.7%) cases of oligohydramnios were reported.
Maternal effects
Hypertensive disease was the major maternal complication, affecting 67 (30.2%) pregnancies. There were two recorded cases of gestational diabetes.
There were five (2.3%) cases of peritonitis, four of which occurred in patients on peritoneal dialysis (Table 3). These four patients represented 28.6% of the total number of women on peritoneal dialysis.
Table 3.
Details of the five cases of peritonitis
| Case | Mode of dialysis | Details |
|---|---|---|
| 1 | PD | Intraperitoneal spillage of infected amniotic fluid during caesarean section9 |
| 2 | PD | Antenatal sterile eosinophilic peritonitis12 |
| 3 | PD | Antenatal bacterial peritonitis19 |
| 4 | PD | Postpartum peritonitis39 |
| 5 | HD | Uterine infarction with infected haematoma post hysterotomy11 |
PD, peritoneal dialysis; HD, haemodialysis
Three patients on peritoneal dialysis presented during their pregnancies with bloody dialysate drainage.9,36,38 This resolved without sequelae in one patient,9 but significant bleeding necessitated surgery in the other two. The first patient was found to have active bleeding of a serosal tear of the uterine fundus – haemostasis could not be secured and she had a hysterotomy with delivery of a dead fetus at 19 weeks.36 The second patient had multiple areas of uterine bleeding; haemostasis was achieved with sutures and cauterization but the patient miscarried the day after surgery.38
Of the 157 pregnancies for which data regarding mode of delivery was available, 58 (36.9%) babies were delivered by caesarean section. Of these, the majority were performed for indications such as fetal distress (20, 34.5%) and preterm labour (9, 15.5%).
Neonatal outcomes
There were 19 neonatal deaths and 28 perinatal deaths among the 159 live births for which such data were reported. This gives neonatal and perinatal mortality rates of 11.9% and 17.6%, respectively. Mean birth weight was 1810 (±1193.8) g and average Apgar score at five minutes was 8.0 (±4).
DISCUSSION
Outcomes and complications
The 79.1% live birth rate was more favourable than those from earlier studies utilizing registry data and national surveys. While outcomes may have indeed improved as a result of advances in care,21,29 it must be noted that the available literature largely comprises case reports and small case series. This inherently carries the problem of publication bias as successful cases are more likely to be reported. The spontaneous miscarriage rate is also likely to be underestimated, as many pregnancies are lost before they are clinically confirmed. True survival rates may therefore be somewhat less optimistic.
The preterm delivery rate was high at 71.1%, with the majority (64, 60.4%) due to medical intervention for complications such as fetal distress and intrauterine growth restriction, and the remainder (42, 39.6%) because of spontaneous labour or premature rupture of membranes. These data are however limited by the lack of information regarding indications for preterm delivery in about a third (33.8%) of the babies born prematurely.
Our preterm delivery rate is in keeping with those observed in previous studies.13,36 Apart from polyhydramnios, higher blood urea concentration (as measured by predialysis urea levels), hypercalcaemia, hypomagnesaemia and post-dialysis dips in progesterone levels have also been put forward as contributory factors for spontaneous preterm delivery in patients on dialysis.3,12 The high risk for preterm delivery and significant numbers that required delivery for fetal distress and intrauterine growth restriction means that such pregnancies are best managed in centres with facilities for close fetal surveillance and intensive neonatal care.
Renal failure results in impairment of one of the major physiological means of regulating blood pressure, and this, together with the increase in fluid volume in pregnancy and the higher risk of developing preeclampsia, makes it unsurprising that hypertensive disease was the main maternal complication observed.
One of the common problems with hypertension in pregnant dialysis patients is the difficulty in differentiating superimposed preeclampsia from inadequate dialysis.12,39 This is because the majority of such women are anuric, making it impossible to test for proteinuria; non-anuric patients may also have pre-existing proteinuria secondary to their underlying renal pathology, making interpretation of urine chemistry more difficult.
Clinicians may use clinical symptoms such as epigastric or right upper quadrant pain and biochemical parameters such as thrombocytopenia and raised liver transaminases to help in distinguishing preeclampsia from suboptimal dialysis. The distinction is however often difficult, and a high index of suspicion, particularly when blood pressure remains poorly controlled despite fluid restriction and intensified dialysis, is required.12,39
There were 72 (32%) cases of polyhydramnios. The high incidence of polyhydramnios in pregnant dialysis patients has been postulated as being due to increased diuresis by the fetal kidneys in response to raised urea and creatinine concentrations.55,56 Several studies have also reported26,33,56 an association between more intensive haemodialysis regimens – resulting in lower circulating concentrations of metabolites – and a lower incidence of polyhydramnios.
