Abstract
Objective:
In order to investigate the antioxidant activities of polysaccharides (BPL-1 and BPL-2), one of the most important functional constituents in Brasenia schreberi was isolated from the external mucilage of B. schreberi (BPL-1) and the plant in vivo (BPL-2). This paper examines the relationship between the content of sulfuric radicals and uronic acid in BPL and the antioxidant activity of BPL.
Materials and Methods:
The free radicals, 2,2'-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS+) and 1,1-diphnyl-2-picrylhydrazyl (DPPH-), were used to determine the antioxidant activity of BPL. The Fourier-transform infrared spectroscopy of BPL-1 and BPL-2 revealed typical characteristics of polysaccharides.
Results:
The two sample types had different contents. This was proved by their different adsorption peak intensities. The IC50 values of BPL-1 (31.189 mg/ml) and BPL-2 (1.863 mg/ml) showed significant DPPH radical scavenging activity. Based on the quantification of ABTS radical scavenging, the IC50 value of BPL-1 (5.460 mg/ml) was higher than that of BPL-2 (0.239 mg/ml). Therefore, in terms of the reducing power, the IC50 value of BPL-1 was too high to determine, and the IC50 value of BPL-2 was found to be 50.557 mg/ml. Hence, the antioxidant activity and total reducing power were high, and they were greater in BPL-2 than in BPL-1. In addition, BPL-2 was found to have more sulfuric radicals and uronic acid than BPL-1.
Conclusion:
The contents of sulfuric radicals and uronic acid are significantly correlated to the antioxidant activity and reducing power of BPL; the more sulfuric radicals and uronic acid, the more antioxidant activity and reducing power BPL has.
SUMMARY
The water-soluble crude polysaccharides obtained from the external mucilage and the Brasenia schreberi plant in vivo were confirmed to have high contents of sulfuric radicals and uronic acid
Both BPL-1 and BPL-2 exhibited antioxidative activity and reducing power, and their antioxidative activity gradually improved with increasing concentrations
The content of sulfuric radicals and uronic acid in BPL-1 and BPL-2 might explain their high antioxidant activity.
Abbreviations used: BPL-1:Polysaccharide were isolated from the external mucilage of B. Schreberi; BPL-2: Polysaccharide were isolated from the plant in vivo of B. schreberi; BPL:Polysaccharide were isolated from B. Schreberi.
Keywords: Antioxidant, Brasenia schreberi, polysaccharide, sulfuric radical, uronic acid
INTRODUCTION
Oxidation is essential to many organisms. It helps in the production of energy to fuel biological processes. However, reactive oxygen species are often overproduced under pathological conditions, resulting in oxidative stress.[1,2] The overproduction of various forms of activated oxygen species, such as free radical and nonfree radical species, is involved in the onset of many diseases, such as cancer, cardiovascular disease, rheumatoid arthritis, and atherosclerosis as well as in degenerative processes associated with aging.[3] Synthetic antioxidants inevitably have unwanted side effects that can damage people's health, and they are used for industrial processing at the present time. Therefore, in order to reduce damage to the human body, the development and utilization of natural antioxidants is essential and urgent.[4] In past studies, it has been found that some natural substances have antioxidant activity, such as ascorbic acid, tocopherol, beta-carotene, flavonoids, tannins, and anthocyanins.
Brasenia schreberi is native to China. It grows in ponds, lakes, and swamps. It is a kind of rare and valuable edible vegetable. B. schreberi is mainly produced in Zhejiang, Jiangsu, Hunan, and Sichuan in China. As resources with natural antioxidants, much attention has been paid to plants and other organisms. B. schreberi is a famous traditional Chinese herbal medicine. It nourishes the liver and brightens the eyes. It has been eaten since ancient times due to its high nutritional and medicinal values. The compendium of materia medica records the following:
B. schreberi is cold, sweet, and nontoxic, and it has many medicinal values, such as diuresis, swelling, heat cure dysentery, jaundice, and boils. Simultaneously, the most notable is that the highest proportion of the various nutrients of B. schreberi is a polysaccharide. Polysaccharides and their conjugates, used in the food industry and in medicine for a long time, have attracted much attention, in the recent years, due to their biological activities.[5] Therefore, it is highly possible that the polysaccharide extracted from B. schreberi has great antioxidant activity.
