Abstract
Iron deficiency (ID) and related anaemia (IDA) during pregnancy are highly prevalent worldwide in both developed and developing nations although the causes are often different. At conception, many women lack sufficient iron stores to meet the increased requirements of pregnancy, which are calculated at approximately 1200 mg. Appraisal of iron status in pregnant women is problematic, however the most reliable available diagnostic test is a serum ferritin < 20 µg/L. ID is often associated with other nutritional disorders, and there is frequently a secondary cause or association. A greater oral intake is usually insufficient to meet the increased demands of pregnancy, however regular oral supplements (given either daily or intermittently) can often meet maternal needs and avoid associated neonatal complications of IDA. Over-treatment with iron should be avoided, but intravenous administration is useful when deficiency is discovered late, is severe, or if the woman is intolerant of oral formulations. This paper reviews the current literature, and addresses differences in the prevalence and causes of ID betwen developed and developing nations. It examines gestational iron requirements, distinguishes between ID and IDA, and highlights difficulties in diagnostic testing. Finally, it appraises the evidence for and against different treatment regimens, ranging from food fortification to intravenous iron infusions, according to availability and to need.
Keywords: haematology, nutrition
INTRODUCTION
Iron deficiency (ID) in women of child-bearing age has been recognized since at least the 17th century,1 yet it remains a public health issue in both developed and developing countries today, with an estimated prevalence of over 3 billion people.2 The condition is aggravated by and may present during pregnancy, when the need for iron is increased. Whether iron deficiency anaemia (IDA) will arise during pregnancy depends on two factors: the woman's iron stores at the time of conception and the amount of iron absorbed during gestation. The fact that anaemia frequently does arise indicates both that pre-existing stores are often inadequate and that physiological adaptations are insufficient to meet the increased requirements.
This review will address the nature and extent of iron storage at conception, and the requirements and adaptive changes that occur during pregnancy; it will examine inherent difficulties in the appraisal of iron status, associated and differential diagnoses, and treatment strategies. Although clearly a global issue, it is important to acknowledge that the causes of IDA and available treatment options, whether before, during or after the pregnancy, frequently differ between developed and developing nations.
PREVALENCE AND GLOBAL ISSUES
Developing nations
ID and IDA have a high prevalence in the developing world. Studies of pregnant women from Zimbabwe, China, India and Mexico between 1996 and 2008 indicated that between 43% and 73% of women are iron deficient (usually diagnosed as a low ferritin concentration), and between 7% and 33% of women had IDA.3–6 The Chinese study of 3591 pregnant women found the prevalence of both ID and IDA to be highest in the third trimester, with over 85% of women affected. Of the 3721 non-pregnant premenopausal women in the study, 34% were ID and 15% had IDA. Interestingly, the prevalence rate of ID (but not IDA) in the first trimester in both pregnant and non-pregnant women was higher in urban (42% and 36%) compared with rural dwellers (36% and 32%). The prevalence of IDA in 746 pregnant women from four provinces in Zimbabwe was 33%, compared with the overall rate of 24% of the 3151 study participants (including preschool children, 18%; lactating women, 30%; and adult men, 17%). A study from Mexico examined 186 adolescents who attended local health institutions in their first trimester, where only 37% of prospective mothers displayed normal iron indices and haemoglobin concentrations ([Hb]).
Sub-communities in developed nations are also prone to a high prevalence of IDA. Seventy-two percent of women aged over 14 from an aboriginal population in North-West Australia had ID, many of whom were also anaemic; and IDA (serum ferritin <12 µg/L) developed in 86% of Asian and 40% of black women in another study from South Africa.7,8 Such data are consistent with the findings of de Benoist and co-workers, who ascertained the global prevalence of anaemia in non-pregnant women to be 30.2%, rising to 47.4% during pregnancy.9
Developed nations
Surprisingly, the prevalence of ID and IDA in more affluent cultures and societies appears to be similar to poorer communities, without substantial evidence of improvement in the last 60 years. In 1951, a study of 2087 pregnant women from Manchester, UK, demonstrated higher [Hb] in the 1249 women taking oral iron during the pregnancy.10 The prepregnancy haemoglobin was also attained more quickly in the treated group postpartum, taking over a year on average in the non-treated group. A study from London 35 years later demonstrated that 82% of 669 non-anaemic (Hb ≥110 g/L) pregnant women had serum ferritin concentrations <50 µg/L at 16 weeks gestation. Ferritin concentrations were below 12 µg/L in 12%, and only 8% had levels above 80 µg/L.11 In a 1994 study from the USA, 69% of a group of 88 adolescent teenagers had depleted iron stores refractory to prescribed treatment (possibly due to poor compliance). At two weeks postpartum, stores remained depleted in 80%, with 55% still with IDA. One interesting feature was that all low birth weight infants (8%) were born to mothers who had ID by 16 weeks. Other studies from the USA and from Europe have reported similar findings.12–15
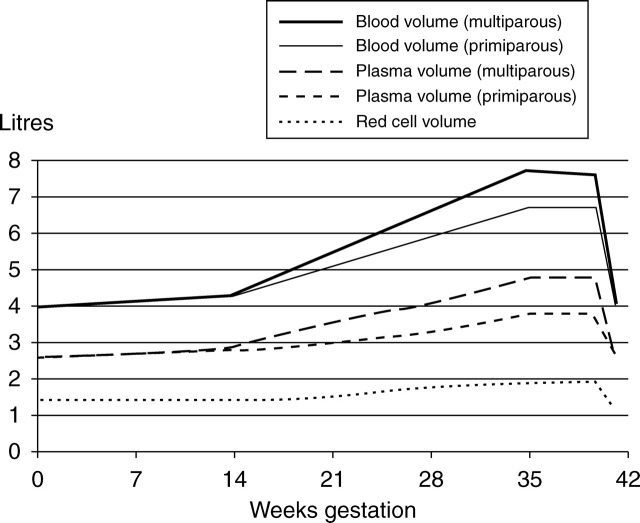
Gestational iron requirements (see Table 1 and Figure 1)
Table 1.
Average iron requirements and changes during and after pregnancy for a woman with a pre-gestational bodyweight 55 kg to 60 kg
| Stage | Amount (mg) |
|---|---|
| Pre-partum | |
| Fetus | 270 |
| Placenta | 90 |
| Expansion of RBC mass | 450 |
| Obligatory losses | 230 |
| Sub-total | 1040 |
| Intra-partum | |
| Maternal blood loss at delivery | 150 |
| Overall requirement | 1190 |
| Post-partum changes | |
| Contraction of maternal RBC mass | −450 |
| Loss of menstruation during gestation | −160 |
| Sub-total | −610 |
| Net requirement | 580 |
Figure 1.
