Abstract
Background:
Baicalin is a bioactive ingredient extracted from the root of Scutellariae radix, which is used to treat ulcerative colitis (UC).
Objective:
We investigated the activity of baicalin on lipopolysaccharide-stimulated RAW264.7 cells and 2,4,6-trinitrobenzene sulfonic acid-induced rats, including the attenuation of oxidant stress and apoptosis.
Materials and Methods:
The severity of colitis was assessed by disease activity index. The activities of catalase (CAT), glutathione peroxidase (GSH-PX), superoxide dismutase (SOD), and the content of malondialdehyde (MDA) were determined by their corresponding kits. The terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling (TUNEL) was performed to study whether experimental colitis was associated with intestinal epithelial cell (IEC) apoptosis and the effect of baicalin on IEC apoptosis. Western blot analysis and immunocytochemistry assay were applied to determine the protein expressions. The reactive oxygen species (ROS) level in the colon of UC rats treated with baicalin was determined by ROS assay kit.
Results:
Baicalin remarkably upregulated the activities of CAT, GSH-PX, and SOD and decreased the content of MDA in a dose-dependent manner in vitro and in vivo. The TUNEL-positive cells in rats treated baicalin were remarkably reduced. Both Western blot analysis and immunocytochemistry assay indicated that baicalin significantly decreased the expressions of transforming growth factor beta-1, Bax protein and upregulated the expression of Bcl-2 protein. In addition, the expressions of total and cleaved caspase-3, total and cleaved caspase-9 protein, Fas, and FasL in vitro were downregulated by the treatment with baicalin. Baicalin of different doses reduced the generation of ROS in UC rats.
Conclusion:
Taken together, these evidences provide scientific basics for the application of baicalin in the treatment of UC and suggest that baicalin exerts its effect via suppression of oxidant stress and apoptosis.
SUMMARY
Baicalin remarkably upregulated the activities of catalase, glutathione peroxidase, and superoxide dismutase and decreased the content of MDA, both in vivo and in vitro
The terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling-positive cells in rats treated baicalin remarkably reduced in a concentration-dependent manner
Western blot analysis and immunocytochemistry assay indicated that baicalin significantly decreased the expressions of transforming growth factor beta-1, Bax protein, and upregulated the expression of Bcl-2 protein
The expressions of total and cleaved caspase-3, total and cleaved caspase-9 protein, Fas, and FasL in vitro were downregulated by the treatment with baicalin.
Abbreviations used: UC: Ulcerative colitis, LPS: Lipopolysaccharide, TNBS: 2,4,6-trinitrobenzene sulfonic acid, DAI: Disease activity index, CAT: Catalase, GSH-PX: Glutathione peroxidase, SOD: Superoxide dismutase, MDA: Malondialdehyde, TUNEL: Terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling, ROS: Reactive oxygen species, IEC: Intestinal epithelial cell, SD: Sprague-Dawley, HE: H and E, DNTB: 5,5'-dithiobis-2-nitrobenzoic acid, TBA: Thiobarbituric acid, TBARS: Thiobarbituric acid-reactive substances, S.D: Standard deviation, and PBS: Phosphate-buffered saline.