Management
Haemodialysis was the predominant mode of renal replacement therapy among patients included in our review. Potential problems associated with haemodialysis in pregnancy include fetal distress from hypotension secondary to rapid fluid shifts during dialysis; transmission of blood-borne infections such as hepatitis B; and thrombosis of vascular access due to the physiological procoagulant state of pregnancy.39 These complications were however uncommon in our review.
Frequency and duration of dialysis were increased in the majority of patients included in our study. More intensive haemodialysis regimens are associated with improved fetal outcomes.13,33 Apart from reducing the incidence of polyhydramnios, this is also likely because amniotic fluid urea and creatinine levels fall in tandem with maternal levels of these metabolic waste products, thus providing a less azotaemic intrauterine environment for the fetus. Maternal risk of developing worsening hypertension is also reduced57 as fluid balance is more tightly regulated. This has prompted some centres31,50 to propose daily nocturnal haemodialysis as a means of further improving the chance of a successful pregnancy outcome. Clinicians should however remember that patients on intensified dialysis regimens need to be carefully monitored to ensure adequate gain in dry weight over the course of the pregnancy and to avoid electrolyte and acid–base imbalances.15,56
Peritoneal dialysis had previously been proposed as the preferred mode of dialysis in pregnancy56 because of tighter control of metabolite and electrolyte levels and lower risk of acute hypotensive episodes. Subsequent studies have however failed to show significant differences in obstetrical outcomes between the two methods of dialysis.4,36 We observed that the live birth rates in patients on haemodialysis (79.1%) and peritoneal dialysis (78.6%) were similar, but were unable to draw definite conclusions regarding the benefits of either method as the sample size of the peritoneal dialysis group was small and dialysis regimes were not standardized.
There is a concern that peritoneal dialysis increases the risk of peritonitis, particularly in already immunosuppressed pregnant women; the incidence was 28.6% (4 of 14 patients) in our analysis.9,12,19,39 Clinicians should be alert to this potential complication in patients on peritoneal dialysis presenting with abdominal pain and fever. Attention should also be paid to patients complaining of bloody peritoneal effluent, as haemoperitoneum may be the first clue to the presence of abdominopelvic pathology and significant bleeding can result in serious morbidity to both the mother and fetus. Of the three pregnancies9,36,38 in our review complicated by haemoperitoneum, two had bleeding significant enough to warrant surgery and eventually result in miscarriage.36,38
Limitations
This review is based on case series and case reports, which represent level 3 evidence. Such studies are also associated with the problems of publication bias and heterogeneity of data concerning patient characteristics, duration of dialysis and management regimens. We have attempted to contain this by limiting our analysis to only patients who were already on chronic dialysis prior to conception.
CONCLUSIONS
Pregnancy outcomes appear to have improved, with 83.6% of conceptions continuing beyond 20 weeks and almost four in five resulting in live births. However, the high rates of preterm delivery and the challenges of diagnosing and managing maternal hypertensive disease remain areas of concern.
In view of the multifaceted problems that potentially complicate such pregnancies, multidisciplinary involvement comprising physicians, obstetricians, midwives, dialysis nurses and neonatologists, starting from prepregnancy care and continuing through the antenatal period to delivery and beyond, is extremely important.