Figurative representation of changes in blood and plasma volume during pregnancy
The physiology of iron absorption and distribution in humans is now well understood but beyond the scope of this article to describe in detail. It has been well summarized elsewhere.2,16,17 During pregnancy, iron requirements are not uniform.16 In the first trimester, daily needs decrease due to the absence of menstruation. This represents a saving of approximately 0.56 mg of iron per day, or 160 mg for the pregnancy.18 Ongoing losses from the gut, skin and urine (which continue throughout the pregnancy) are calculated at approximately 14 µg/kg/day (about 230 mg for the pregnancy).19 In addition, several studies have suggested that erythropoietic activity might be reduced during this period.10,20–22
In the second trimester, blood volume increases by 45%, with an increase in plasma volume of 50%; red cell mass is raised by 35% in iron-replete women. This amounts to approximately 450 mg of iron in a 55 kg woman. Although this represents a significant demand during the gestation, long-term demands are substantially less as, apart from fetal requirements, the iron is redistributed rather than lost.23,24
Fetal demands for iron are maximal during the third trimester. The iron content of a 3 kg fetus has been estimated at 270 mg.25 Parturition presents additional losses, which can be difficult to quantify in individual cases. However, maternal losses following a normal delivery average about 150 mg of iron, with an additional 90 mg from the placenta and umbilical cord.25 Lactation losses (0.3 mg/day) are usually offset by the absence of menstruation, except when prolonged.26
Therefore, the mother must be able to mobilize almost 1200 mg of iron during pregnancy, although her net obligations are approximately 600 mg. Effectively, despite being reduced in the first trimester, average daily dose requirements rise to between 4 and 6 mg per day in the second and third trimesters, respectively.16 Since changes in red cell mass in the second trimester do not begin until relatively late, the actual requirements can be as much as 10 mg per day in the last 6–8 weeks. Even in well-educated, nutritionally replete women, it is most unlikely such requirements can be met through diet alone, a restriction that has been confirmed in at least one recent observational study.27
RECOGNITION AND DIAGNOSIS
ID versus IDA
Anaemia represents the final stage of ID, and occurs as tissue and cellular stores are progressively exhausted, yet non-anaemic ID is under-recognized as a pathological entity. Animal and human studies have identified reduced exercise capacity (thought related to mitochondrial dysfunction) and impaired temperature regulation in such subjects.21–24,28 Substantial cognitive defects have also been identified.29,30 Reductions in both exercise and cognition may be responsive to treatment.30 Infants with ID have also been reported to have a reduced mental development index and persistent developmental delay.29,31 However, whether such abnormalities are amenable to iron supplementation during pregnancy remains contentious, with conflicting reports suggesting the severity of ID might be important.32,33
Anaemia
The National Health and Nutrition Examination Survey (NHANES) II study identified 117 g/L (2 SD below a mean of 135 g/L) as the lower normal limit of haemoglobin for non iron-deficient premenopausal women.34,35 In pregnancy however, the complex physiological changes that occur progressively throughout the gestation make it difficult to distinguish anaemia from physiological adaptation, particularly when a single test is performed at a specific gestational stage. Increases in both plasma volume (≥50%) and red cell mass (up to 30%) contribute to a marked increase in total blood volume; however, the haemoglobin falls because of a greater increase in plasma volume. The rise in plasma volume is more marked in multiple gestations and parous women, and reduced iron stores restrict elevations in the red cell mass, an effect that becomes more evident later in the pregnancy.36–40
Thus, what [Hb] is normal in a particular woman at a certain gestational age is inherently problematic. By simple calculation and with some empirical support, a [Hb] of 105 g/L could be regarded as an acceptable physiological lower limit at any stage of gestation.24 A cut-off of 110 g/L has also proved acceptable and workable for many and has the advantage of corresponding to the World Health Organization (WHO) and Centers for Disease Control and Prevention guidelines,16,34,35,41 although both of these guidelines have been criticized because of inadequate sampling data and statistical flaws.42 In addition, there are no prospective data to support a worse maternal outcome from anaemia per se (as opposed to associated complications), and low birth weight and other morbidities have been shown to have a U-shaped association with maternal [Hb], most likely due to associated pathologies.43,44
However, mortality reports do not address changes in symptomatic wellbeing or functional capacity. Poor quality of life is well recognized in other conditions such as chronic kidney disease, particularly when the [Hb] falls below 100 g/L.45,46 Furthermore, as discussed above the consequences of ID (which affect both mother and fetus irrespective of the [Hb]) are well recognized, prevail for substantial periods postpartum, and affect subsequent pregnancies and childhood development.5,30,47
Iron status (see Table 2)
Table 2.
Tests used to diagnose iron deficiency in pregnancy
| Test | Advantages | Limitations |
|---|---|---|
| Standard iron studies Iron Transferrin saturation Ferritin | Readily available; widely used in research studies Low ferritin diagnostic of iron deficiency | Parameters are subject to substantial physiological change during pregnancy |
| Non-standard iron studies Circulating transferrin | Likely of value in determining ID Probably little change | Not widely available; limited data in pregnancy |
| receptor | during pregnancy | |
| Reticulocyte haemoglobin content | ||
| Zinc protoporphyrin | ||
| %hypochromic RBC | ||
| Hepcidin | Key determinant in iron absorption | Difficult to measure and interpret; very limited data in pregnancy |
| Bone marrow | Direct measure of iron presence in bone marrow | Subject to physiological change during pregnancy |
| Recognised gold standard |
RBC: red blood cells; ID: iron deficient
Whatever [Hb] is considered abnormal in a specific patient, iron studies are usually the first of the relevant investigations that should be performed. However, these too are not without inherent difficulties in pregnancy. Early in the gestation there is an initial fall in serum iron, which stabilizes by mid-pregnancy.48 Increased transferrin concentrations reflect a 50% increase in total iron-binding capacity, with a corresponding fall in transferrin saturation.48 The serum ferritin rises slightly in response to reduced erythropoietic activity initially, but then a progressive reduction by mid-term to about 50% of pre-pregnancy values occurs in response to haemodilution and iron mobilization, with a nadir reached by week 32.22,49–52 It is important to recognize also that plasma ferritin concentrations and transferrin saturation display significant day-to-day variability, and that substantial rises in ferritin occur concurrently with infection or inflammation as part of the acute phase reaction.53
Iron parameters that may be more constant in pregnancy include circulating transferrin receptor (cTfR), reticulocyte haemoglobin content, zinc protoporphyrin levels, hypochromic red cell (or reticulocyte) percentages and bone marrow sampling. Some studies suggest that cTfR is not subject to the physiological changes induced by gestation and rises only in ID. Although other studies have failed to confirm this, appropriate complementary changes in cTfR have been identified in marginally iron-deficient women who were treated with oral iron supplements.50,51,54
Reticulocyte haemoglobin content has also been shown to be useful in assessing marginal iron stores,55 but studies in pregnant women are few. Zinc protoporphyrin assays during pregnancy demonstrate an enhanced sensitivity compared with ferritin in the appraisal of iron status, although it has not been embraced by the majority of clinical laboratories.54,56,57 An elevated percentage (>10%) of hypochromic red cells and reticulocytes has also been deployed with high success in diagnosing ID in patients with kidney failure.58 Although the technique has not been addressed substantially in pregnancy, there is no reason it could not be. The data can be ascertained automatically from routine analysis, but a specific analyser (coulter counter) is required.
