Abstract
The thymus is a lymphatic organ that plays a vital role in the development of immunity in childhood. The thymus involutes during periods of stress and may acutely decrease in size but usually recovers to its normal size. The thymus also involutes during pregnancy, a process that is possibly hormonally mediated and thought to be necessary for fetal survival.
This report describes two pregnant patients with signs and symptoms suggestive of pulmonary embolism who were incidentally found to have thymic enlargement on computed tomography. Follow-up imaging postpartum in both cases demonstrates a significant reduction in thymus size, suggesting thymic hyperplasia. Both patients delivered healthy babies at term.
Thymic involution does not universally occur in pregnancy, challenging the theory of its necessity to fetal survival.
Keywords: thymic hyperplasia, pregnancy, multidetector computed tomography, pulmonary embolism
INTRODUCTION
Thymic hyperplasia presents in two different histological forms, true thymic hyperplasia and lymphoid hyperplasia. True thymic hyperplasia has been associated with the clinical entity ‘thymic rebound,’ in which thymic enlargement occurs in a patient recovering from recent stress.1 Lymphoproliferative hyperplasia of the thymus is associated with autoimmune diseases, most commonly myasthenia gravis.2 Radiographically, these two entities are indistinguishable.
Pregnancy has been described as one of the conditions associated with thymic involution. This phenomenon is thought to occur under the influence of increased levels of oestrogen and progesterone.3,4
Multidetector computed tomography (CT) is increasingly used in the diagnosis of pulmonary embolism (PE) in the pregnant and non-pregnant population. Incidental findings and alternative diagnoses are relatively common occurrences on CT studies. The following two cases describe incidental thymic enlargement during pregnancy that improved in the postpartum period.
Case 1
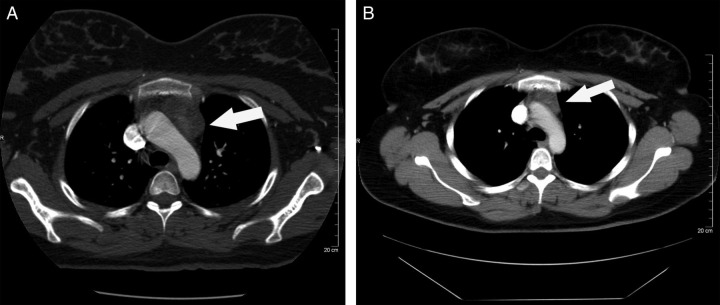
A 30-year-old G1P0 woman with a history of hypertriglyceridaemia presented at 36 weeks' gestation with dyspnoea of three weeks' duration. The symptoms usually lasted about two hours and were not associated with a specific time of day or activity. She described occasional nighttime awakenings secondary to dyspnoea and denied any chest pain, palpitations, dizziness or loss of consciousness. Physical exam revealed a heart rate of 88 beats per minute (bpm) at rest and 120 bpm with minimal exertion, a respiratory rate of 22 bpm, an oxygen saturation of 97% on room air and a 2/6 systolic ejection murmur heard at the left sternal border. Work-up included routine labs as well as spirometry, multidetector CT-pulmonary angiogram (MDCT-PA), echocardiogram and bilateral lower-extremity ultrasound. The work-up was negative except that the MDCT-PA revealed an enlarged thymus measuring 4.8 × 2.7 cm (normal size = 2.0 × 2.0 cm) (Figure 1A). The patient had persistent, intermittent dyspnoea throughout her pregnancy, which was attributed to gestational dyspnoea. She had a vaginal delivery at 40 weeks of a 3630 g healthy baby. Fifteen weeks postpartum, repeat CT revealed a decrease in thymus size to 3.4 × 2.5 cm (Figure 1B). Dyspnoea resolved postpartum.
Figure 1.
(A) Computed tomography (CT) chest of case 1, antepartum at 36 weeks. Arrow indicates an enlarged thymus measuring 4.8 × 2.7 cm. (B) CT chest of case 1, 15 weeks postpartum. Arrow indicates thymus measuring 3.4 × 2.5 cm
Case 2
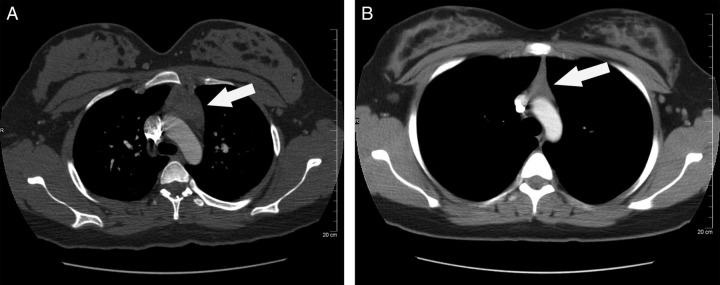
A 21-year-old G2P1 woman with a history of depression, asthma and marijuana abuse presented at 36 weeks' gestation with worsening dyspnoea over two months. Symptoms occurred 3–4 times daily and were precipitated by exertion and relieved with rest. On physical exam, the patient had a heart rate of 100 bpm at rest and 160 bpm with minimal exertion. Electrocardiogram and holter monitor revealed sinus tachycardia, and an echocardiogram was normal. Pulmonary function tests demonstrated no significant abnormalities. Routine labs were all normal, and lactate dehydrogenase as well as beta-2 microglobulin, acetylcholine receptor antibodies and thyroid-stimulating hormone were also normal. A MDCT-PA was negative for pulmonary embolus but demonstrated a 4.2 × 2.7 cm anterior mediastinal mass consistent with thymic hyperplasia (Figure 2A). The patient had a vaginal delivery at 39 weeks of a healthy 3665 g baby. A repeat chest CT at seven weeks postpartum revealed resolution of thymic enlargement with a thymus measuring 2.1 × 1.5 cm (Figure 2B).