REFERENCES
- 1. Registration Committee of the European Dialysis and Transplant Association. Successful pregnancies in women treated by dialysis and kidney transplantation. Br J Obstet Gynaecol 1980;87:839–45 [DOI] [PubMed] [Google Scholar]
- 2. Toma H, Tanabe K, Tokumoto T, Kobayashi C, Yagisawa T. Pregnancy in women receiving renal dialysis or transplantation in Japan: a nationwide survey. Nephrol Dial Transplant 1999;14:1511–6 [DOI] [PubMed] [Google Scholar]
- 3. Souqiyyeh MZ, Huraib SO, Saleh AG, Aswab S. Pregnancy in chronic haemodialysis patients in the Kingdom of Saudi Arabia. Am J Kidney Dis 1992;19:235–8 [DOI] [PubMed] [Google Scholar]
- 4. Okundaye I, Abrinko P, Hou S. Registry of pregnancy in dialysis patients. Am J Kidney Dis 1998;31:766 [DOI] [PubMed] [Google Scholar]
- 5. Chang CT, Wu MS, Chien HC. Successful twin pregnancy in a patient on long-term haemodialysis. Nephrol Dial Transplant 1999;14:2487–8 [DOI] [PubMed] [Google Scholar]
- 6. Yoo J, Unnikrishnan D, Lwin LN, Villanueva HJ, Tannenberg AM. Successful triplet pregnancy in a patient on chronic haemodialysis. Nephrol Dial Transplant 2004;19:994–7 [DOI] [PubMed] [Google Scholar]
- 7. Amoedo ML, Fernández E, Borrás M, Pais B, Montoliu J. Successful pregnancy in a hemodialysis patient treated with erythropoietin. Nephron 1995;70:262–3 [DOI] [PubMed] [Google Scholar]
- 8. Hou CH, Lee CN, Hung KY, Huang CH, Tsai TJ, Chen CY. An unexpected pregnancy causes poor drainage in automated peritoneal dialysis. Nephrol Dial Transplant 1996;11:2335–7 [DOI] [PubMed] [Google Scholar]
- 9. Tison A, Lozowy C, Benjamin A, Usher R, Prichard S. Successful pregnancy complicated by peritonitis in a 35-year old CAPD patient. Perit Dial Int 1996;16(Suppl. 1):S489–91 [PubMed] [Google Scholar]
- 10. Krawczyk W, Egiert A, Krzywicka A, Rózyc P. The successful outcome of pregnancy in a woman with end-stage renal failure chronically hemodialyzed without change of treatment regimen. Nephron 1997;77:492–3 [DOI] [PubMed] [Google Scholar]
- 11. Malik GH, al-Wakeel JS, Shaikh JF, et al. Three successive pregnancies in a patient on haemodialysis. Nephrol Dial Transplant 1997;12:1991–3 [DOI] [PubMed] [Google Scholar]
- 12. Romão JE Jr Luders C, Kahhale S, et al. Pregnancy in women on chronic dialysis. A single-center experience with 17 cases. Nephron 1998;78:416–22 [DOI] [PubMed] [Google Scholar]
- 13. Bagon JA, Vernaeve H, De Muylder X, Lafontaine JJ, Martens J, Van Roost G. Pregnancy and dialysis. Am J Kidney Dis 1998;31:756–65 [DOI] [PubMed] [Google Scholar]
- 14. Vidal ML, Ursu M, Martinez A, et al. Nutritional control of pregnant women on chronic hemodialysis. J Ren Nutr 1998;8:150–6 [DOI] [PubMed] [Google Scholar]
- 15. Giatras I, Levy DP, Malone FD, Carlson JA, Jungers P. Pregnancy during dialysis: case report and management guidelines. Nephrol Dial Transplant 1998;13:3266–72 [DOI] [PubMed] [Google Scholar]
- 16. Nakabayashi M, Adachi T, Itoh S, Kobayashi M, Mishina J, Nishida H. Perinatal and infant outcome of pregnant patients undergoing chronic hemodialysis. Nephron 1999;82:27–31 [DOI] [PubMed] [Google Scholar]
- 17. Reister F, Reister B, Heyl W, et al. Dialysis and pregnancy – a case report and review of the literature. Ren Fail 1999;21:533–9 [DOI] [PubMed] [Google Scholar]
- 18. Ralph C. Pregnancy in a hemodialysis patient with an ethical/cultural challenge. CANNT J 2000;10:35–8 [PubMed] [Google Scholar]
- 19. Tuncer M, Trak B, Sapan M, et al. Successful pregnancy complicated with peritonitis in a 25-year-old Turkish CAPD patient. Perit Dial Int 2000;20:349–50 [PubMed] [Google Scholar]
- 20. Chang H, Miller MA, Bruns FJ. Tidal peritoneal dialysis during pregnancy improves clearance and abdominal symptoms. Perit Dial Int 2002;22:272–4 [PubMed] [Google Scholar]