In the limited studies performed, bone marrow sampling is an accurate appraisal of body stores, however at least one study has suggested this might not always be so.59 Marrow iron stores can also reduce during pregnancy, making a ‘normal’ marrow difficult to define, especially later in the gestation.48 In addition, its deployment has limitations on several practical fronts.
The discovery of hepcidin, a 25-amino acid protein processed from a larger precursor (pro-hepcidin), in 2000 represented a significant advance in our understanding of iron homeostasis.60–62 Unable to be measured directly until recently, reliable assays are still not widely available. Of the few reports available in pregnant patients, serum pro-hepcidin estimates do not appear to correlate well with iron status in pregnancy.63 However, a recent study using a urinary assay of hepcidin by mass spectrometry in pregnant women did suggest a close correlation with ferritin and transferrin receptor status, but not with haemoglobin, inflammatory markers or erythropoietin concentrations.64 Further studies will be of interest.
Many of the tests described above are confined to specialist laboratories and are unsuitable for routine screening of pregnant patients, even in the developed world. In a clinical practice setting, a low or falling mean corpuscular volume – regardless of [Hb] – will be the first initial suggestion of ID, and there are numerous reports of the utility of a low serum ferritin to confirm the diagnosis at any stage of the pregnancy. The specific concentration of ferritin used to define ID varies, but levels between 20 and 50 µg/L have been cited, with convincing specificity particularly for the lower value.22,56,57,65,66
Causes of ID (see Table 3)
Table 3.
Causes and important associations of iron deficiency and iron deficiency anaemia in women of child-bearing age
| Developing nations | Developed nations |
|---|---|
Food shortage
|
Dietary
High socio-economic status Blood donation Low socio-economic status Ethnic minorities
|
Developing nations
The causes of ID in developing populations do depend to some extent on local factors; however, the primary issues relate to public health.7 A cross-sectional survey of six villages in rural India in 2000 and 2001 found that the primary causes of micronutrient deficiency – including iron – were low dietary intake and food of poor nutrient value.5 Such findings are echoed in other studies, where food insecurity either in quantity or in content, and lack of contraception resulting in shorter reproductive and pregnancy cycles are facts of life.3,67,68 Whenever studied, ID is but one of several micronutrient deficits identified in pregnancy.3,8 Reduced serum levels of zinc (73%), copper (3%), magnesium (44%), folic acid (26%) and iodine (6%) were identified in one cross-sectional study of rural pregnant Indian women from various areas; multiple deficiencies were common.8 At least one other population study has found similar results.9 Other deficiencies also frequently co-exist with iron, particularly vitamins A and B12, and should always be considered.59,67,69Although such deficiencies are widely prevalent, some caution is advised when extrapolating tissue deficiency of nutrients from low serum concentrations, particularly in a clinical context. Other important causes of ID in developing societies include malaria, HIV infection, parasite infestations and short interpregnancy intervals.5 Haemoglobinopathies (e.g. sickle cell disease and variants) can contribute to ID through both chronic and acute haemolysis. In areas of endemic infection or where there is a high prevalence of disease, particularly when [Hb] and ferritin concentrations are very low, concurrent ID is likely.
Developed nations
In comparison to their counterparts in developing nations, and given iron losses due to menstruation are relatively constant, the predominant cause of ID in otherwise healthy groups of women in developed nations most commonly relate to a net imbalance between iron ingested and iron lost. If iron stores are reduced at conception (which is common), it is unlikely progressive deficiency can be averted without specific iron treatment. A recent observational study from the USA demonstrated that premenopausal women from mid- to upper-income brackets were unable to consume sufficient amounts of iron from food to meet the needs of pregnancy.27 These findings are corroborated in a recent Australian study, although studies from the developing world do suggest that substantial gains in both iron stores and haemoglobin can be achieved.70,71
In an otherwise healthy woman, attention should be paid firstly to dietary habits. Many women restrict their iron intake through selective red meat avoidance. Similarly, a vegetarian or vegan diet and recognized nutritional disorders such as anorexia can predispose to ID, which might only be recognized during pregnancy. In women with severe ID, additional studies are warranted, particularly to ensure concurrent deficiencies (such as coeliac disease) are not present. One important group of women who can be overlooked are those from ethnic minority groups, either native or immigrant, within nutritionally replete societies who may well be iron deficient from causes more commonly associated with developing nations.
TREATMENT
The treatment of ID and IDA in pregnancy relates directly to its necessity, its effectiveness and its potential to cause harm. As previously discussed, there are few if any reports to support deleterious maternal outcomes from ID or IDA. Apart from symptomatic and functional limitations mentioned earlier, there are several studies highlighting the risks of low birth weight and preterm delivery in both developed and developing countries, particularly as anaemia becomes more marked.72–75 There is also evidence that treatment with iron improves perinatal outcome, an effect especially evident in women who are anaemic early in pregnancy but also seen in women given small doses of regular iron from 20 weeks gestation.75–78 In addition, the iron status of children up to 12 months of age is improved even when iron supplementation is begun late in pregnancy.72–74 Such data suggest that treatment is both desirable and effective.
When taken orally iron replacement can cause gastrointestinal discomfort (particularly nausea and constipation) and on occasion may produce iron intoxication from overdose (mostly in children). There are also reports of elevations of ‘free iron’ in the plasma and lipid peroxidation, indicative of oxidative stress.79–81 Numerous reports, with some support from animal studies,82–84 now also suggest that excessive iron supplementation can cause increased prematurity, low birth weight and infant mortality, most likely due to an increase in [Hb] above physiological levels. Effects appear most marked as haemoglobin rises above 130 g/L.36,37,85–90 Thus, oral iron supplementation during pregnancy appears safe and efficacious but is not necessarily harmless. As discussed earlier, a woman of 55–60 kg body weight has to mobilize approximately 1200 mg of iron during the pregnancy, with a net cost of 600 mg. Based on the NHANES III study (1988–1994) most women of reproductive age, even in the industrialized world, have less than 300 mg of iron storage at the start of pregnancy.91 Therefore, most women will require iron treatment during the gestation. Evidence from numerous reports suggests that iron absorption, similar to demand, increases through pregnancy. Lowest rates are in the first trimester at around 7% of a given dose, rising to between 15% and 35% by 35 weeks gestation. Absorption is markedly affected by iron dose, total body iron status, food type (high or poor iron bioavailability) and by other foods (such as calcium and cereals) ingested at that time.20,92–96
The average weekly absorbed iron dose required for women during the latter trimesters has been calculated at 3–4 mg/day.16,42 Assuming an average absorption of 20%, iron needs could be supplied from a total weekly oral dose of 120–160 mg/week for women with a starting [Hb] of 90 g/L, less with higher values. This is achievable in a variety of ways as will be discussed, but it is important to realize that such a regimen is unlikely to increase markers of iron stores (such as ferritin) significantly during the pregnancy; rather it will maintain the [Hb] as iron is diverted into the circulating erythron mass. Measurement of ferritin and [Hb] on a monthly basis (more regularly if clinically indicated) is probably the best means of monitoring the response to iron therapy. Elevations of 10 g/L per month can be expected with appropriate dosing. Failure to respond to treatment usually indicates ongoing iron loss (usually gastrointestinal) or poor compliance with treatment.
