Abstract
Context:
Recent studies have demonstrated that baicalin has antihyperglycemic effects by inhibiting lipid peroxidation. Baicalin is low hydrophilic and poorly absorbed after oral administration. Thus, a suitable formulation is highly desired to overcome the disadvantages of baicalin.
Objective:
The objective of this work was to prepare baicalin-loaded nanostructured lipid carriers (B-NLCs) for enhanced antidiabetic effects.
Materials and Methods:
B-NLCs were prepared by high-pressure homogenization method using Precirol as the solid lipid and Miglyol as the liquid lipid. The properties of the NLCs, such as particle size, zeta potential (ZP), and drug encapsulation efficiency (EE), were investigated. The morphology of NLCs was observed by transmission electron microscopy. In addition, drug release and antidiabetic activity were also studied.
Results:
The results revealed that B-NLCs particles were uniformly in the nanosize range and of spherical morphology with a mean size of 92 ± 3.1 nm, a ZP of −31.35 ± 3.08 mV, and an EE of 85.29 ± 3.42%. Baicalin was released from NLCs in a sustained manner. In addition, B-NLCs showed a significantly higher antidiabetic efficacy compared with baicalin.
Conclusion:
B-NLCs described in this study are well-suited for the delivery of baicalin.
SUMMARY
Currently, herbal medicines have attracted increasing attention as a complementary approach for type 2 diabetes
Baicalin has antihyperglycemic effects by inhibiting lipid peroxidation
A suitable formulation is highly desired to overcome the disadvantages (poor solubility and low bioavailability) of baicalin
Nanostructured lipid carriers could enhance the antidiabetic effects of baicalin.
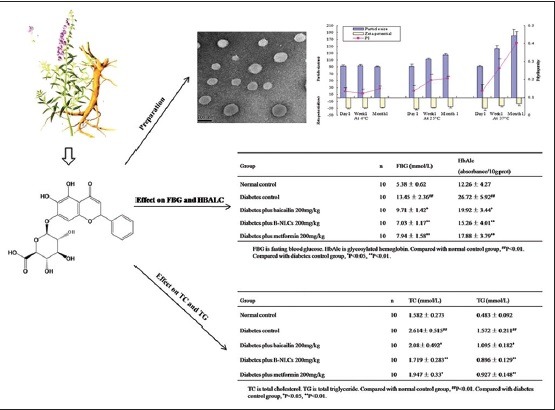
Abbreviations used: B-NLCs: Baicalin-Loaded Nanostructured Lipid Carriers, B-SUS: Baicalin Water Suspension, EE: Encapsulation Efficiency, FBG: Fasting Blood Glucose, HbAlc: Glycosylated Hemoglobin, HPLC: High-performance Liquid Chromatography; NLCs: Nanostructured Lipid Carriers, PI: Polydispersity Index, SD: Sprague-Dawley, SLNs: Solid lipid nanoparticles, STZ: Streptozotocin, TC: Total cholesterol, TEM: Transmission Electron Microscope, TG: Total Triglyceride, ZP: Zeta Potential.
Keywords: Antidiabetic efficacy, baicalin, drug release, high-pressure homogenization, nanostructured lipid carriers
INTRODUCTION
Diabetes mellitus, which characterizes by hyperglycemia resulting from defects in insulin secretion or action, is a metabolic disorder in the endocrine system. There are two major types of diabetes including type 1 diabetes and type 2 diabetes, and more than 90% of patients suffer from the latter.[1] Type 2 diabetes mellitus is characterized by insulin insensitivity, hyperglycemia, and hyperinsulinemia followed by long-term complications.[2] Currently available drugs for type 2 diabetes have a number of limitations, such as adverse effects and high rates of secondary failure.[3] Therefore, herbal medicines have attracted increasing attention as a complementary approach.[4]
Flavonoids extracted from herbal medicines possess antioxidant potential by scavenging ROS, and therefore, flavonoids are considered therapeutic remedies for diabetic complications.[5,6] Baicalin [structure shown in Figure 1] is one of the main flavonoid components isolated from Scutellaria radix. Baicalin has a lot of pharmacological effects, such as anticancer, antibacterial, and antidiabetic activities.[7,8,9] Baicalin has been reported to exhibit antihyperglycemic effects by inhibiting lipid peroxidation.[10] However, despite the pharmacological functions, more attention should be paid to the side effects of baicalin. Baicalin is low hydrophilic and poorly absorbed after oral administration due to its glycosyl group on the ring.[11] To overcome these drawbacks, one of the strategies is to encapsulate baicalin into biodegradable and biocompatible nanoparticles.