Keywords: Apoptosis, baicalin, oxidative stress, ulcerative colitis
INTRODUCTION
Ulcerative colitis (UC) is a chronic and inflammatory disease of intestinal tract and possesses the characteristics of relapsing-remitting course.[1] Patients suffering from UC are more likely to develop colorectal cancer in comparison with general population, which is a common malignancy worldwide.[2] The overexpression of prooxidant molecules was associated with the increased activities of infiltrating leukocytes, neutrophils, and macrophages in the development of UC.[3] Moreover, these cytokines could upregulate the level of myeloperoxidase, resulting in the generation of reactive oxygen species (ROS), which could induce the apoptosis.[4,5] A growing body of evidences highlighted the essential roles of the promotion of intestinal epithelial cell (IEC) apoptosis and the suppression of inflammatory cell apoptosis in the injury of colonic tissue, and the abnormality of immunology in UC. Consequently, oxidative stress and IEC apoptosis were potential drivers in the induction and progression of UC.[6,7] Therefore, the inhibition of oxidative stress and apoptosis are the main programs to cure UC.[8,9]
Baicalin (5,6,7-trihydroxyflavone-7-β-D-glucuronide, molecular weight = 446.36, Figure 1), a bioactive flavonoid, is extracted from the root of Scutellaria baicalensis Georgi (Scutellariae radix).[10] Baicalin has shown its great effects on anti-inflammatory, antioxidant, and antimicrobial properties.[11,12] Growing evidence suggested that baicalin was a protective agent against oxidative stress-induced diseases, such as type-2 diabetic, hepatic, and global cerebral ischemia/reperfusion injury.[13,14,15] In addition, baicalin possessed anti-apoptotic property.[16] It has been reported that baicalin inhibited oxidative stress-induced apoptosis in several cells, such as HepG2, H9c2 rat ventricular, and PC-12 cells.[17,18,19] Oxidative stress and apoptosis contributed to the development of UC, and we have demonstrated that baicalin hold an anti-inflammatory effect on UC through inhibiting Toll-like receptor-4/nuclear factor kappa-light-chain-enhancer of activated B cells pathway activation.[20] However, whether the suppression of oxidant stress and apoptosis was another underlying mechanism of baicalin against UC remains unclear.
Figure 1.
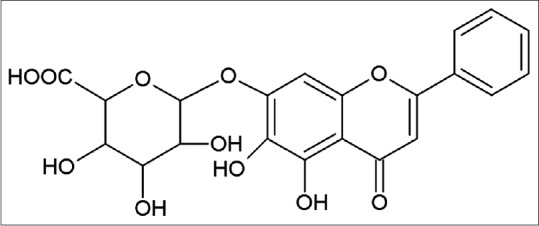
Chemical structure of baicalin
In the present study, we explored the underlying molecular mechanism of baicalin on lipopolysaccharide (LPS)-stimulated RAW264.7 cells and 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced rats. The protective effect of baicalin on UC via antioxidant stress and anti-apoptosis was studied. Our findings may provide a distinctive insight for comprehending the treatment of baicalin on UC.
MATERIALS AND METHODS
Chemicals and drugs
Baicalin (purity: ≥98%) was purchased from Sigma-Aldrich (St. Louis, MO, USA). The murine macrophage cell line, RAW264.7, was obtained from American Type Culture Collection (Manassas, VA, USA). All the antibodies, transforming growth factor beta-1 (TGF-β1), total and cleaved caspase-3, total and cleaved caspase-9, Bax, Bcl-2, Fas, and FasL, were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). In addition, basal Dulbecco's modified Eagle's medium (DMEM) and fetal bovine serum (FBS) were provided by Gibco (Grand Island, NY, USA). The quantification kits of catalase (CAT), glutathione peroxidase (GSH-PX), superoxide dismutase (SOD), and malondialdehyde (MDA) were provided by Jiancheng Bioengineering Institute (Nanjing, China). The other chemicals and reagents used were of analytical grade.
Study design
RAW264.7 cells were cultured in high-glucose DMEM supplemented with 10% FBS, 100 U/mL penicillin and 100 μg/mL streptomycin at 37°C in a 5% CO2 atmosphere. Medium was changed every day. RAW264.7 cells were stimulated by 1 μg/mL LPS (Sigma, St. Louis, MO, USA), and then treated with different concentration of baicalin. Total proteins were extracted for further experiments.