The correct approach to replenishment of iron stores and/or haemoglobin in ID and IDA is still debated. The options currently available include iron fortification of foods (either in food sources or as an additive), intermittent oral iron tablets, daily oral iron, parenteral iron and blood transfusion.
Food fortification is an attractive option for developing countries, where the logistics of oral iron supplementation among the most needy are highly challenging, and the rate of compliance with treatment is poor.72–75 Iron fortification of sugar (mean intake 4 mg/day) in non-pregnant women over three years resulted in a substantial increase in iron stores, with reserves still increasing by about 40 mg/year after the third year.97 Importantly, substantial change was evident even in women with severe hookworm infection. Two Vietnamese studies showed similar improvements in iron stores following ingestion of iron-fortified fish sauce for six and 12 months (in respective studies).98,99 In these studies, many of the women were anaemic at study commencement. By study end, prevalence rates of anaemia had decreased substantially and iron stores had increased in the majority of the women. However, such replacement alternatives are best suited to long-term strategies in the public health domain where they would appear to promise a cost-effective means of addressing a substantial global problem.
Oral iron, either as iron sulphate or fumarate, with or without folic acid, is the most commonly used treatment for ID and IDA in pregnancy. The issues of weekly versus daily supplementation, timing and duration of treatment, and total weekly dose are still debated. Arguments for intermittent supplementation include that it is more physiological by avoiding mucosal absorption block and excessive pooling of intestinal iron with associated oxidative stress, it has logistic advantages in distribution particularly in areas of limited supply, that it avoids many of the gastrointestinal side-effects of daily iron, and that it works.79,80,82,83,100–102 Apart from logistic advantages, which may partly explain the enthusiasm shown by the WHO for its use in developing nations, each of these arguments has been contested.103–109 Proponents of a daily iron regimen suggest instead that concentrating on compliance (which is arguably easier with regular dosing) and limiting the daily dose provide a more sound approach to iron replenishment. Non-compliance is certainly an ongoing issue, both in the developing and the developed world, where it has been associated with smoking, low socioeconomic status and maternal [Hb].110
The timing and dosage of oral iron are also controversial. Most studies have focused on treatment from mid-pregnancy, at or before 20 weeks gestation. This accords with screening tests common to regular clinical practice in many countries. A randomized, double-blind study from Denmark examined doses of between 20 and 80 mg of oral iron daily in 427 pregnant women. A dose of 40 mg was sufficient to prevent ID in 90% of women (and IDA in at least 95%) both during pregnancy and postpartum.15 No difference in gastrointestinal side-effects was observed. In a more recent Australian study of anaemic pregnant patients, a similar dosage regimen showed an improved side-effect profile with no substantial difference in haemoglobin response compared with higher doses.111 Regular low dosage of oral iron would appear to benefit the majority of women, preventing ID and IDA and avoiding the potential problems of raising the [Hb] too high. Furthermore, the gastrointestinal side-effect profile would appear to be acceptable in most cases.
Reports of parenteral iron use are predominantly, but not exclusively, from the developed world. Reports of its use in pregnancy have been scant, possibly because of concerns of adverse reactions; however, intravenous iron sucrose in particular has been used in several recent studies. Perhaps because of its immediate bioavailability, it may result in a more rapid rise in [Hb] in anaemic patients compared with oral iron but probably does not confer an advantage in preventing anaemia in pregnancy.112,113 The side-effect profile of iron sucrose is encouraging, it can be given rapidly (within ten minutes), and it represents a real alternative to oral iron in refractory patients or those intolerant of oral formulations. Two small recent studies have also identified a similar replenishment of iron stores and haemoglobin with low molecular weight iron dextran and iron dextrin (polymaltose).114,115 Each of these agents is able to be given as a total dose infusion. Care must be taken with the overall dose of parenteral iron that is used, as saturation of stores and too great a rise in haemoglobin are considerations. In contrast, iron sucrose can only be given in smaller aliquots (maximum 200 mg), providing some innate safeguards and allowing for incremental dosing. The use of intramuscular iron is to be discouraged: it is painful, can cause permanent scarring, is probably less effective than intravenous dosing and there are no data to suggest the risk of adverse events systemically is less than with the intravenous route.116
Postpartum management of ID and IDA is often treated haphazardly. Combinations of observation, oral iron, parenteral iron, blood transfusion and use of erythropoietic stimulating agents (ESAs) have been used. It is useful to recall that a substantial amount of iron will be returned to body stores from the erythron mass in the first two months postpartum and, together with the concurrent contraction in plasma volume, there is a substantial rise in [Hb] over this period. Blood transfusion is probably used too frequently in many centres; it should be reserved for patients with acute blood loss and cardiovascular compromise. Iron infusion with or without an ESA for some weeks will augment the rise in haemoglobin,117,118 particularly in patients who received little or no iron pre-partum and who are profoundly anaemic. However, most patients with ID and IDA postpartum will receive sufficient benefit from regular oral iron not to require such an escalation in treatment.
SUMMARY AND CONCLUSIONS
ID is highly prevalent worldwide, is often associated with other nutritional disorders (particularly folic acid, zinc, vitamin A), and frequently has a secondary cause or association. The prevalence is greater in parous women and in multiple pregnancies;
The causes of ID and IDA in the developing world are often different from those in developed countries;
Appraisal of iron stores in pregnancy is problematic because of complex physiological changes. The most reliable available current diagnostic test for ID is a low serum ferritin. Concentrations <20 µg/L are a very good index of ID;
Dietary intake alone to maintain iron stores in pregnancy is unlikely to succeed because of the increased requirements for iron during pregnancy;
Oral supplements at a low dose (40 mg elemental iron per day), starting from at least mid-gestation, can ameliorate ID and IDA and improve neonatal outcome and maternal wellbeing;
Intermittent oral treatment regimens (weekly or twice weekly) are an alternative therapeutic option, particularly in areas of limited supply or access;
Over-treatment with iron in pregnancy may be associated with an increased risk of prematurity and infant mortality;
Intravenous iron has a role in the treatment and avoidance of ID and IDA, particularly in women who present late, and/or display severe deficiency or anaemia, or who are intolerant of oral iron.