Figure 1.
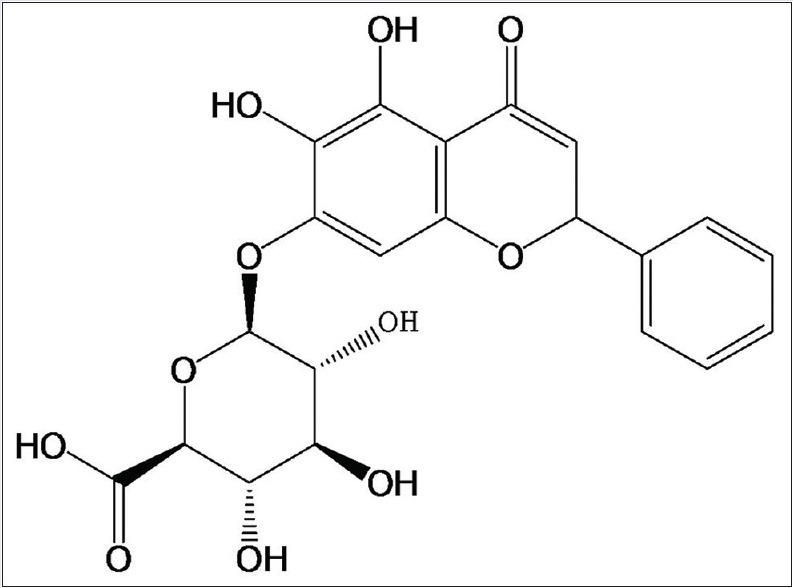
Structure of baicalin
Lipid nanoparticle formulations with solid matrix have gained tremendous attention in pharmaceutical fields during recent years owing to the excellent tolerability.[12] Solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs) are new generations of lipid nanoparticles produced from solid lipids. SLNs consist of pure solid lipids while NLCs are made of both solid lipids and liquid lipids. NLCs are reported to be evolved as second-generation lipid nanoparticles to overcome the disadvantages of SLNs, including larger particle sizes, limited drug-loading capacity, and risk of gelation.[13] Hence, NLCs may be feasible as carriers for baicalin delivery.
Therefore, in the present study, we aimed at preparing baicalin-loaded NLCs (B-NLCs) to enhance its antidiabetic activity. B-NLCs were prepared using a hot melting high-pressure homogenization method. Physical properties of B-NLCs such as particle size, zeta potential (ZP), and other properties were evaluated. In addition, in vitro release study and in vivo antidiabetic study were also performed.
MATERIALS AND METHODS
Reagents
Baicalin (purity > 95%) was purchased from Zhucheng Haotian Medicine Co., Ltd (China). Precirol® ATO 5 (glycerol distearate) was a kind gift from Gattefosse (France). Miglyol® 812 was purchased from Beijing Fengli Jingqiu Commerce and Trade Co., Ltd (China). Pluronic® F68 (polyoxyethylene-polyoxypropylene (150:29) block copolymer) was purchased from Sigma (USA). Streptozotocin (STZ, lot: 031M1287V) were purchased from Sigma (USA) and dissolved in citrate buffer (pH 4.2) before use. Metformin tablets (lot: 120706) were purchased from Bristol-Myers Squibb pharmaceutical Co., Ltd (Shanghai, China). Double-distilled water was obtained through the use of a Millipore® Simplicity System (Millipore, MA, USA). Other chemicals and reagents used were chromatographic or analytical grade.
Experimental animals
Six-week-old male Sprague-Dawley (SD) rats (180–200 g, grade of specific pathogen free) were supplied by Beijing HFK Bio-Technology Co., Ltd (Beijing, China). All the rats were maintained in the Experimental Animal Center of Jiangsu University (Jiangsu, China). The rats were housed in the 12 h dark/light room with the temperature at 22–25°C and the humidity at 45–70%. All experimental procedures were performed in accordance with the approval of the Animal Ethical Committee, Jiangsu University.