According to the above, it is worthwhile to extract and isolate polysaccharide from B. schreberi and assay its antioxidant activity. There are no specific reports regarding the relationship between the content of sulfuric radicals and uronic acid in BPL or about the antioxidant activity of BPL. Hence, in this study, the BPL was prepared from the external mucilage (BPL-1) and the plant in vivo (BPL-2), respectively. The present work investigates the possible antioxidant effects of BPL-1 and BPL-2. In the study, we, the researchers, examined the antioxidant activity of the BPL, specifically quantifying their reducing powers, 1,1-diphnyl-2-picrylhydrazyl (DPPH-) radical scavenging abilities, and 2,2'-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS+) radical scavenging abilities. This study establishes the research about the antioxidant activity of water-soluble polysaccharide from B. schreberi. Our research was inspired by previous studies that show that many polysaccharides of different plants have strong antioxidant activity.
MATERIALS AND METHODS
Materials and chemicals
All plant materials were purchased from Horse Lake (Sichuan). Sodium hydroxide, N-butyl alcohol, trichloromethane, anhydrous EtOH, D-glucose, D-glucuronic acid, Vitamin C, and trichloroacetic acid were used in this study. The ABTS+ was purchased from Merck Co., and the DPPH was purchased from Sigma-Aldrich Co.(St. Louis, MO, USA). All other chemicals and reagents used were of analytical purity.
Preparation of the extracts
Polysaccharides from the external mucilage were extracted and isolated from B. schreberi. The fresh B. schreberi were obtained and thoroughly washed with tap water to remove the preservation solution. All the clear samples were extracted by reflux with sodium hydroxide at 40°C for 30 min (1:15, w/v). After the mixture was filtered, the residues were air dried, and the filtrate was concentrated in a rotary evaporator under reduced pressure at 55°C (S1). The dried leaves were mashed and mixed with some water, and then the mixture was extracted by reflux at 100°C for 1 h. After the mixture was filtered, the residues were extracted with the same procedure 3 times, and then all of the filtrates were concentrated in a rotary evaporator under reduced pressure at 55°C (S2). The S1 and S2 were repeatedly treated with the same volume of SEVAG reagent to remove free proteins. The supernatants were precipitated using anhydrous EtOH (1:4) (v/v) with the magnetic stirrer at 4°C for one night. The mixtures (S1, S2) were then centrifuged for 10 min at 4000 rotations per minute to obtain the sediments. The sediments were successively eluted with acetone and ethyl acetate and filtrated. Then the BPL-1 and BPL-2 were obtained and dried.
Analysis of the content of sulfuric radicals and uronic acid
The sulfuric radical content in the BPL-1 and BPL-2 were determined using the phenol-sulfuric acid method, using D-glucose as the standard.[6] First, D-glucose was used to prepare the reference solution (0.1 mg/ml). Second, the reference solution was put in tubes (0.2 ml, 0.4 ml, 0.6 ml, 0.8 ml, and 1.0 ml), and phenol solution was added to shock them. Then concentrated sulfuric acid was quickly added to shock them. The tubes were left to stand for 10 min until they cooled to room temperature, and the absorbance at 490 nm followed. Redistilled water was used as a blank control. The results were determined using linear regression. The absorbance levels of the samples were then determined to determine the content of sulfuric radicals. The uronic acid content in the BPL-1 and BPL-2 were also determined using the carbazole-sulfuric acid method, with D-glucuronic acid as the standard.[7,8] First, D-glucuronic acid was used to prepare reference solution (0.2 mg/ml). Secondly, the reference solution was placed in tubes (0 ml, 0.1 ml, 0.2 ml, 0.4 ml, 0.6 ml, and 0.8 ml), and redistilled water was added to shock them. Then concentrated sulfuric acid was added to them, and they were quickly shocked using ice water. Then the mixtures were incubated at 50°C for 20 min. After the samples cooled to room temperature, carbazole solution was added (0.2 ml). The tubes were left to stand for 2 h followed by the absorbance at 490 nm. The sample was determined using the same method. The results were determined through linear regression, and then the absorbance of the samples was determined in order to quantify the content of uronic acid.