References
- 1. Beutler E. History of iron in medicine. Blood Cells Mol Dis 2002;29:297–308 [DOI] [PubMed] [Google Scholar]
- 2. Andrews NC. Disorders of iron metabolism. N Engl J Med 1999;341:1986–95 [DOI] [PubMed] [Google Scholar]
- 3. Sikosana PL, Bhebhe S, Katuli S. A prevalence survey of iron deficiency and iron deficiency anaemia in pregnant and lactating women, adult males and pre-school children in Zimbabwe. Cent Afr J Med 1998;44:297–305 [PubMed] [Google Scholar]
- 4. Liao QK. [Prevalence of iron deficiency in pregnant and premenopausal women in China: a nationwide epidemiological survey.]. Zhonghua Xue Ye Xue Za Zhi 2004;25:653–7 [PubMed] [Google Scholar]
- 5. Pathak P, Kapil U, Kapoor SK, et al. Prevalence of multiple micronutrient deficiencies amongst pregnant women in a rural area of Haryana. Indian J Pediatr 2004;71:1007–14 [DOI] [PubMed] [Google Scholar]
- 6. Mendez Estrada RO, Pacheco B, Noriega Verdugo H, Quihui L, Morales G, Valencia ME. [Prevalence of iron deficiency and iron deficiency anemia in pregnant adolescents from northwest Mexico, 2007–2008]. Arch Latinoam Nutr 2009;59:147–51 [PubMed] [Google Scholar]
- 7. Hopkins RM, Gracey MS, Hobbs RP, Spargo RM, Yates M, Thompson RC. The prevalence of hookworm infection, iron deficiency and anaemia in an aboriginal community in north-west Australia. Med J Aust 1997;166:241–4 [PubMed] [Google Scholar]
- 8. Mayet FG. Anaemia of pregnancy. S Afr Med J 1985;67:804–9 [PubMed] [Google Scholar]
- 9. World Health Organization. Weekly Iron-Folic Acid Supplementation (WIFS) in Women of Reproductive Age: Its Role in Promoting Optimal Maternal and Child Health. Position Statement. Geneva: World Health Organization, 2009. [PubMed] [Google Scholar]
- 10. Magee HE, Milligan EH. Haemoglobin levels before and after labour. Br Med J 1951;2:1307–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. deSwiet M. Antihypertensive drugs in pregnancy. Br Med J (Clin Res Ed) 1985;291:365–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hallberg L. Iron balance in pregnancy and lactation. In: Foman S, Zlotkin S, eds. Nutritional Anemias. New York: Raven Press, 1992:13–28 [Google Scholar]
- 13. Cook JD, Skikne BS, Lynch SR, Reusser ME. Estimates of iron sufficiency in the US population. Blood 1986;68:726–31 [PubMed] [Google Scholar]
- 14. English RM, Bennett SA. Iron status of Australian children. Med J Aust 1990;152:582–6 [PubMed] [Google Scholar]
- 15. Milman N, Bergholt T, Eriksen L, et al. Iron prophylaxis during pregnancy – how much iron is needed? A randomized dose–response study of 20–80 mg ferrous iron daily in pregnant women. Acta Obstet Gynecol Scand 2005;84:238–47 [DOI] [PubMed] [Google Scholar]
- 16. Bothwell TH. Iron requirements in pregnancy and strategies to meet them. Am J Clin Nutr 2000;72:257S–64S [DOI] [PubMed] [Google Scholar]
- 17. Andrews NC. Forging a field: the golden age of iron biology. Blood 2008;112:219–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hallberg L, Rossander-Hulten L. Iron requirements in menstruating women. Am J Clin Nutr 1991;54:1047–58 [DOI] [PubMed] [Google Scholar]
- 19. Green R, Charlton R, Seftel H, et al. Body iron excretion in man: a collaborative study. Am J Med 1968;45:336–53 [DOI] [PubMed] [Google Scholar]
- 20. Hallberg L, Hulten L. Iron requirements, iron balance and iron deficiency in menstruating and pregnant women. In: Hallberg L, Asp N-G, eds. Iron Nutrition in Health and Disease. London: George Libbey, 1996:165–82 [Google Scholar]
- 21. Taylor DJ, Lind T. Red cell mass during and after normal pregnancy. Br J Obstet Gynaecol 1979;86:364–70 [DOI] [PubMed] [Google Scholar]
- 22. Kaufer M, Casaneuva E. Relation of pregnancy serum ferritin levels to hemoglobin levels throughout pregnancy. Eur J Clin Nutr 1990;44:709–15 [PubMed] [Google Scholar]
- 23. Bothwell TH, Charlton RW. Current problems of iron overload. Recent Results Cancer Res 1979;69:87–95 [DOI] [PubMed] [Google Scholar]
- 24. De Leeuw NK, Lowenstein L, Hsieh YS. Iron deficiency and hydremia in normal pregnancy. Medicine (Baltimore) 1966;45:291–315 [DOI] [PubMed] [Google Scholar]
- 25. Widdowson EM, Spray CM. Chemical development in utero . Arch Dis Child 1951;26:205–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fransson GB, Lonnerdal B. Iron in human milk. J Pediatr 1980;96:380–4 [DOI] [PubMed] [Google Scholar]
- 27. Turner RE, Langkamp-Henken B, Littell RC, Lukowski MJ, Suarez MF. Comparing nutrient intake from food to the estimated average requirements shows middle- to upper-income pregnant women lack iron and possibly magnesium. J Am Diet Assoc 2003;103:461–6 [DOI] [PubMed] [Google Scholar]
- 28. Brownlie TT, Utermohlen V, Hinton PS, Giordano C, Haas JD. Marginal iron deficiency without anemia impairs aerobic adaptation among previously untrained women. Am J Clin Nutr 2002;75:734–42 [DOI] [PubMed] [Google Scholar]
- 29. Lozoff B, Jimenez E, Wolf AW. Long-term developmental outcome of infants with iron deficiency. N Engl J Med 1991;325:687–94 [DOI] [PubMed] [Google Scholar]
- 30. Murray-Kolb LE, Beard JL. Iron treatment normalizes cognitive functioning in young women. Am J Clin Nutr 2007;85:778–87 [DOI] [PubMed] [Google Scholar]
- 31. Oski FA. Iron deficiency – facts and fallacies. Pediatr Clin North Am 1985;32:493–7 [DOI] [PubMed] [Google Scholar]
- 32. Idjradinata P, Pollitt E. Reversal of developmental delays in iron-deficient anaemic infants treated with iron. Lancet 1993;341:1–4 [DOI] [PubMed] [Google Scholar]
- 33. Zhou SJ, Gibson RA, Crowther CA, Baghurst P, Makrides M. Effect of iron supplementation during pregnancy on the intelligence quotient and behavior of children at 4 y of age: long-term follow-up of a randomized controlled trial. Am J Clin Nutr 2006;83:1112–7 [DOI] [PubMed] [Google Scholar]
- 34. CDC. Current trends CDC criteria for anemia in children and childbearing-aged women. MMWR 1989;38:400–4 [PubMed] [Google Scholar]