Preparation of B-nanostructured lipid carriers
The NLCs were prepared by a high-pressure homogenization technique.[14] In brief, lipid phase comprised Precirol (5.0% w/w) and Miglyol (2.0% w/w) were dissolved in chloroform and mixed with the drug (0.5% w/w) which was dissolved in dehydrated alcohol. Later, the mixture was heated at 10°C above the melting point of the solid lipid to remove the organic solvents. The aqueous phase, which contained pluronic (2.0% w/w) surfactant in double-distilled water, was prepared at the same temperature. The lipid phase was dispersed into the aqueous phase under high-speed stirring using the Ultra Turrax® (IKA Works GmbH and Co, Germany) at 8000 rpm for 8 min to yield a hot pre-emulsion. The resulting pre-emulsion was passed through a high-pressure homogenizer (EmulsiFlex C-50, Avestin Inc, Canada) at 1000 psi for 8 cycles. The B-NLCs suspension was obtained by solidification at room temperature.
Particle size, zeta potential, and polydispersity index
The analysis of average particle diameter, ZP, and polydispersity index (PI) were performed using a Malvern Zetasizer Nano ZS (Malvern Instruments, UK). The ZP was calculated using the Smoluchowski equation. Particle size was evaluated using volume distribution. Prior to measurements, all samples were diluted with distilled water to yield a suitable scattering intensity. All measurements were performed at 25°C, and each value was measured in triplicates.
Physical stability
The physical stability of freshly prepared B-NLCs was assessed under shade environment at three different temperatures (4°C, 25°C, and 37°C) for 1 month. Changes of the particle size, ZP, and PI were measured as previously described. All measurements were conducted in triplicates.
High-performance liquid chromatography analysis
A modified version of high-performance liquid chromatography (HPLC) method was developed to assess the content of baicalin. The HPLC system (Waters, USA) consisted of a 600 controller pump and a multiple-wavelength ultraviolet/visible detector. A reversed-phase C18 Wakosil HPLC column (5 µm, 150 mm × 4.6 mm) was used for separation. The analysis was performed using the mobile phase: Methanol and 0.1% phosphoric acid in water (42:58, v/v) under column temperature 30°C. The detection wavelength was set at 278 nm with a flow rate of 1 mL/min HPLC. Chromatograms were shown in Figure 2. The HPLC method was established for measurement of entrapment efficiency and in vitro release.
Figure 2.
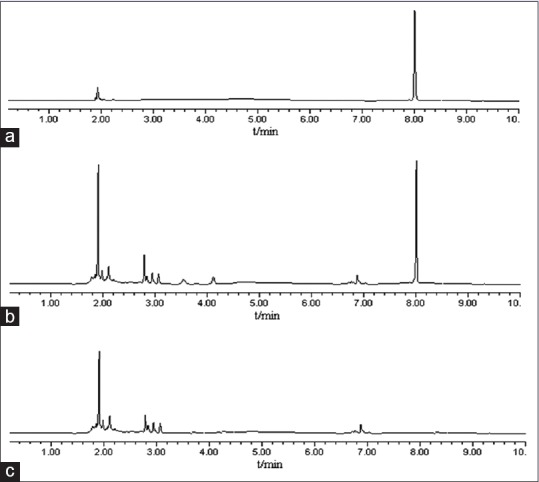
High-performance liquid chromatography chromatogram of baicalin high-performance liquid chromatography is high-performance liquid chromatography. (a) Chromatogram of standard baicalin sample, (b) chromatogram of B-nanostructured lipid carriers sample, peak 1 is baicalin with tR of 8 min
Entrapment efficiency
The EE of baicalin in the NLCs was assessed indirectly by measuring the concentration of free drug in the aqueous phase of the nanoparticles suspension. Free drug was separated from B-NLCs using Sephadex-G50 column (1.5 cm × 25 cm).[15] In brief, approximately 0.5 mL of NLCs suspension was added into a Sephadex G-50 gel column and eluted with distilled water at the rate of 1 mL/min. The free drug was separated and collected, and its concentration was determined by HPLC. Finally, the EE of B-NLCs was calculated using the following equation:
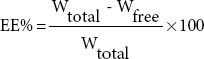
where Wtotal and Wfree are the total amount of baicalin present in B-NLCs suspension and the amount of untrapped baicalin, respectively.