Assay of antioxidant activity in vitro of the polysaccharides extracted from Brasenia schreberi
Determination of total reducing power
The ferric iron ion (Fe3+) reduction method is often used in experiments to observe the reducing power of the materials.[9,10] Each extract (0.05–3.2 mg/ml) in water was mixed with 2.5 ml of 0.2 M phosphate buffer saline (with a pH of 6.6) and 2.5 ml of 30 mM potassium ferricyanide, and the mixture was incubated at 50°C for 20 min. Thereafter, 2.5 ml of 10% trichloroacetic acid was added, and the mixture was centrifuged at 1000 rpm for 10 min. The upper layer (2.5 ml) was mixed with 2.5 ml of deionized water and 0.5 ml of 6 mM ferric chloride, and its absorbance was measured spectrophotometrically at 700 nm against a blank. Ascorbic acid was used as the reference standard. The high absorbance of the reaction mixture indicated greater reductive potential.[11]
Assay of 1,1-diphnyl-2-picrylhydrazyl radical-scavenging activity
The scavenging effects of polysaccharide on DPPH radicals were assayed using the method proposed by Blois,[12] with some modifications. The antioxidant activity of the polysaccharides was determined using the DPPH method. About 0.9 ml of the DPPH solution (0.05–3.2 mg/ml) was added to 0.3 ml of the extract or standard solution (0.05–3.2 mg/ml) and allowed to react at room temperature in the dark for 30 min. The absorbance was measured against a corresponding blank at 517 nm. Deionized water (0.3 ml) in place of the extract solution was used as a control, and all measurements were done in triplicate.
The DPPH scavenging effect was calculated as follows:
Scavenging effect (%) = ([A0-A1]/A0) ×100%, where A0 is the absorbance of the control and A1 is the absorbance of the polysaccharides or standard.
Assay of 2,2'-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) radical-scavenging activity
The analysis and quantification was performed on various concentrations of samples in order to determine the absorbance of ABTS+ radical cation, which helped the researchers measure the samples' abilities to scavenge ABTS+ radical cations. This method was firstly proposed by Miller et al.[13] and modified by Re et al.[14] The oxidized ABTS solution was allowed to stand for 16 h in the dark at room temperature. About 1 ml of ABTS solution was added to 0.1 ml of various concentrations of the polysaccharides, and the absorbance of each mixture was measured against a corresponding blank at 734 nm after 20 min. All measurements were done in triplicate.
The ABTS scavenging effect was calculated as follows:
Scavenging effect (%) = ([A0-A1]/A0) ×100%, where A0 is the absorbance of the control and A1 is the absorbance of the polysaccharides/standard.
RESULTS AND DISCUSSION
Contents of sulfuric radical and uronic acid
The sulfuric radical content in the BPL-1 and BPL-2 were determined through the phenol-sulfuric acid method, using D-glucose as a standard. The uronic acid content in the BPL-1 and BPL-2 were determined by the carbazole-sulfuric acid method, with D-glucuronic acid as a standard.
The total content of sulfuric radicals in BPL-1 and BPL-2 were calculated by referencing the calibration curve (y = 8.5000x + 0.0160, r2 = 0.9945). The contents were expressed as a percent. Furthermore, the total content of uronic acid in the BPL-1 and BPL-2 samples were calculated by referencing the calibration curve (y = 41.085x + 0.0878, r2 = 0.9847). The contents were expressed as a percent. The contents of sulfuric radicals and uronic acid in the samples are shown in Table 1. There were significant differences among them. The results show that BPL-2 had a higher content of sulfuric radicals and uronic acid than BPL-1.