Male Sprague-Dawley rats (220 ± 20 g) were purchased from Shanghai SLAC Laboratory Animal Co. Ltd. (Shanghai, China). All rats were housed five per cage and maintained in an animal holding room controlled at a constant temperature of 25 ± 2°C with a relative humidity of 50 ± 5% and a 12 h light-dark cycle. The animals were allowed access to food and tap water ad libitum throughout the acclimatization and experimental periods. Permission for the animal studies was obtained from the Animal Ethics Committee of Jiangsu Provincial Academy of Chinese Medicine. Before experiments, the animals were fasted 12 h with free access to tap water. Then, 10% chloral hydrate (0.3 mL/100 g) was injected to the abdominal cavity of rats to make them anesthetic. The UC model rats were induced by a single intracolonic injection of 0.2 mL 5% TNBS in 0.25 mL of 50% ethanol. The rats in control blank group were given equal volume of physiological saline instead of TNBS by intracolonic injection. Following instillation of the TNBS solution, rats were maintained in a head-down position for a few minutes to prevent leakage of the intracolonic instillation.[21] The rats were divided into six groups (n = 15/group): control blank group (physiological saline), model group (physiological saline), positive group (100 mg/kg mesalazine), high-dose baicalin group (120 mg/kg baicalin), medium-dose baicalin group (60 mg/kg baicalin), and low-dose baicalin group (30 mg/kg baicalin). The rats in each group were treated with corresponding drugs per day by gastric lavage for 15 days. Disease activity indices (DAI) were calculated to evaluate the degree of colitis.[22]
Histological assessment
Colon tissues were fixed in 10% formalin and obtained from embedded paraffin samples. The tissues were deparaffinized with xylene and rehydrated using graded alcohol for the staining analyses. The sections were stained with H and E and then visualized and photographed under a microscope using a camera system (Olympus LK2, Japan). Histological injuries were evaluated according to the previously described method, considering both inflammatory cell infiltration and tissue damage. Scores for infiltration were 0: no infiltration; 1: increased number of inflammatory cells in the lamina propria; 2: inflammatory cells extending into the submucosa; and 3: transmural inflammatory infiltrates; and for tissue damage, 0: no mucosal damage; 1: discrete epithelial lesions; 2: erosions or focal ulcerations; and 3: severe mucosal damage with extensive ulceration extending into the bowel wall.
Immunohistochemical analysis
Rat colonic tissues were removed under anesthesia and were preserved by perfusion fixation with a solution of 4% paraformaldehyde. After that, tissues were blocked in paraffin and then cut into 5 µm thick sections. Tissue sections were incubated in citrate buffer (pH 6.0) at 95–100°C for 20 min in water bath for antigen retrieval. According to endogenous peroxidase, slides were incubated in hydrogen peroxide in methanol to reduce nonspecific background staining. Sequentially, tissues were boiled in citrate buffer solution for 10 min. They were cooled and then washed by phosphate-buffered saline (PBS) before the application of blocking serum. Primary antibodies, TGF-β1 (1:200), Bax (1:200), and Bcl-2 (1:200) were incubated with tissues to detect their targeted proteins, and then probed with secondary antibody. Elivison two-step method was performed for the immunohistochemical staining, and photographs of slides were taken using DM2500 optical microscope (Jilin Province Glory Trading Co., Ltd, Jilin, China).
Western blot analysis
To further evaluate the effect of baicalin on oxidative stress and apoptosis-related protein expressions and explore the possible mechanism underlying, Western blot analysis was performed for the protein expressions of TGF-β1, total and cleaved caspase-3, total and cleaved caspase-9, Bax, Bcl-2, Fas, and FasL. Briefly, RAW264.7 cells were washed with ice-cold PBS after treatment with drugs for the specified time, and lysed in cell lysis buffer (1% Triton X-100, 0.015 M NaCl, 10 mM Tris–HCl, 1 mM EDTA, 1 mM PMSF, 10 lg/mL of each leupeptin and pepstain A), and then incubated on ice for 30 min. The cell lysates were centrifuged at 13,000 g for 15 min at 4°C. Protein extracts were separated by 6–15% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred onto polyvinylidene difluoride membranes (Millipore Corporation, MA, USA). The membranes were blocked with 5% skimmed milk for 2 h, and then given an overnight incubation with the primary antibodies, TGF-β1 (1:400), caspase-3 (1:400), cleaved caspase-3 (1:400), caspase-9 (1:400), cleaved caspase-9 (1:400), Bax (1:400), Bcl-2 (1:400), Fas (1:400), and FasL (1:400) at 4°C. After thorough washing, the membranes were incubated with horseradish peroxidase-bound secondary antibody (1:5000) for 1 h at room temperature. Chemiluminescence reagents were added for the visualization of the protein bands (Millipore Corporation, MA, USA). The quantification of proteins was analyzed by Image-pro plus software (IPP 6.0, Media Cybernetics Inc., Silver Spring, Maryland, USA).