- 21. Chao AS, Huang JY, Lien R, Kung FT, Chen PJ, Hsieh PC. Pregnancy in women who undergo long-term hemodialysis. Am J Obstet Gynecol 2002;187:152–6 [DOI] [PubMed] [Google Scholar]
- 22. Walsh AM. Management of a pregnant woman dependent on haemodialysis. EDTNA ERCA J 2002;28:91–4 [DOI] [PubMed] [Google Scholar]
- 23. Luciani G, Bossola M, Tazza L, et al. Pregnancy during chronic hemodialysis: a single dialysis-unit experience with five cases. Ren Fail 2002;24:853–62 [DOI] [PubMed] [Google Scholar]
- 24. Guida B, Pollio F, Nastasi A, et al. Nutritional intervention in a hemodialysis pregnant woman: a case report. Clin Nutr 2003;22:205–7 [DOI] [PubMed] [Google Scholar]
- 25. Molaison EF, Baker K, Bordelon MA, Brodie P, Powell K. Successful management of pregnancy in a patient receiving hemodialysis. J Ren Nutr 2003;13:229–32 [DOI] [PubMed] [Google Scholar]
- 26. Prasad S, Parkhurst D, Morton MR, Henning P, Lawton J, Bannister K. Increased delivery of haemodialysis assists successful pregnancy outcome in end-stage renal failure. Nephrology (Carlton) 2003;8:311–4 [DOI] [PubMed] [Google Scholar]
- 27. Kazancioglu R, Sahin S, Has R, et al. The outcome of pregnancy among patients receiving hemodialysis treatment. Clin Nephrol 2003;59:379–82 [DOI] [PubMed] [Google Scholar]
- 28. Al-Wadei KH, Abu-Asba NW, Donia AF. The first reported case of successful pregnancy in a haemodialysis patient in Yemen. Nephrol Dial Transplant 2004;19:264 [DOI] [PubMed] [Google Scholar]
- 29. Eroğlu D, Lembet A, Ozdemir FN, et al. Pregnancy during hemodialysis: perinatal outcome in our cases. Transplant Proc 2004;36:53–5 [DOI] [PubMed] [Google Scholar]
- 30. Demant AW, Schmiedel A, Simula SM, et al. High-risk dialysis: pregnancy in a patient with extended Stanford-B-aneurysm of the aorta and end-stage renal disease. Nephrol Dial Transplant 2004;19:1634–6 [DOI] [PubMed] [Google Scholar]
- 31. Gangji AS, Windrim R, Gandhi S, Silverman JA, Chan CT. Successful pregnancy with nocturnal hemodialysis. Am J Kidney Dis 2004;44:912–6 [PubMed] [Google Scholar]
- 32. Smith WT, Darbari S, Kwan M, O Reilly-Green C, Devita MV. Pregnancy in peritoneal dialysis: a case report and review of adequacy and outcomes. Int Urol Nephrol 2005;37:145–51 [DOI] [PubMed] [Google Scholar]
- 33. Haase M, Morgera S, Bamberg C, et al. A systematic approach to managing pregnant dialysis patients – the importance of an intensified haemodiafiltration protocol. Nephrol Dial Transplant 2005;20:2537–42 [DOI] [PubMed] [Google Scholar]
- 34. Hussain S, Savin V, Piering W, Tomasi J, Blumenthal S. Phosphorus-enriched hemodialysis during pregnancy: two case reports. Hemodial Int 2005;9:147–52 [DOI] [PubMed] [Google Scholar]
- 35. Moranne O, Samouelian V, Lapeyre F, et al. A systematic approach to managing dialysis patients: the importance of an intensified haemodiafiltration protocol. Nephrol Dial Transplant 2006;21:1443; author reply 1443 [DOI] [PubMed] [Google Scholar]
- 36. Chou CY, Ting IW, Hsieh FJ, Lee CN. Haemoperitoneum in a pregnant woman with peritoneal dialysis. Nephrol Dial Transplant 2006;21:1454–5 [DOI] [PubMed] [Google Scholar]
- 37. Malik GH, Al-Harbi A, Al-Mohaya S, et al. Pregnancy in patients on dialysis – experience at a referral center. J Assoc Physicians India 2005;53:937–41 [PubMed] [Google Scholar]
- 38. Lew SQ. Persistent hemoperitoneum in a pregnant patient receiving peritoneal dialysis. Perit Dial Int 2006;26:108–10 [PubMed] [Google Scholar]
- 39. Tan LK, Kanagalingam D, Tan HK, Choong HL. Obstetric outcomes in women with end-stage renal failure requiring renal dialysis. Int J Gynaecol Obstet 2006;94:17–22 [DOI] [PubMed] [Google Scholar]