Table 1.
Contents of sulfuric radical and uronic acid

Fourier-transform infrared spectroscopy spectroscopy
To investigate the chemical composition of the B. schreberi, Fourier-transform infrared spectroscopy (FTIR) technology was used, and the results are displayed in Figures 1 and 2. The FTIR spectrum of BPL-1 is shown in Figure 1. It shows a broad intensity peak at around 3760 cm−1, which is the characteristic absorption of amine, and relatively weak O-H bands at around 3458 cm−1 and 1635 cm−1. Weak CH3 and CH2 bands were also identified at 1461 cm−1 and 1455 cm−1, respectively. The FTIR absorption at around 901 cm−1 651 cm−1 is also characteristic of benzene.
Figure 1.
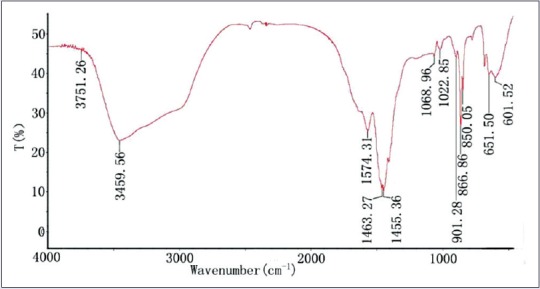
Fourier-transform infrared spectroscopy spectra of the polysaccharide of BPL-1
Figure 2.
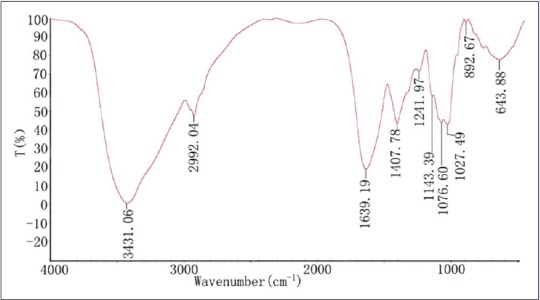
Fourier-transform infrared spectroscopy spectra of the polysaccharide of BPL-2
The FTIR spectrum of the BPL-2 is shown in Figure 2. A strong and broad absorption peak at 3431 cm− 1 for O-H stretching vibrations, a peak at 2929 cm− 1 for C-H stretching vibrations, and a strong extensive absorption in the region of 900–1200 cm− 1 for coupled C-O and C-C stretching and C-OH bending vibrations were observed in BPL-2, indicating the characteristic absorptions of polysaccharides. In addition, the absorption peak at 1241 cm− 1 was assigned to the asymmetric stretching vibrations of so, an evidence of sulfuric radicals, indicating that BPL-2 was sulfated polysaccharides.
The spectrograph indicated that BPL-1 and BPL-2 had some similar adsorption peaks, which may be because they both contain sulfuric radicals. There were some different contents in the two kinds of samples. This was proved by different adsorption peaks. The intensities of peaks were also different between them. The results of FTIR characterizations were consistent with those of Table 1.
Antioxidant activity analysis
Scavenging activity of 1,1-diphnyl-2-picrylhydrazyl radicals
DPPH is a free radical compound that has been widely used to determine the free radical scavenging abilities of various samples.[15,16,17] The free radical-scavenging activity of BPL-1 and BPL-2 were examined using the DPPH method, and the results were compared with the free radical-scavenging activity of Vitamin C. DPPH is a stable free radical that shows maximum absorption at 517 nm in methanol. When DPPH encounters a proton-donating substance, such as an antioxidant, the radical is scavenged, and the absorbance at 517 nm is reduced. This is visually noticeable as a color change from purple to yellow. Based on this principle, the antioxidative activity of a substance can be expressed as its ability to scavenge DPPH free radicals. In the study, the DPPH free radical scavenging effect of each simple was measured. The results are shown in Figure 3.