Figure 2.
(A) Computed tomography (CT) chest of case 2, antepartum at 36 weeks. Arrow indicates an enlarged thymus measuring 4.2 × 2.7 cm. (B) CT chest of case 2, seven weeks postpartum. Arrow indicates an enlarged thymus measuring 2.1 × 1.5 cm
DISCUSSION
The thymus is a bi-lobed, pyramid-shaped organ located in the upper anterior thorax superior to the heart. It develops from the third and fourth branchial pouches and is derived from all three germinal layers. Histologically, the thymus is divided into the central medulla (predominantly epithelial cells) and peripheral cortex (predominantly lymphoid cells). Differentiation into the medulla and cortex is completed between 14 and 16 weeks of gestation.5
The thymus plays a critical role in adaptive immunity by producing T-lymphocytes derived from stem cells in the bone marrow. T-cells then differentiate into Th1 (cell-mediated immunity) and Th2 (humoral immunity) cells.6 The thymus is most active during the neonatal and preadolescent periods and then, under the influence of circulating sex hormones, undergoes age-related atrophy during puberty. With age, the thymus further decreases in mass, or ‘involutes’, but while still functional, this organ lacks the capacity to respond to a sudden decline in peripheral T-cells with an increase in T-cell output.4
Acute thymic involution occurs during certain situations such as stress, infection, malnutrition, use of glucocorticoids and pregnancy.2,4 Thymic atrophy causes lymphocyte depletion, which in turn downregulates inflammatory responses. It is thought that thymic involution is a critical adaptive mechanism for the regulation and control of the maternal immune system which helps prevent an immune reaction against a semi-allogeneic fetus. This phenomenon is, in theory, important for fetal survival.4,7 There is a shift towards Th2-type humoral immunity in pregnancy, which is thought to be mediated by progesterone and glucocorticoids.8 This finding is consistent with the observation that Th1-mediated autoimmune diseases (i.e. rheumatoid arthritis) improve and Th2-mediated diseases (i.e. systemic lupus erythematosus) seem to worsen in pregnancy.6,9 Thymic involution in pregnancy may be hormonally mediated, as progesterone, oestrogen and cortisol levels all rise during the course of gestation. It has been noted in numerous studies that the protective effects of progesterone are possible through interaction with intracellular progesterone receptors. Progesterone-receptor knockout mice do not demonstrate thymic involution during pregnancy, and have decreased fertility and increased fetal loss.10 However, it is not clear as to whether these adverse fetal effects are mediated by the lack of thymic involution or by other effects of progesterone on the reproductive system. However, progesterone-receptor-dependent paracrine mechanisms that block T-cell lymphopoiesis in pregnancy are thought to be required for normal fertility.6 Hormonal effects on the thymus are, however, quite complex and activation of these receptors is not limited to one specific hormone. Progesterone signalling is often intertwined with other hormones, such as oestrogen, which can induce expression of the progesterone receptor.11 Oestrogen also induces thymic atrophy, an effect that is primarily mediated through oestrogen receptors alpha.3,7
In both patient cases described in this report, atypical thymic enlargement occurred during pregnancy and improved spontaneously postpartum. As there were no signs or symptoms of myasthenia gravis or other autoimmune disease in these two patients, thymic enlargement in these cases was likely due to true thymic hyperplasia rather than thymic lymphoid hyperplasia. Thymic hyperplasia has been demonstrated to occur with hyperthyroidism, after completion of glucocorticoid treatment and after recovery from physical stress.1,2 Neither patient had known thyroid disease, recently received glucocorticoid treatment or were recovering from a recent stressful event and therefore, the true aetiology of their thymic hyperplasia remains unknown. It is possible that the enlarged thymus gland placing pressure on the trachea could have contributed to these patients' dyspnoea.