Morphology
The morphology of B-NLCs was determined by transmission electron microscope (TEM, JEM-1200, JEOL Co. Ltd., Japan). Samples were diluted 50 times with the distilled water prior to observation. After dilution, a drop of sample was placed on a 200-mesh copper grid and was negatively stained with 2% phosphotungstic acid. The grid was dried at room temperature and then observed by transmission electron microscopy (TEM).
In vitro release
A dialysis bag diffusion technique was utilized to evaluate the in vitro drug release of B-NLCs. About 2 mL of B-NLCs dispersion and baicalin water suspention (B-SUS) were placed into a dialysis bag with a molecular weight cutoff of 8 kDa. The dialysis bag was placed in 100 mL release medium (phosphate-buffer at pH 6.8) to maintain the sink condition. The medium was maintained at 37 ± 0.5°C with the rotation at 100 rpm. At 0.5, 1, 2, 3, 4, 6, 8, and 12 h, 1 mL dispersion sample was withdrawn and replaced with the same volume of fresh medium. The amount of baicalin was determined by the established HPLC method.
In vivo antidiabetic
About 80 male SD rats were assigned randomly into two groups, i.e. the normal control group (n = 10, given normal pallet diet), the diabetic group (given high-fat diet). After 8 weeks, the rats in diabetic group were intraperitoneally injected 30 mg/kg STZ to induce diabetes mellitus. The rats in normal group were treated with the same volume citrate buffer. Diabetes was confirmed 72 h in the STZ-injected rats by measuring the glucose concentrations of peripheral blood obtained from the tail vein. The plasma glucose levels above 16.7 mmol/L were considered as diabetic and were used in the experiment.[16] The diabetic rats were randomly allotted into 4 groups (n = 10 each group): Diabetes plus drinking water group (model group), diabetes plus metformin 200 mg/kg group, diabetes plus baicalin 200 mg/kg group, and diabetes plus B-NLCs 200 mg/kg group. The rats received daily i.g. of corresponding drug from the next day after grouping. After the end of the 4 weeks, the overnight-fasted rats were weighed and anesthetized with intraperitoneal injection of sodium pentobarbital (30 mg/kg). Plasma samples were collected from abdominal aorta and centrifuged (4°C, 3500 rpm, and 15 min) to recover the plasma which was stored at − 20°C pending analysis. The fasting blood glucose (FBG), total cholesterol (TC), and total triglyceride (TG) in plasma were detected with automatic biochemistry analyzer (Hitachi 7080, Japan). The concentration of glycosylated hemoglobin (HbAlc) in plasma was detected using HbAlc kit (Nanjing Jiancheng Institute of Bioengineering, China).
Statistical analysis
All values were expressed as mean ± standard deviation. The data were assessed by SPSS statistical software (SPSS version 15.0; SPSS Inc., Chicago, IL, USA). Statistical analyses of data were performed by one-way analysis of variance: post hoc multiple comparisons. A P < 0.05 was considered to be statistically significant.
RESULTS AND DISCUSSION
Several techniques for NLCs fabrication have been developed in the last decade, including solvent emulsification-evaporation methods, microemulsion techniques, and high-pressure homogenization methods.[17] High-pressure homogenization, used in this study, is considered to be a promising approach, which could be utilized on a large scale in the pharmaceutical industry. In this study, Precirol was used as the solid lipid to form the outer shell of the nanoparticles whereas Miglyol was adopted as the liquid lipid to enhance the EE of baicalin in NLCs. In our pre-experiment, we found that baicalin could be dissolved in several solid and liquid lipid, such as GMS, Compritol 888 ATO, Precirol, Maisine 35–1, and Miglyol. However, stable NLC particles could not be prepared using GMS as the solid lipid and Maisine 35–1 as the liquid lipid. Hence, Precirol and Miglyol were used as the solid lipid and liquid lipid, respectively. Pluronic was selected as the surfactant based on a single factor experiment, using the particle size and PI as the index. Formulation optimization of B-NLCs was then performed using the orthogonal experiment taking the EE as the index. As a result, optimal formulation was set as follows: Precirol (5.0% w/w), Miglyol (2.0% w/w), drug (0.5% w/w), and pluronic (2.0% w/w).