Figure 3.
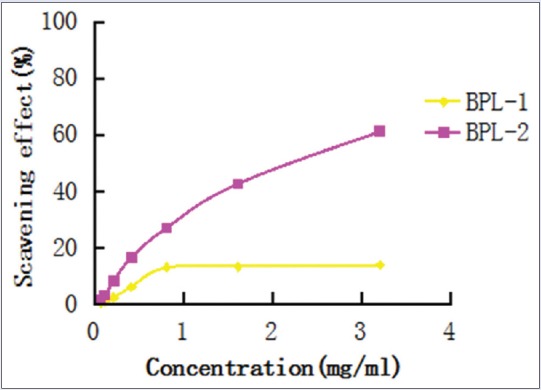
The 1,1-diphnyl-2-picrylhydrazyl free radical scavenging effect
Both BPL-1 and BPL-2 exhibited antioxidative activity and their DPPH scavenging effects gradually improved with increasing concentrations. The IC50 value of BPL-1 and BPL-2 were 31.189 mg/ml and 1.863 mg/ml, respectively [Table 2].
Table 2.
IC50 of different extracts for various antioxidant systems

Scavenging activity to 2,2'-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid)-radical
ABTS+ radical cation is reactive towards most antioxidants, and the decolorization reflects the capacity of an antioxidant species to donate electrons or hydrogen atoms to deactivate the radical species. Currently, this method is widely used to evaluate the antioxidant activities of natural products.[18,19,20]
In the study, the trends in ABTS+ scavenging activity of the BPL-1 and BPL-2 gradually improved as their concentrations increased. However, BPL-2 demonstrated higher antioxidative activity than BPL-1. Meanwhile, BPL-2 was also determined to have more sulfuric radicals and uronic acid than BPL-1. When the concentration of BPL-2 was approximately 0.8 mg/ml, the rate of ABTS+ radical scavenging was close to 100%. Hence, BPL-2 demonstrated high ABTS+ scavenging effects. The IC50 value of BPL-1 and BPL-2 were 5.460 mg/ml and 0.239 mg/ml, respectively [Table 2 and Figure 4].
Figure 4.
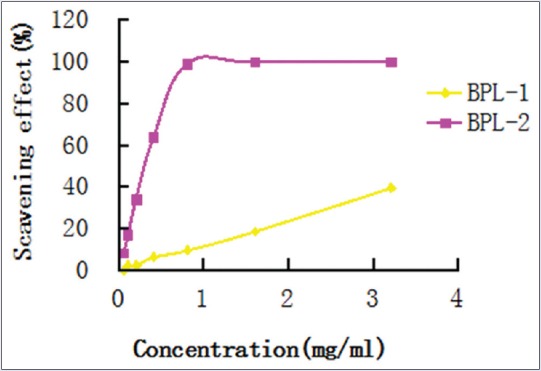
The 2,2’-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) free radical scavenging effect
Reducing power
The reduction capacity of a substance is not only important in terms of the performance of antioxidant activity but also a reasonable explanation of its antioxidant capacity.[21] BPL uses a Fe3 + reduction method.[22] The yields of sulfuric radicals of BPL-1 and BPL-2 were 0.78% and 16.86%, respectively, and the yields of uronic acid of BPL-1 and BPL-2 were 0.079% and 1.449%, respectively. The reduction capacity of a compound may serve as a significant indicator of its potential antioxidant activity. In this study, the polysaccharides exhibited effective reduction capacities at all concentration levels. The reduction capacities of the polysaccharides increased with increasing concentrations [Figure 5]. According to changing concentration trends, the researchers of this study concluded that the reducing power of BPL-2 was stronger than that of BPL-1. The IC50 of BPL-1 is too high to determine, and the IC50 of BPL-2 was 50.557 mg/ml.