Determination of catalase and glutathione peroxidase activity
To investigate the attenuation of baicalin on oxidative stress status in LPS-stimulated RAW264.7 cells and TNBS-induced rats, we analyze the CAT and GSH-PX activity. CAT activity was assayed by the ammonium molybdate spectrophotometric method, which was based on the decreased absorbance because of the consumption of H2O2.[23] GSH-PX activity was measured by the method of Hafeman.[24] GSH-PX could degrade H2O2 in the presence of GSH, leading to the decrease of GSH. Then, the remaining GSH was reacted with 5,5'-dithiobis-2-nitrobenzoic acid (DTNB). Absorbance was read spectrophotometrically at 405 nm for CAT and 412 nm for GSH-PX at the end of reaction on a microplate reader.
Determination of superoxide dismutase activity and malondialdehyde content
To further study the effect of baicalin on oxidative stress status in vitro and in vivo, SOD activity and MDA content were evaluated. The activity of SOD was measured by the method of Marklund and Marklund,[25] owing to the ability of SOD to inhibit the autoxidation of pyrogallol. The MDA levels in cell supernatant and tissue homogenate were measured according to the thiobarbituric acid (TBA) method previously described. The method was based on the reaction of MDA with TBA to form thiobarbituric acid-reactive substances (TBARS), which was evaluated as the end product of lipid peroxidation. The SOD activity and MDA content were measured spectrophotometrically at 550 and 450 nm, respectively.
Detection of intestinal epithelial cell apoptosis by terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling assay
Terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling (TUNEL) staining was performed using the in situ Cell Death Detection Kit according to the manufacturer's protocol (Roche Diagnostics GmbH, Mannheim, Germany) with slight modifications. Briefly, paraffin-embedded colon tissue sections of 5-μm thickness were cut onto glass slides, dewaxed in xylene, hydrated, and treated with proteinase-K. The activity of endogenous peroxidase was blocked when the samples were immersed in 3% H2O2 in methanol. TUNEL reaction mixture was then added to the samples. Subsequently, colon tissue sections were rinsed twice with PBS and mounted with cover slips and glycerin. Positive signals were illustrated as distinct brown nuclear staining. TUNEL-positive cells per field were counted in 10 randomly chosen fields at 400 power magnification, and positive cell percentages were averaged.
Reactive oxygen species assay
The ROS level in the colon of UC rats treated with baicalin was determined by ROS assay kit according to manufacturer's instructions (KeyGEN, Nanjing, China). The content of ROS was calculated according to the standard curve. The result was expressed as fluorescence intensity/mg protein.
Statistical analysis
All data were expressed as the means ± standard deviation. Multigroup comparisons were performed using one-way ANOVA multiple comparisons among means. Student's t-test was used for comparisons of two groups. The data were analyzed by SPSS 17.0 statistical software (Chicago, IL, USA). P < 0.05 were considered statistically significant.
RESULTS
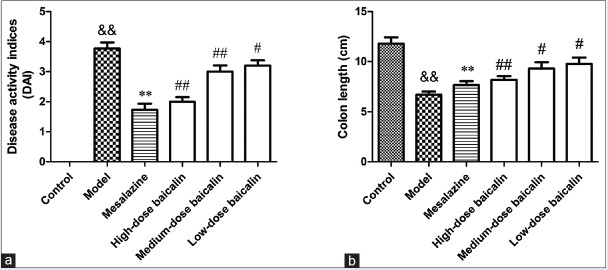
Effect of baicalin on 2,4,6-trinitrobenzene sulfonic acid-induced disease activity index and colon length in ulcerative colitis rats
UC led to a significant increase in the DAI of the rats as compared with the control blank group. Severe rectal bleeding was observed in the model group. Baicalin treatment, at the highest administered dose, significantly reduced UC-induced increase in the DAI of animals [Figure 2a]. Rats with UC in high-dose baicalin group did not show any significant difference in the body weight in comparison with the control blank, and no rectal bleeding was observed at the end of the experiment. Furthermore, UC led to a significant decrease in the colon length in comparison with the control blank group while the treatment with baicalin could significantly reverse the phenomenon [Figure 2b].