- 35. WHO/UNICEF/UNU. Iron Eficiency Anaemia. Assessment, Prevention, and Control. A Guide for Programme Managers. Geneva: World Health Organization, 2001. [Google Scholar]
- 36. Lund CJ, Donovan JC. Blood volume during pregnancy. Significance of plasma and red cell volumes. Am J Obstet Gynecol 1967;98:394–403 [PubMed] [Google Scholar]
- 37. Wallenburg HC, van Eijk HG. Effect of oral iron supplementation during pregnancy on maternal and fetal iron status. J Perinat Med 1984;12:7–12 [DOI] [PubMed] [Google Scholar]
- 38. Fullerton WT, Hytten FE, Klopper AI, McKay E. A case of quadruplet pregnancy. J Obstet Gynaecol Br Commonw 1965;72:791–6 [DOI] [PubMed] [Google Scholar]
- 39. Rovinsky JJ, Jaffin H. Cardiovascular hemodynamics in pregnancy. I. Blood and plasma volumes in multiple pregnancy. Am J Obstet Gynecol 1965;93:1–15 [DOI] [PubMed] [Google Scholar]
- 40. Pirani BB, Campbell DM, MacGillivray I. Plasma volume in normal first pregnancy. J Obstet Gynaecol Br Commonw 1973;80:884–7 [DOI] [PubMed] [Google Scholar]
- 41. World Health Organization. Nutritional anaemias. Technical Report Series No. 503 Geneva, 1972. [Google Scholar]
- 42. Viteri FE, Berger J. Importance of pre-pregnancy and pregnancy iron status: can long-term weekly preventive iron and folic acid supplementation achieve desirable and safe status? Nutr Rev 2005;63:S65–S76 [DOI] [PubMed] [Google Scholar]
- 43. Xiong X, Buekens P, Alexander S, Demianczuk N, Wollast E. Anemia during pregnancy and birth outcome: a meta-analysis. Am J Perinatol 2000;17:137–46 [DOI] [PubMed] [Google Scholar]
- 44. Allen LH. Anemia and iron deficiency: effects on pregnancy outcome. Am J Clin Nutr 2000;71:1280S–4S [DOI] [PubMed] [Google Scholar]
- 45. McMahon LP, Smith J. The HELLP syndrome at 16 weeks gestation: possible association with the antiphospholipid syndrome. Aust N Z J Obstet Gynaecol 1997;37:313–4 [DOI] [PubMed] [Google Scholar]
- 46. McMahon LP, Mason K, Skinner SL, Burge CM, Grigg LE, Becker GJ. Effects of haemoglobin normalization on quality of life and cardiovascular parameters in end-stage renal failure. Nephrol Dial Transplant 2000;15:1425–30 [DOI] [PubMed] [Google Scholar]
- 47. Sachdev H, Gera T, Nestel P. Effect of iron supplementation on physical growth in children: systematic review of randomised controlled trials. Public Health Nutr 2006;9:904–20 [DOI] [PubMed] [Google Scholar]
- 48. Svanberg B, Arvidsson B, Norrby A, Rybo G, Solvell L. Absorption of supplemental iron during pregnancy – a longitudinal study with repeated bone-marrow studies and absorption measurements. Acta Obstet Gynecol Scand Suppl 1975;48:87–108 [DOI] [PubMed] [Google Scholar]
- 49. Bodnar LM, Scanlon KS, Freedman DS, Siega-Riz AM, Cogswell ME. High prevalence of postpartum anemia among low-income women in the United States. Am J Obstet Gynecol 2001;185:438–43 [DOI] [PubMed] [Google Scholar]
- 50. Carriaga MT, Skikne BS, Finley B, Cutler B, Cook JD. Serum transferrin receptor for the detection of iron deficiency in pregnancy. Am J Clin Nutr 1991;54:1077–81 [DOI] [PubMed] [Google Scholar]
- 51. Cook J, Skikne B, Baynes R. The use of serum transferrin receptor for the assessment of iron status. In: Hallberg L, Asp N-G, eds. Iron Nutrition in Health and Disease. London: George Libbey, 1996:49–58 [Google Scholar]
- 52. Fenton V, Cavill I, Fisher J. Iron stores in pregnancy. Br J Haematol 1977;37:145–9 [PubMed] [Google Scholar]
- 53. Beard JL. Iron deficiency: assessment during pregnancy and its importance in pregnant adolescents. Am J Clin Nutr 1994;59:502S–8S discussion 508S–510S [DOI] [PubMed] [Google Scholar]
- 54. Suominen P, Punnonen K, Rajamaki A, Irjala K. Serum transferrin receptor and transferrin receptor-ferritin index identify healthy subjects with subclinical iron deficits. Blood 1998;92:2934–9 [PubMed] [Google Scholar]
- 55. Mast AE, Blinder MA, Lu Q, Flax S, Dietzen DJ. Clinical utility of the reticulocyte hemoglobin content in the diagnosis of iron deficiency. Blood 2002;99:1489–91 [DOI] [PubMed] [Google Scholar]
- 56. Haram K, Hervig T, Ulvik RJ. [Hemoglobin, iron deficiency and anemia in pregnant women. Diagnostic aspects]. Tidsskr Nor Laegeforen 1997;117:962–6 [PubMed] [Google Scholar]
- 57. Madan N, Prasannaraj P, Rusia U, Sundaram KR, Nath LM, Sood SK. Monitoring oral iron therapy with protoporphyrin/heme ratios in pregnant women. Ann Hematol 1999;78:279–83 [DOI] [PubMed] [Google Scholar]
- 58. Richardson D, Bartlett C, Jolly H, Will EJ. Intravenous iron for CAPD populations: proactive or reactive strategies? Nephrol Dial Transplant 2001;16:115–9 [DOI] [PubMed] [Google Scholar]
- 59. Amoa AB, Lavu E, Ray U, Sapuri M, Kariwiga G, Heywood S. The aetiology of severe anaemia among antenatal patients of the Port Moresby General Hospital. P N G Med J 2003;46:143–51 [PubMed] [Google Scholar]
- 60. Krause A, Neitz S, Magert HJ, et al. LEAP-1, a novel highly disulfide-bonded human peptide, exhibits antimicrobial activity. FEBS Lett 2000;480:147–50 [DOI] [PubMed] [Google Scholar]
- 61. Park CH, Valore EV, Waring AJ, Ganz T. Hepcidin, a urinary antimicrobial peptide synthesized in the liver. J Biol Chem 2001;276:7806–10 [DOI] [PubMed] [Google Scholar]
- 62. Pigeon C, Ilyin G, Courselaud B, et al. A new mouse liver-specific gene, encoding a protein homologous to human antimicrobial peptide hepcidin, is overexpressed during iron overload. J Biol Chem 2001;276:7811–9 [DOI] [PubMed] [Google Scholar]
- 63. Roe MA, Spinks C, Heath AL, et al. Serum prohepcidin concentration: no association with iron absorption in healthy men; and no relationship with iron status in men carrying HFE mutations, hereditary haemochromatosis patients undergoing phlebotomy treatment, or pregnant women. Br J Nutr 2007;97:544–9 [DOI] [PubMed] [Google Scholar]