- 40. Haase M, Morgera S, Bamberg C, et al. Successful pregnancies in dialysis patients including those suffering from cystinosis and familial Mediterranean fever. J Nephrol 2006;19:677–81 [PubMed] [Google Scholar]
- 41. Giofrè F, Pugliese C, Alati G, Messina A, Tramontana D. Three successive pregnancies in a patient with chronic renal disease progressing from chronic renal dysfunction through to institution of dialysis during pregnancy and then on to maintenance dialysis. Nephrol Dial Transplant 2007;22:1236–40 [DOI] [PubMed] [Google Scholar]
- 42. Shah A, Bailey E, Hughes S. Goodpasture's syndrome, haemodialysis and pregnancy. Br J Hosp Med (Lond) 2007;68:48–9 [DOI] [PubMed] [Google Scholar]
- 43. Bamberg C, Diekmann F, Haase M, et al. Pregnancy on intensified hemodialysis: fetal surveillance and perinatal outcome. Fetal Diagn Ther 2007;22:289–93 [DOI] [PubMed] [Google Scholar]
- 44. Altay M, Yavuz I, Bagdatoglu O, Duranay M. Unexpected and late diagnosis (28th week) of pregnancy in a 39-year-old patient on chronic haemodialysis. Nephrol Dial Transplant 2007;22:1799 [DOI] [PubMed] [Google Scholar]
- 45. Dhir S, Fuller J. Case report: pregnancy in hemodialysis-dependent end-stage renal disease: anesthetic considerations. Can J Anaesth 2007;54:556–60 [DOI] [PubMed] [Google Scholar]
- 46. Asgari E, Bramham K, Shehata H, Makanjuola D. Successful pregnancy in a patient with end-stage renal failure secondary to HIV nephropathy on peritoneal dialysis. Nephrol Dial Transplant 2007;22:3671 [DOI] [PubMed] [Google Scholar]
- 47. Altay M, Akay H, Parpucu H, Duranay M, Oguz Y. A rare case: full-term delivery in a lupus patient on CAPD. Perit Dial Int 2007;27:711–2 [PubMed] [Google Scholar]
- 48. Ferrannini M, Vischini G, Miani N, Staffolani E, Di Daniele N. Successful pregnancy in a uremic patient treated with single needle hemodialysis. Int J Artif Organs 2007;30:1122–5 [DOI] [PubMed] [Google Scholar]
- 49. Chou CY, Ting IW, Lin TH, Lee CN. Pregnancy in patients on chronic dialysis: a single center experience and combined analysis of reported results. Eur J Obstet Gynecol Reprod Biol 2008;136:165–70 [DOI] [PubMed] [Google Scholar]
- 50. Barua M, Hladunewich M, Keunen J, et al. Successful pregnancies on nocturnal home hemodialysis. Clin J Am Soc Nephrol 2008;3:392–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Khurana A, Nickel AE, Greene JF Jr, Narayanan M, High WA, Foulks CJ. Successful pregnancy in a hemodialysis patient and marked resolution of her nephrogenic systemic fibrosis. Am J Kidney Dis 2008;51:e29–32 [DOI] [PubMed] [Google Scholar]
- 52. Al Saran K, Sabry A. Pregnancy in dialysis patients: two successful cases from a Saudi renal center and resulting management guidelines. Clin Nephrol 2008;70:265–9 [DOI] [PubMed] [Google Scholar]
- 53. Nonaka T, Kikuchi A, Kido N, et al. Prenatal diagnosis of unilateral pulmonary agenesis in a pregnant woman undergoing chronic hemodialysis due to chronic renal failure. Prenat Diagn 2009;29:707–9 [DOI] [PubMed] [Google Scholar]
- 54. Saito T, Ubara Y, Suwabe T, et al. A patient with pregnancy-related acute abdomen after hemodialysis for over 18 years. Clin Nephrol 2009;71:345–9 [DOI] [PubMed] [Google Scholar]
- 55. Nagotte MP, Grundy HO. Pregnancy outcome in women requiring chronic haemodialysis. Obstet Gynecol 1988;72:456–9 [PubMed] [Google Scholar]
- 56. Hou S. Pregnancy in women on haemodialysis and peritoneal dialysis. Baillieres Clin Obstet Gynaecol 1994;8:481 [DOI] [PubMed] [Google Scholar]
- 57. Schneider KT. Pregnancy-induced hypertension: maternal and fetal hemodynamics. Arch Gynecol Obstet 1989;245:240–4 [DOI] [PubMed] [Google Scholar]