Particle size is one critical factor in the aerosolization process. PI is a measure of particle homogeneity. Generally, the closer the value of polydispersity to zero the higher the homology among particles. The average particle size of the B-NLCs determined by dynamic light scattering measurement was 92 ± 3.1 nm [Figure 3a], with PI of 0.13 ± 0.02, indicating that the NLCs were well-distributed in the system. ZP could be regarded as a prediction for the storage stability of nanoparticles.[18] The ZP of B-NLCs was found to be − 31.35 ± 3.08 mV [Figure 3b] indicating the surface of the nanoparticles were negatively charged, and the created nanoparticles were relatively stable.
Figure 3.
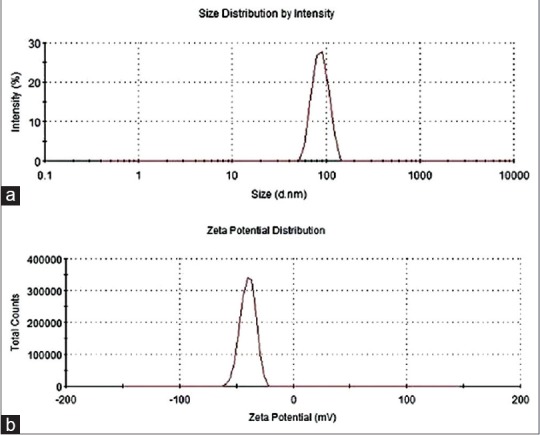
Particle size and zeta potential of B-nanostructured lipid carriers. (a) Particle size (nm) and (b) zeta potential (mV)
The freshly made samples were stored at different temperatures (4°C, 25°C, and 37°C) and analyzed for their particle size, ZP, and PI after 1 day, 1 week, and 1 month of storage. No aggregation and gelation was found by visual observation during 1 month of storage. As shown in Figure 4, the particle size, ZP, and PI did not change significantly at 4°C, indicating good physical stability of B-NLCs dispersion. A small increase in both size and PI was observed for samples stored at 25°C. In addition, the ZP decreased slightly. It is reported that high temperature may break the hydrogen bonds of surfactants, leading to a reduced stability of NLCs.[19] As shown in Figure 4, after incubating at 37°C for 1 week and 1 month, the particle and PI were significantly increased whereas the ZP was reduced about 25–50% after 1-week and 1-month storage, respectively. Previous studies reported that stability reduction might result from the decrease of ZP.[20] Statistical analysis did not show any significant difference in the ZP of samples under 4°C and 25°C, indicating relative good physical stability of B-NLCs.
Figure 4.
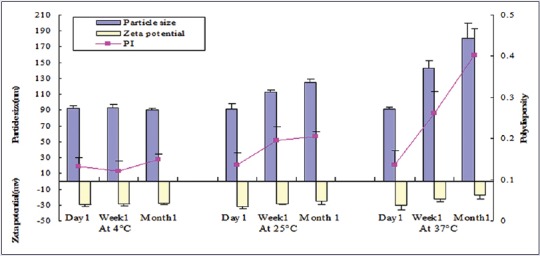
Changes of particle size, zeta potential, and polydispersity index of B-nanostructured lipid carriers at different temperatures
EE is a very important characteristic used to judge the quality of the lipid nanoparticles. Generally, several methods have been used to evaluate EE including ultrafiltration, dialysis, ultracentrifugation, and Sephadex minicolumn method used in the present study.[15] It has been reported that the liquid state of Miglyol oil might help to encapsulate higher amount of drugs.[21] The average EE of the freshly prepared B-NLCs was 85.29 ± 3.42%, indicating that being a hydrophobic drug, baicalin could be incorporated well into lipid carriers.
In this current study, TEM was used to assess the morphology of B-NLCs. TEM is a method to evaluate the microstructure of rather delicate systems such as vesicles, emulsions, liquid crystalline phases, and also lipid nanoparticles. As indicated in Figure 5, the particles were uniformly in the nanosize range and of spherical morphology.
Figure 5.