Figure 2.
(a) Effect of baicalin on 2,4,6-trinitrobenzene sulfonic acid-induced disease activity index in ulcerative colitis rats. (b) Effect baicalin on 2,4,6-trinitrobenzene sulfonic acid-induced colon length (cm) in ulcerative colitis rats
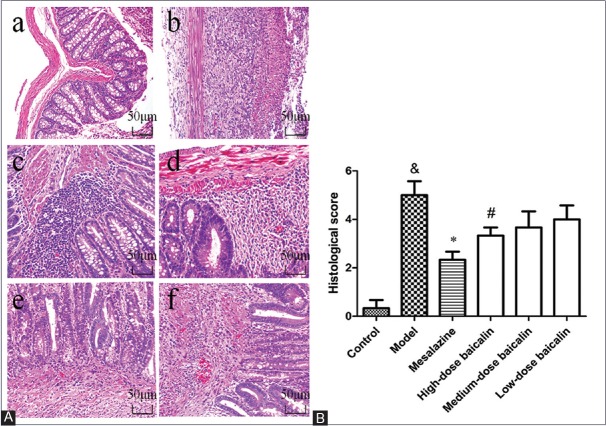
Baicalin alleviates the colonic mucosal injuries induced by 2,4,6-trinitrobenzene sulfonic acid
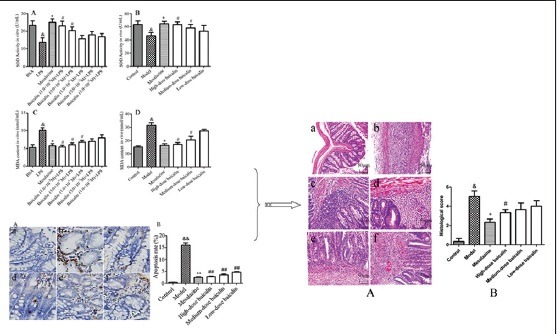
These colonic mucosal injuries persisted till day 15, including epithelial necrosis, epithalaxy, impaired mucosa involving submucosa with hyperemia and edema, and ulcer accompanied with numerous inflammatory cell infiltrations [Figure 3Ab] compared with the control group [Figure 3Aa] but could be alleviated by the treatment of mesalazine [Figure 3Ac] and baicalin [Figure 3Ad–f]. The colonic histological injury scores were higher [Figure 3B] in TNBS group compared with the control blank group, which were remarkably ameliorated by baicalin treatment. These results revealed a distinct improvement of histology in baicalin + TNBS group compared with TNBS group.
Figure 3.