- 64. Schulze KJ, Christian P, Ruczinski I, et al. Hepcidin and iron status among pregnant women in Bangladesh. Asia Pac J Clin Nutr 2008;17:451–6 [PMC free article] [PubMed] [Google Scholar]
- 65. Guyatt GH, Oxman AD, Ali M, Willan A, McIlroy W, Patterson C. Laboratory diagnosis of iron-deficiency anemia: an overview. J Gen Intern Med 1992;7:145–53 [DOI] [PubMed] [Google Scholar]
- 66. van den Broek NR, Letsky EA, White SA, Shenkin A. Iron status in pregnant women: which measurements are valid? Br J Haematol 1998;103:817–24 [DOI] [PubMed] [Google Scholar]
- 67. Seshadri S. Prevalence of micronutrient deficiency particularly of iron, zinc and folic acid in pregnant women in South East Asia. Br J Nutr 2001;85 (Suppl. 2):S87–S92 [PubMed] [Google Scholar]
- 68. Gautam CS, Saha L, Sekhri K, Saha PK. Iron deficiency in pregnancy and the rationality of iron supplements prescribed during pregnancy. Medscape J Med 2008;10:283 [PMC free article] [PubMed] [Google Scholar]
- 69. van den Broek NR, Rogerson SJ, Mhango CG, Kambala B, White SA, Molyneux ME. Anaemia in pregnancy in southern Malawi: prevalence and risk factors. BJOG 2000;107:445–51 [DOI] [PubMed] [Google Scholar]
- 70. Patterson AJ, Brown WJ, Roberts DC, Seldon MR. Dietary treatment of iron deficiency in women of childbearing age. Am J Clin Nutr 2001;74:650–6 [DOI] [PubMed] [Google Scholar]
- 71. Makola D, Ash DM, Tatala SR, Latham MC, Ndossi G, Mehansho H. A micronutrient-fortified beverage prevents iron deficiency, reduces anemia and improves the hemoglobin concentration of pregnant Tanzanian women. J Nutr 2003;133:1339–46 [DOI] [PubMed] [Google Scholar]
- 72. Puolakka J, Janne O, Vihko R. Evaluation by serum ferritin assay of the influence of maternal iron stores on the iron status of newborns and infants. Acta Obstet Gynecol Scand Suppl 1980;95:53–6 [DOI] [PubMed] [Google Scholar]
- 73. Colomer J, Colomer C, Gutierrez D. Anemia during pregnancy as a risk factor for infant iron deficiency. Report from the Valencia infant anemia cohort. In: Hercberg S, Galan P, Dupin H, eds. Recent Knowledge on Iron and Folate Deficiencies in the World. Colloque: INSERM, 1990:577–82 [Google Scholar]
- 74. Preziosi P, Prual A, Galan P, Daouda H, Boureima H, Hercberg S. Effect of iron supplementation on the iron status of pregnant women: consequences for newborns. Am J Clin Nutr 1997;66:1178–82 [DOI] [PubMed] [Google Scholar]
- 75. Rasmussen K. Is there a causal relationship between iron deficiency or iron-deficiency anemia and weight at birth, length of gestation and perinatal mortality? J Nutr 2001;131:590S–601S; discussion 601S–603S [DOI] [PubMed] [Google Scholar]
- 76. Ronnenberg AG, Wood RJ, Wang X, et al. Preconception hemoglobin and ferritin concentrations are associated with pregnancy outcome in a prospective cohort of Chinese women. J Nutr 2004;134:2586–91 [DOI] [PubMed] [Google Scholar]
- 77. Berger J, Thanh HT, Cavalli-Sforza T, et al. Community mobilization and social marketing to promote weekly iron-folic acid supplementation in women of reproductive age in Vietnam: impact on anemia and iron status. Nutr Rev 2005;63:S95–S108 [DOI] [PubMed] [Google Scholar]
- 78. Menendez C, Todd J, Alonso PL, et al. The effects of iron supplementation during pregnancy, given by traditional birth attendants, on the prevalence of anaemia and malaria. Trans R Soc Trop Med Hyg 1994;88:590–3 [DOI] [PubMed] [Google Scholar]
- 79. Breuer W, Ronson A, Slotki IN, Abramov A, Hershko C, Cabantchik ZI. The assessment of serum nontransferrin-bound iron in chelation therapy and iron supplementation. Blood 2000;95:2975–82 [PubMed] [Google Scholar]
- 80. Mertz S, Woodhouse L, Donangelo C, et al. Breath ethane excretion rate in young women is increased in daily iron but not in daily zinc supplementation. FASEB J 1999;13:A216 [Google Scholar]
- 81. Lachili B, Hininger I, Faure H, et al. Increased lipid peroxidation in pregnant women after iron and vitamin C supplementation. Biol Trace Elem Res 2001;83:103–10 [DOI] [PubMed] [Google Scholar]
- 82. Knutson MD, Walter PB, Ames BN, Viteri FE. Both iron deficiency and daily iron supplements increase lipid peroxidation in rats. J Nutr 2000;130:621–8 [DOI] [PubMed] [Google Scholar]
- 83. Walter PB, Knutson MD, Paler-Martinez A, et al. Iron deficiency and iron excess damage mitochondria and mitochondrial DNA in rats. Proc Natl Acad Sci USA 2002;99:2264–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Steer PJ. Maternal hemoglobin concentration and birth weight. Am J Clin Nutr 2000;71:1285S–7S [DOI] [PubMed] [Google Scholar]
- 85. Hemminki E, Starfield B. Routine administration of iron and vitamins during pregnancy: review of controlled clinical trials. Br J Obstet Gynaecol 1978;85:404–10 [DOI] [PubMed] [Google Scholar]
- 86. Hytten F, Leitch I, Baird D. The Physiology of Human Pregnancy. 2nd edn Oxford: Blackwell, 1971. [Google Scholar]
- 87. Mahomed K, Hytten F. Iron and folate supplementation in pregnancy. In: Chalmers I, ed. Effective Care in Pregnancy. Oxford: Oxford University Press, 1989:301–7 [Google Scholar]
- 88. Murphy JF, O'Riordan J, Newcombe RG, Coles EC, Pearson JF. Relation of haemoglobin levels in first and second trimesters to outcome of pregnancy. Lancet 1986;1:992–5 [DOI] [PubMed] [Google Scholar]
- 89. Scanlon KS, Yip R, Schieve LA, Cogswell ME. High and low hemoglobin levels during pregnancy: differential risks for preterm birth and small for gestational age. Obstet Gynecol 2000;96:741–8 [DOI] [PubMed] [Google Scholar]
- 90. Huisman A, Aarnoudse JG. Increased 2nd trimester hemoglobin concentration in pregnancies later complicated by hypertension and growth retardation. Early evidence of a reduced plasma volume. Acta Obstet Gynecol Scand 1986;65:605–8 [DOI] [PubMed] [Google Scholar]
- 91. Cook JD, Flowers CH, Skikne BS. The quantitative assessment of body iron. Blood 2003;101:3359–64 [DOI] [PubMed] [Google Scholar]