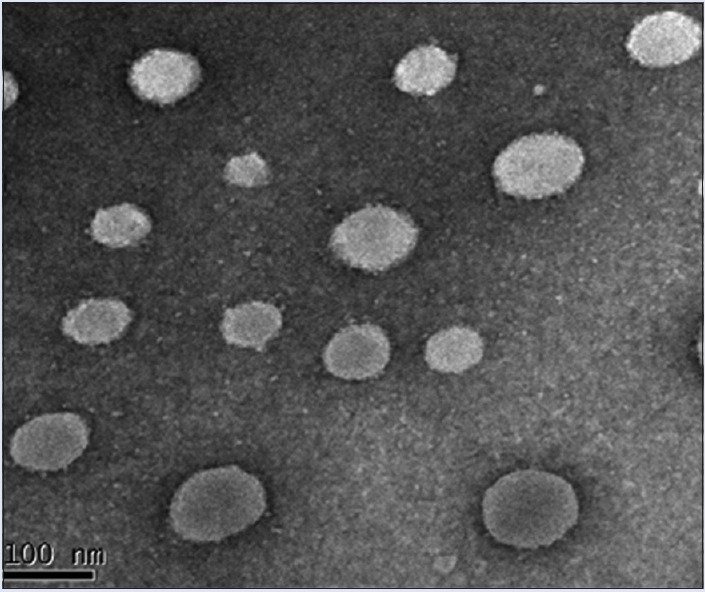
Transmission electron microscope image of B-nanostructured lipid carriers
To provide necessary guidance for clinical administration, it is essential to determine the drug-release profiles based on model-dependent methods such as zero, first, and Higuchi models.[22] In vitro release of baicalin from the NLCs was evaluated using a dialysis bag diffusion technique, where the sink conditions were maintained using 100 mL phosphate buffer (pH 6.8), and the results were graphically represented in Figure 6. For B-SUS, more than 90% baicalin was released within 4 h, indicating that the dialysis bag was not a limiting factor in the controlled release of baicalin from the nanoparticles. However, there was approximately 65% of baicalin in B-NLCs was released at the end of 4 h. The linear regression analysis Table 1 reveals that the baicalin release profile of B-NLCs formulation best fits to zero order kinetics. Overall, baicalin was released from NLCs in a sustained manner, and complete drug (80%) was released after 12 h.
Figure 6.
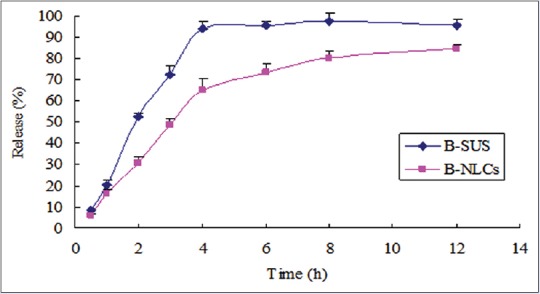
In vitro drug release of baicalin from the B-SUS and B-nanostructured lipid carriers’ formulations
Table 1.
Linear regression analysis of baicalin-loaded nanostructured lipid carriers release kinetics

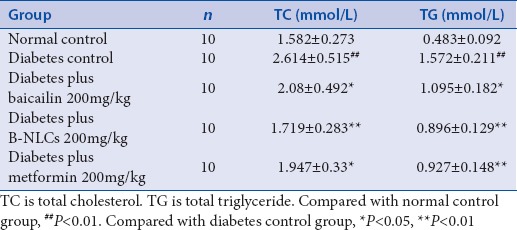
As shown in Tables 2 and 3, the FBG, TC, TG, and HbAlc in plasma were significantly higher in the diabetic control group than those in the normal control group (P < 0.01), the baicalin and B-NLCs significantly decreased the FBG, HbAlc, TC, and TG (P < 0.01 or P < 0.05). The data showed that the baicalin have hypoglycemic effect and regulate the lipid metabolism in type 2 diabetes mellitus rats. Recent years, the importance of baicalin was underscored in the treatment of diabetes.[7,23] However, the physical property of baicalin restrained its application in pharmacology. The B-NLCs might possess relatively good physical stability. In our research, compared with the baicalin group, the B-NLCs have a better hypoglycemic and hypolipidemic effect. The B-NLCs may be more effective in the treatment of diabetes.
Table 2.