Representative histological images and scores of colonic epithelial cell. (A) Representative histological images stained by H and E. a: Control blank (rats were not induced but administrated by physiological saline); b: 2,4,6-trinitrobenzene sulfonic acid (rats were induced by 2,4,6-trinitrobenzene sulfonic acid and administrated by physiological saline); c: 2,4,6-trinitrobenzene sulfonic acid + mesalazine (rats were induced by 2,4,6-trinitrobenzene sulfonic acid and administrated by mesalazine); d: 2,4,6-trinitrobenzene sulfonic acid + high-dose baicalin (rats were induced by 2,4,6-trinitrobenzene sulfonic acid and administrated by high-dose baicalin); e: 2,4,6-trinitrobenzene sulfonic acid + medium-dose baicalin (rats were induced by 2,4,6-trinitrobenzene sulfonic acid and administrated by medium-dose baicalin); f: 2,4,6-trinitrobenzene sulfonic acid + low-dose baicalin (rats were induced by 2,4,6-trinitrobenzene sulfonic acid and administrated by low-dose baicalin). (B) Histological score. Data were mean ± standard deviation (n = 6). &P < 0.05 versus control blank group; *P < 0.05 versus 2,4,6-trinitrobenzene sulfonic acid group; #P < 0.05 versus mesalazine group
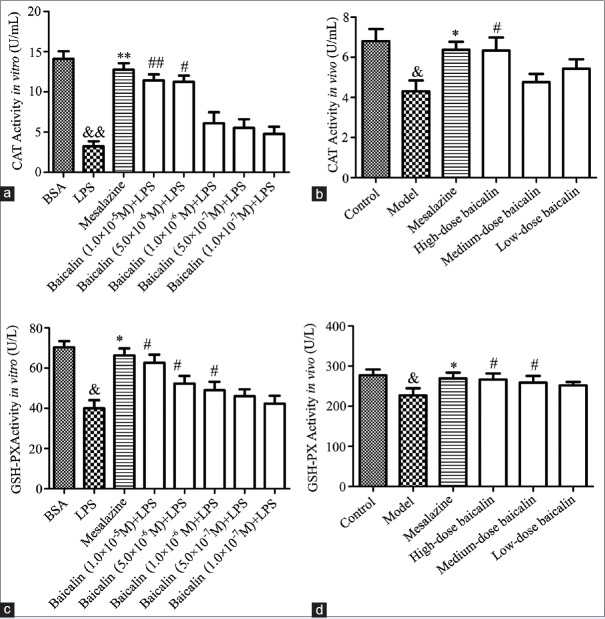
Baicalin upregulated the activities of catalase and glutathione peroxidase in the supernatant of lipopolysaccharide-stimulated RAW264.7 cells and serum of ulcerative colitis rats
To estimate the antioxidant activity of baicalin on colon injury, the activities of CAT and GSH-PX in the supernatant of LPS-stimulated RAW264.7 cells and the serum of UC rats were evaluated. As shown in Figure 4, the activities of CAT and GSH-PX in RAW264.7 cells or UC rat serum were observably downregulated by exposure to 1 μg/mL LPS or 0.2 mL 5% TNBS (P < 0.05, P < 0.01). However, mesalazine (100 mg/kg), positive drug, could upregulate the activity of these two antioxidant enzymes. As anticipated, the activities of CAT and GSH-PX in baicalin treatment group (1.0 × 10−7 M, 5.0 × 10−7 M, 1.0 × 10−6 M, 5.0 × 10−6 M, and 1.0 × 10−5 M for cells, and 30 mg/kg, 60 mg/kg, and 120 mg/kg for UC rats) were evidently increased in comparison with that in model group. These results suggested that baicalin could attenuate the oxidant damage in colon, which was induced by LPS in vitro or TNBS in vivo.
Figure 4.