Figure 5.
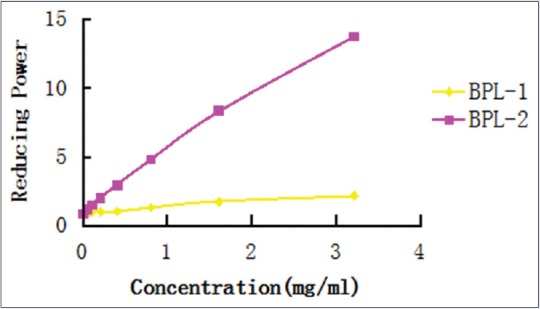
Reducing power of the samples
The correlation of antioxidant activity and the content of sulfuric radicals and uronic acid
The DPPH and ABTS radical scavenging activities and reducing power of BPL-1 and BPL-2 have significant correlations with the content of sulfuric radicals and uronic acid. Meanwhile, BPL-2 was determined to have more sulfuric radicals and uronic acid than BPL-1, and BPL-1 had greater antioxidant activity, scavenging activities of DPPH and ABTS+ radicals, and reducing power than BPL-1. Therefore, the content of sulfuric radicals and uronic acid in BPL-1 and BPL-2 might explain their high antioxidant activity.
CONCLUSIONS
On the one hand, the sulfuric radical content in the BPL-1 and BPL-2 were determined using the phenol-sulfuric acid method, and the uronic acid content in the BPL-1 and BPL-2 were using the carbazole-sulfuric acid method. On the other hand, the antioxidant activity from B. schreberi was determined by the reducing power scavenging of DPPH radicals and ABTS+ radicals.
According to the above results, it was concluded that the water-soluble crude polysaccharides obtained from the external mucilage and the B. schreberi plant in vivo were confirmed to have high contents of sulfuric radicals and uronic acid. The assessment of the plant's in vitro antioxidation properties showed that BPL possessed strong free radical scavenging activities for DPPH and ABTS+ radicals. In addition, the BPL-2 showed a higher rate of ABTS+ radical scavenging, which is comparable to that of Vitamin C.
On the one hand, BPL-2 had higher antioxidant activities in terms of scavenging DPPH and ABTS+ radicals and reducing power. On the other hand, BPL-2 also had more sulfuric radicals and uronic acid than BPl-1. According to these correlations [Table 2], sulfuric radical and uronic acid content are significantly related to the antioxidant activities and the reducing power of BPL. The more sulfuric radicals and uronic acid that plants have, the more antioxidant activities and reducing power they have. The results of this study suggest that the water-soluble polysaccharides obtained from B. schreberi should be explored as a potentially novel and effective natural antioxidant.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
ABOUT AUTHOR

Aoxue Luo
Aoxue Luo, is a associate professor and master adviser of Sichuan Agriculture University. The resource development of plants and study of plant secondary metabolites are her research interests. She is also the corresponding author of this research.