Effect of baicalin-loaded nanostructured lipid carriers on fasting blood glucose and glycosylated hemoglobin in type 2 diabetic rats (mean±standard deviation)
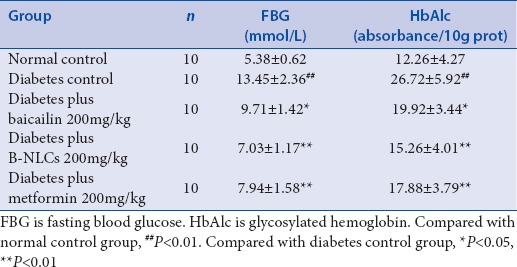
Table 3.
Effect of baicalin–loaded nanostructured lipid carriers on total cholesterol and total triglyceride in type 2 diabetic rats (mean±standard deviation)
CONCLUSION
In the proposed study, B-NLCs with high EE were successfully prepared using high-pressure homogenization method. B-NLCs were spherical with a mean size of approximately 92 nm. The results indicated that B-NLCs possess relatively good physical stability and have a better ability to retain drugs. Furthermore, the NLCs formulation increased the antidiabetic efficacy of baicalin. Taken together, the NLCs developed in this study demonstrate a promising carrier for baicalin.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
ABOUT AUTHOR

Prof. Ximing Xu
Prof. Ximing Xu, is a professor at the School of Pharmacy, Jiangsu University, Zhenjiang. His research interest is in the area of pharmaceutical sciences. He has hosted more than ten national and provincial major scientific projects and published more than one hundred academic papers on authoritative journals at home and abroad, such as Advanced Functional Materials, Biomaterials, and Small. Furthermore, he was elected to the National talents project, 333 Project of Jiangsu Province and Leaders of Advanced Disciplines in Jiangsu Province.
Acknowledgments
This work was supported by the China Postdoctoral Science Foundation (2014M551523) and Scientific Research Foundation of Jiangsu University (14JDG165).
REFERENCES
- 1.Xie JT, Wang A, Mehendale S, Wu J, Aung HH, Dey L, et al. Anti-diabetic effects of Gymnema yunnanense extract. Pharmacol Res. 2003;47:323–9. doi: 10.1016/s1043-6618(02)00322-5. [DOI] [PubMed] [Google Scholar]
- 2.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–20. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 3.Deng YX, Shi QZ, Chen B, Zhang XJ, Liu SZ, Qiu XM. Comparative pharmacokinetics of baicalin in normal and the type 2 diabetic rats after oral administration of the Radix scutellariae extract. Fitoterapia. 2012;83:1435–42. doi: 10.1016/j.fitote.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 4.Deng YX, Chen YS, Zhang WR, Chen B, Qiu XM, He LH, et al. Polysaccharide from Gynura divaricata modulates the activities of intestinal disaccharidases in streptozotocin-induced diabetic rats. Br J Nutr. 2011;106:1323–9. doi: 10.1017/S0007114511001693. [DOI] [PubMed] [Google Scholar]
- 5.Halliwell B, Rafter J, Jenner A. Health promotion by flavonoids, tocopherols, tocotrienols, and other phenols: Direct or indirect effects? Antioxidant or not. Am J Clin Nutr. 2005;81(1 Suppl):268S–76S. doi: 10.1093/ajcn/81.1.268S. [DOI] [PubMed] [Google Scholar]
- 6.Williamson G, Barron D, Shimoi K, Terao J. In vitro biological properties of flavonoid conjugates found in vivo. Free Radic Res. 2005;39:457–69. doi: 10.1080/10715760500053610. [DOI] [PubMed] [Google Scholar]
- 7.Li HT, Wu XD, Davey AK, Wang J. Antihyperglycemic effects of baicalin on streptozotocin-nicotinamide induced diabetic rats. Phytother Res. 2011;25:189–94. doi: 10.1002/ptr.3238. [DOI] [PubMed] [Google Scholar]
- 8.Waisundara VY, Hsu A, Huang D, Tan BK. Scutellaria baicalensis enhances the anti-diabetic activity of metformin in streptozotocin-induced diabetic Wistar rats. Am J Chin Med. 2008;36:517–40. doi: 10.1142/S0192415X08005953. [DOI] [PubMed] [Google Scholar]