- 92. Svanberg B. Iron absorption in early pregnancy – a study of the absorption of non-haeme iron and ferrous iron in early pregnancy. Acta Obstet Gynecol Scand Suppl 1975:69–85 [DOI] [PubMed] [Google Scholar]
- 93. Svanberg B. Absorption of iron in pregnancy. Acta Obstet Gynecol Scand Suppl 1975:1–108 [PubMed] [Google Scholar]
- 94. Svanberg B, Arvidsson B, Bjorn-Rasmussen E, Hallberg L, Rossander L, Swolin B. Dietary iron absorption in pregnancy – a longitudinal study with repeated measurements of non-haeme iron absorption from whole diet. Acta Obstet Gynecol Scand Suppl 1975;48:43–68 [DOI] [PubMed] [Google Scholar]
- 95. Heinrich HC, Bartels H, Heinisch B, et al. [Intestinal 59Fe resorption and prelatent iron deficiency during human pregnancy]. Klin Wochenschr 1968;46:199–202 [DOI] [PubMed] [Google Scholar]
- 96. Whittaker PG, Lind T, Williams JG. Iron absorption during normal human pregnancy: a study using stable isotopes. Br J Nutr 1991;65:457–63 [DOI] [PubMed] [Google Scholar]
- 97. Viteri FE, Alvarez E, Batres R, et al. Fortification of sugar with iron sodium ethylenediaminotetraacetate (FeNaEDTA) improves iron status in semirural Guatemalan populations. Am J Clin Nutr 1995;61:1153–63 [DOI] [PubMed] [Google Scholar]
- 98. Thuy PV, Berger J, Davidsson L, et al. Regular consumption of NaFeEDTA-fortified fish sauce improves iron status and reduces the prevalence of anemia in anemic Vietnamese women. Am J Clin Nutr 2003;78:284–90 [DOI] [PubMed] [Google Scholar]
- 99. Thuy P, Berger J, Nakanishi Y, et al. Effectiveness of NaFeEDTA fortified fish sauce in controlling iron deficiency in Vietnam. Abstract presented at the International Nutritional Anemia Group meeting Lima, Peru, 2004. [Google Scholar]
- 100. Viteri FE, Liu X, Tolomei K, Martin A. True absorption and retention of supplemental iron is more efficient when iron is administered every three days rather than daily to iron-normal and iron-deficient rats. J Nutr 1995;125:82–91 [DOI] [PubMed] [Google Scholar]
- 101. Srigiridhar K, Nair KM, Subramanian R, Singotamu L. Oral repletion of iron induces free radical mediated alterations in the gastrointestinal tract of rat. Mol Cell Biochem 2001;219:91–8 [DOI] [PubMed] [Google Scholar]
- 102. Pena-Rosas JP, Nesheim MC, Garcia-Casal MN, et al. Intermittent iron supplementation regimens are able to maintain safe maternal hemoglobin concentrations during pregnancy in Venezuela. J Nutr 2004;134:1099–104 [DOI] [PubMed] [Google Scholar]
- 103. Pizarro F, Olivares M, Arredondo M, et al. Does Daily Iron Administration Produce a Mucosal Blockade? Wageningen, The Netherlands: European Academy of Nutritional Sciences, 1997. [Google Scholar]
- 104. Hallberg L. Combating iron deficiency: daily administration of iron is far superior to weekly administration. Am J Clin Nutr 1998;68:213–7 [DOI] [PubMed] [Google Scholar]
- 105. Cook JD, Reddy MB. Efficacy of weekly compared with daily iron supplementation. Am J Clin Nutr 1995;62:117–20 [DOI] [PubMed] [Google Scholar]
- 106. Beard JL. Weekly iron intervention: the case for intermittent iron supplementation. Am J Clin Nutr 1998;68:209–12 [DOI] [PubMed] [Google Scholar]
- 107. Hallberg L. Oral iron therapy: factors affecting the absorption. In: Hallberg L, Harwerth H, Vannotti A, eds. Iron Deficiency. New York: Academic Press, 1970:551–72 [Google Scholar]
- 108. Fairweather-Tait SJ, Minski MJ. Studies on iron availability in man, using stable isotope techniques. Br J Nutr 1986;55:279–85 [DOI] [PubMed] [Google Scholar]
- 109. Ridwan E, Schultink W, Dillon D, Gross R. Effects of weekly iron supplementation on pregnant Indonesian women are similar to those of daily supplementation. Am J Clin Nutr 1996;63:884–90 [DOI] [PubMed] [Google Scholar]
- 110. Nordeng H, Eskild A, Nesheim BI, Aursnes I, Jacobsen G. Guidelines for iron supplementation in pregnancy: compliance among 431 parous Scandinavian women. Eur J Clin Pharmacol 2003;59:163–8 [DOI] [PubMed] [Google Scholar]
- 111. Zhou SJ, Gibson RA, Crowther CA, Makrides M. Should we lower the dose of iron when treating anaemia in pregnancy? A randomized dose-response trial. Eur J Clin Nutr 2009;63:183–90 [DOI] [PubMed] [Google Scholar]
- 112. Bayoumeu F, Subiran-Buisset C, Baka NE, Legagneur H, Monnier-Barbarino P, Laxenaire MC. Iron therapy in iron deficiency anemia in pregnancy: intravenous route versus oral route. Am J Obstet Gynecol 2002;186:518–22 [DOI] [PubMed] [Google Scholar]
- 113. Bencaiova G, von Mandach U, Zimmermann R. Iron prophylaxis in pregnancy: intravenous route versus oral route. Eur J Obstet Gynecol Reprod Biol 2009;144:135–9 [DOI] [PubMed] [Google Scholar]
- 114. Ayub R, Tariq N, Adil MM, Iqbal M, Junaid A, Jaferry T. Efficacy and safety of total dose infusion of low molecular weight iron dextran in the treatment of iron deficiency anemia during pregnancy. J Coll Physicians Surg Pak 2008;18:424–7 [PubMed] [Google Scholar]
- 115. van Zyl-Smit R. Diagnosis and management of iron deficiency in chronic dialysis patients. Curr Opin Nephrol Hypertens 2000;9:669–74 [DOI] [PubMed] [Google Scholar]
- 116. Solomons NW, Schumann K. Intramuscular administration of iron dextran is inappropriate for treatment of moderate pregnancy anemia, both in intervention research on underprivileged women and in routine prenatal care provided by public health services. Am J Clin Nutr 2004;79:1–3 [DOI] [PubMed] [Google Scholar]
- 117. Breymann C, Visca E, Huch R, Huch A. Efficacy and safety of intravenously administered iron sucrose with and without adjuvant recombinant human erythropoietin for the treatment of resistant iron-deficiency anemia during pregnancy. Am J Obstet Gynecol 2001;184:662–7 [DOI] [PubMed] [Google Scholar]
- 118. Wagstrom E, Akesson A, Van Rooijen M, Larson B, Bremme K. Erythropoietin and intravenous iron therapy in postpartum anaemia. Acta Obstet Gynecol Scand 2007;86:957–62 [DOI] [PubMed] [Google Scholar]