REFERENCES
- 1.Gülçin I, Oktay M, Küfrevioglu OI, Aslan A. Determination of antioxidant activity of lichen Cetraria islandica (L) Ach. J Ethnopharmacol. 2002;79:325–9. doi: 10.1016/s0378-8741(01)00396-8. [DOI] [PubMed] [Google Scholar]
- 2.Yildirim A, Mavi A, Oktay M, Kara AA, Algur OF, Bilaloglu V. Comparison of antioxidant and antimicrobial activities of tilia (Tilia argentea Desf ex DC), sage (Salvia triloba l.), and black tea (Camellia sinensis) extracts. J Agric Food Chem. 2000;48:5030–4. doi: 10.1021/jf000590k. [DOI] [PubMed] [Google Scholar]
- 3.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–47. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 4.Mau JL, Lin HC, Chen CC. Antioxidant properties of several medicinal mushrooms. 2002;50:6072–7. doi: 10.1021/jf0201273. [DOI] [PubMed] [Google Scholar]
- 5.Chen H, Zhang M, Qu Z, Xie B. Compositional analysis and preliminary toxicological evaluation of a tea polysaccharide conjugate. Journal of Agricultural and Food Chemistry. 2007;55:2256–60. doi: 10.1021/jf0632740. [DOI] [PubMed] [Google Scholar]
- 6.He JZ, Shao P, Ni HD, Chai NJ, Sun PL. Study on the structure and constituents of polysaccharide from Ganoderma lucidum. Spectroscopy and Spectral Analysis. 2010;1:123–7. [PubMed] [Google Scholar]
- 7.Dische Z. A new specific color reaction of hexuronic acids. J Biol Chem. 1947;167:189–98. [PubMed] [Google Scholar]
- 8.Yang W, Pei F, Shi Y, Zhao L, Yong F, Hu Q. Purification, characterization and anti-proliferation activity of polysaccharides from Flammulina velutipes. Carbohydr Polym. 2012;88:474–80. [Google Scholar]
- 9.Reddy PE, Manohar SM, Reddy SV, Bitla AR, Vishnubhotla S, Narasimha SR. Ferric reducing ability of plasma and lipid peroxidation in hemodialysis patients. Intradialytic changes. Int J Nephrol Urol. 2010;3:414–21. [Google Scholar]
- 10.Singh S, Singh RP. In vitro methods of assay of antioxidants: An overview. Food Rev Int. 2008;24:392–415. [Google Scholar]
- 11.Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal Biochem. 1996;239:70–6. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 12.Blois MS. Antioxidant determinations by the use of a stable free radical. Nature. 1957;181:1199–1200. [Google Scholar]
- 13.Miller NJ, Rice-Evans C, Davies MJ, Gopinathan V, Milner A. A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clin Sci (Lond) 1993;84:407–12. doi: 10.1042/cs0840407. [DOI] [PubMed] [Google Scholar]
- 14.Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26:1231–7. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 15.Xu W, Zhang F, Luo Y, Ma L, Kou X, Huang K. Antioxidant activity of a water-soluble polysaccharide purified from Pteridium aquilinum. Carbohydr Res. 2009;344:217–22. doi: 10.1016/j.carres.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 16.Li XM, Zhou AG. Evaluation of antioxidant activity of the polysaccharides extracted from Lycium barbarum fruits in vitro. Eur Polym J. 2007;43:488–97. [Google Scholar]
- 17.Zhao ZY, Huangfu LT, Dong LL, Liu SL. Functional groups and antioxidant activities of polysaccharides from five categories of tea. Ind Crops Prod. 2014;58:31–5. [Google Scholar]
- 18.Durmaz G. Freeze-dried ABTS method: A ready-to-use radical powder to assess antioxidant capacity of vegetable oils. Food Chem. 2012;133:1658–63. [Google Scholar]
- 19.Floegel A, Kim DO, Chung SJ, Kooa SI, Chuna OK. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J Food Compost Anal. 2011;24:1043–8. [Google Scholar]
- 20.Zeng WC, Jia LR, Zhang Y, Cen JQ, Chen X, Gao H, et al. Antibrowning and antimicrobial activities of the water-soluble extract from pine needles of Cedrus deodara. J Food Sci. 2011;76:318–23. doi: 10.1111/j.1750-3841.2010.02023.x. [DOI] [PubMed] [Google Scholar]
- 21.Luchsinger WW, Cornesky RA. Reducing power by the dinitrosalicylic acid method. Anal Biochem. 1962;4:346–7. doi: 10.1016/0003-2697(62)90098-2. [DOI] [PubMed] [Google Scholar]
- 22.Reddy PE, Manohar SM, Reddy SV, Bitla AR, Vishnubhotla S, Narasimha SR. Ferric reducing ability of plasma and lipid peroxidation in hemodialysis patients. Intradialytic changes. Int J Urol Nephrol. 2010;2:414–21. [Google Scholar]