- 9.Yu C, Zhang Z, Zhang H, Zhen Z, Calway T, Wang Y, et al. Pretreatment of baicalin and wogonoside with glycoside hydrolase: A promising approach to enhance anticancer potential. Oncol Rep. 2013;30:2411–8. doi: 10.3892/or.2013.2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ng TB, Liu F, Wang ZT. Antioxidative activity of natural products from plants. Life Sci. 2000;66:709–23. doi: 10.1016/s0024-3205(99)00642-6. [DOI] [PubMed] [Google Scholar]
- 11.Shen YC, Chiou WF, Chou YC, Chen CF. Mechanisms in mediating the anti-inflammatory effects of baicalin and baicalein in human leukocytes. Eur J Pharmacol. 2003;465:171–81. doi: 10.1016/s0014-2999(03)01378-5. [DOI] [PubMed] [Google Scholar]
- 12.Attama AA. SLN, NLC, LDC: State of the art in drug and active delivery. Recent Pat Drug Deliv Formul. 2011;5:178–87. doi: 10.2174/187221111797200524. [DOI] [PubMed] [Google Scholar]
- 13.Tiwari R, Pathak K. Nanostructured lipid carrier versus solid lipid nanoparticles of simvastatin: Comparative analysis of characteristics, pharmacokinetics and tissue uptake. Int J Pharm. 2011;415:232–43. doi: 10.1016/j.ijpharm.2011.05.044. [DOI] [PubMed] [Google Scholar]
- 14.How CW, Abdullah R, Abbasalipourkabir R. Physicochemical properties of nanostructured lipid carriers as colloidal carrier system stabilized with polysorbate 20 and polysorbate 80. Afr J Biotechnol. 2011;10:1684–9. [Google Scholar]
- 15.Gokce EH, Korkmaz E, Dellera E, Sandri G, Bonferoni MC, Ozer O. Resveratrol-loaded solid lipid nanoparticles versus nanostructured lipid carriers: Evaluation of antioxidant potential for dermal applications. Int J Nanomedicine. 2012;7:1841–50. doi: 10.2147/IJN.S29710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dong Z, Chen C. Effect of catalpol on diabetic nephropathy in rats. Phytomedicine. 2013;20:1023–9. doi: 10.1016/j.phymed.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 17.Uner M. Preparation, characterization and physico-chemical properties of solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC): Their benefits as colloidal drug carrier systems. Pharmazie. 2006;61:375–86. [PubMed] [Google Scholar]
- 18.Seetapan N, Bejrapha P, Srinuanchai W, Ruktanonchai UR. Rheological and morphological characterizations on physical stability of gamma-oryzanol-loaded solid lipid nanoparticles (SLNs) Micron. 2010;41:51–8. doi: 10.1016/j.micron.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 19.Sun M, Nie S, Pan X, Zhang R, Fan Z, Wang S. Quercetin-nanostructured lipid carriers: Characteristics and anti-breast cancer activities in vitro. Colloids Surf B Biointerfaces. 2014;113:15–24. doi: 10.1016/j.colsurfb.2013.08.032. [DOI] [PubMed] [Google Scholar]
- 20.Salgin S, Salgin U, Bahadir S. Zeta potentials and isoelectric points of biomolecules: The effects of ion types and ionic strengths. Int J Electrochem Sci. 2012;7:12404–14. [Google Scholar]
- 21.Müller RH, Radtke M, Wissing SA. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv Drug Deliv Rev. 2002;54(Suppl 1):S131–55. doi: 10.1016/s0169-409x(02)00118-7. [DOI] [PubMed] [Google Scholar]
- 22.Jeong SH, Park K. Drug loading and release properties of ion-exchange resin complexes as a drug delivery matrix. Int J Pharm. 2008;361:26–32. doi: 10.1016/j.ijpharm.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 23.Liu SZ, Deng YX, Chen B, Zhang XJ, Shi QZ, Qiu XM. Antihyperglycemic effect of the traditional Chinese scutellaria-coptis herb couple and its main components in streptozotocin-induced diabetic rats. J Ethnopharmacol. 2013;145:490–8. doi: 10.1016/j.jep.2012.11.017. [DOI] [PubMed] [Google Scholar]