Both patient cases were identified as part of a retrospective review of MDCT-PA scans performed in pregnancy to exclude a diagnosis of PE. A total of 343 scans were reviewed in a period of four years, and a total of six patients with a suspicion of thymic hyperplasia were identified. In four cases, no imaging follow-up was available in the postpartum period at our institution and therefore, these cases were not described. These findings suggest that thymic enlargement is not uncommon in pregnant women presenting with dyspnoea. Radiographically, although a hyperplastic thymus may retain its normal shape, it may frequently lose its bi-lobed appearance and appear oval. Diffuse symmetric involvement of the thymus differentiates thymic hyperplasia from neoplasm, which presents as a focal mass. The dynamic physiology of the thymus and its variations in size and shape are a source of misinterpretation of imaging studies8 and may result in unnecessary interventions. If identification cannot be made by morphology alone on CT images, other techniques may be utilized. Chemical shift ratio (CSR) used in combination with magnetic resonance imaging is determined by comparing the signal intensity of the thymus gland with the signal intensity of the paraspinal muscles. CSR appears to be significantly higher in patients with thymic tumours such as thymoma, invasive thymoma, thymic cancer and lymphomas, compared with those with thymic hyperplasia.12 Positron emission tomography with florine 18-fluorodeoxyglucose (FDG) has been used in diagnosing thymic abnormalities and assessing the extent of disease. Striking FDG avidity may be seen in patients with rebound thymic hyperplasia.8 A direct correlation between thymic size (using CT imaging) and increased thymopoiesis has been suggested in some patient populations. This correlation has been documented in pediatric patients recovering postchemotherapy, HIV patients on highly active antiretroviral therapy and adults recovering from autologous transplants.13–15 Outside of these populations, it is not clear as to whether the finding of a radiographic enlargement of the thymus necessarily correlates with increased T-cell output since the differentiation of medullary versus peripheral cortical enlargement may be difficult by imaging alone. The finding of thymic hyperplasia in these two pregnant women who delivered healthy newborns and had no obvious causes for this phenomenon challenges animal data suggesting that thymic involution is necessary for pregnancy viability. Further research and documentation of cases of thymic hyperplasia during pregnancy are needed to understand the physiological importance and consequences of thymic enlargement during pregnancy.
DECLARATIONS
Competing interests: None declared.
Funding: None.
Ethical approval: Study was reviewed and approved by the Institutional Review Board.
Guarantor: SS.
Contributorship: The patients were identified through a study run by GB. All authors contributed to, reviewed and edited the manuscript and approved the final version.
Acknowledgement: We would like to thank Beth Hott for her help with manuscript preparation.
REFERENCES
- 1. Mendelson DS. Imaging of the thymus. Chest Surg Clin N Am 2001;11:269–93 [PubMed] [Google Scholar]
- 2. Strollo DC, Rosado-de-Christenson ML. Tumors of the thymus. J Thorac Imaging 1999;14:152–71 [DOI] [PubMed] [Google Scholar]
- 3. Erlandsson MC, Ohlsson C, Gustafsson JA, Carlsten H. Role of oestrogen receptors alpha and beta in immune organ development and in oestrogen-mediated effects on thymus. Immunology 2001;103:17–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Taub DD, Longo DL. Insights into thymic aging and regeneration. Immunol Rev 2005;205:72–93 [DOI] [PubMed] [Google Scholar]
- 5. Nishino M, Ashiku SK, Kocher ON, Thurer RL, Boiselle PM, Hatabu H. The thymus: a comprehensive review. Radiographics 2006;26:335–48 [DOI] [PubMed] [Google Scholar]
- 6. Miyaura H, Iwata M. Direct and indirect inhibition of Th1 development by progesterone and glucocorticoids. J Immunol 2002;168:1087–94 [DOI] [PubMed] [Google Scholar]
- 7. Zoller AL, Schnell FJ, Kersh GJ. Murine pregnancy leads to reduced proliferation of maternal thymocytes and decreased thymic emigration. Immunology 2007;121:207–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nasseri F, Eftekhari F. Clinical and radiologic review of the normal and abnormal thymus: pearls and pitfalls. Radiographics 2010;30:413–28 [DOI] [PubMed] [Google Scholar]
- 9. Zen M, Ghirardello A, Iaccarino L, et al. Hormones, immune response, and pregnancy in healthy women and SLE patients. Swiss Med Wkly 2010;140:187–201 [DOI] [PubMed] [Google Scholar]
- 10. Li X, O'Malley BW. Unfolding the action of progesterone receptors. J Biol Chem 2003;278:39261–64 [DOI] [PubMed] [Google Scholar]
- 11. Tibbetts TA, DeMayo F, Rich S, Conneely OM, O'Malley BW. Progesterone receptors in the thymus are required for thymic involution during pregnancy and for normal fertility. Proc Natl Acad Sci USA 1999;96:12021–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Inaoka T, Takahashi K, Mineta M, et al. Thymic hyperplasia and thymus gland tumors: differentiation with chemical shift MR imaging. Radiology 2007;243:869–76 [DOI] [PubMed] [Google Scholar]
- 13. Hakim FT, Memon SA, Cepeda R, et al. Age-dependent incidence, time course, and consequences of thymic renewal in adults. J Clin Invest 2005;115:930–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kolte L, Dreves AM, Ersboll AK, et al. Association between larger thymic size and higher thymic output in human immunodeficiency virus-infected patients receiving highly active antiretroviral therapy. J Infect Dis 2002;185:1578–85 [DOI] [PubMed] [Google Scholar]
- 15. Mackall CL, Fleisher TA, Brown MR, et al. Age, thymopoiesis, and CD4+ T-lymphocyte regeneration after intensive chemotherapy. N Engl J Med 1995;332:143–9 [DOI] [PubMed] [Google Scholar]