(a) Effect of baicalin on lipopolysaccharide-stimulated RAW264.7 cells. (b) Effect of baicalin on 2,4,6-trinitrobenzene sulfonic acid-induced ulcerative colitis rats. (c) The effect of baicalin on catalase activity; (d) the effect of baicalin on glutathione peroxidase activity. &P < 0.05 and &&P < 0.01, lipopolysaccharide versus BSA control; *P < 0.05 and **P < 0.01, mesalazine versus lipopolysaccharide group; #P < 0.05 and ##P < 0.01, baicalin versus lipopolysaccharide group. Data from individual experiments are presented as means ± standard deviation (n = 3, in vitro; n = 6, in vivo)
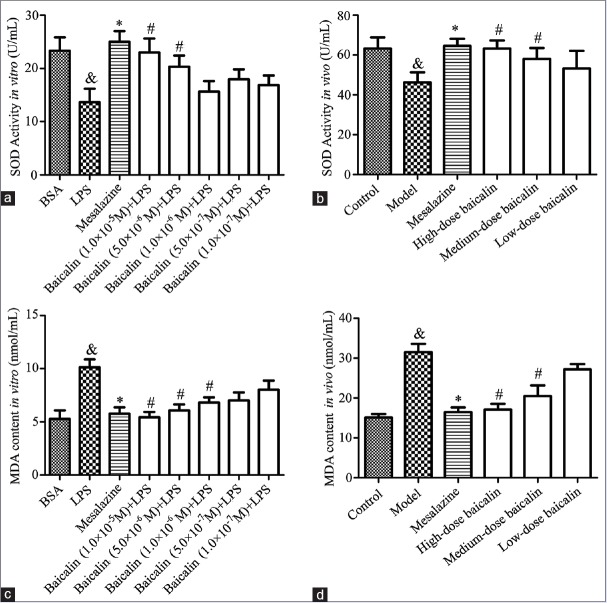
Baicalin upregulated superoxide dismutase activity and downregulated malondialdehyde level in the supernatant of lipopolysaccharide-stimulated RAW264.7 cells and serum of ulcerative colitis rats
To comprehensively discuss the antioxidant activity of baicalin on colon injury, the SOD activity and MDA level in LPS-stimulated RAW264.7 cells supernatant and the serum of UC rats were determined. As presented in Figure 5, the SOD activity was observably decreased by 1 μg/mL LPS in vitro or 0.2 mL 5% TNBS in vivo whereas MDA level was increased. However, the treatment with baicalin could evidently enhance SOD activity while remarkably reduce MDA level in LPS-stimulated RAW264.7 cells supernatant as well as in the serum of UC rats in a concentration-dependent manner. The present observations indicated that baicalin could protect LPS-stimulated RAW264.7 cells and UC rats from colon injury by reducing oxidative stress.
Figure 5.
(a) Effect of baicalin on lipopolysaccharide-stimulated RAW264.7 cells. (b) Effect of baicalin on 2,4,6-trinitrobenzene sulfonic acid-induced ulcerative colitis rats. (c) The effect of baicalin on superoxide dismutase activity; (d) the effect of baicalin on malondialdehyde content. &P < 0.05, model versus blank control; *P < 0.05, mesalazine versus model; #P < 0.05, baicalin versus model. Data from individual experiments are presented as means ± standard deviation (n = 3, in vitro; n = 6, in vivo)
Effect of baicalin on intestinal epithelial cell apoptosis
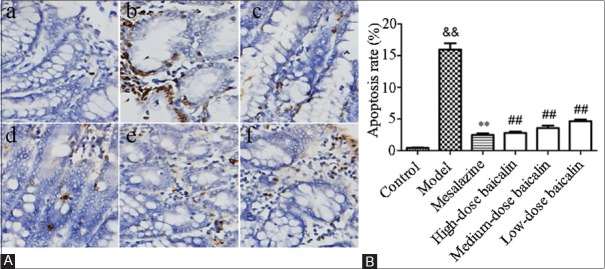
To study whether experimental colitis was associated with IEC apoptosis and the effect of baicalin on IEC apoptosis, we employed TUNEL staining in colonic tissues. As shown in Figure 6, few apoptotic cells were observed in control blank group whereas colon tissues presented a marked appearance of dark-brown apoptotic cells after treatment with TNBS (P < 0.01). In contrast, administration of mesalazine distinctly decreased the number of apoptotic epithelial cells. Treatment with baicalin remarkably reduced the percentages of TUNEL-positive cells in a concentration-dependent manner. Particularly, baicalin in a dose of 120 mg/kg was the most effective in inhibition of IEC apoptosis.
Figure 6.
Effect of baicalin on intestinal epithelial cell apoptosis. (A) Terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling staining in colon tissues. a: Control blank group; b: Model group (2,4,6-trinitrobenzene sulfonic acid); c: Mesalazine group (100 mg/kg); d: High-dose baicalin (120 mg/kg); e: Medium-dose baicalin (60 mg/kg) and f: Low-dose baicalin (30 mg/kg). (B) Apoptosis rate of colon tissues
Baicalin decreased the expression of transforming growth factor beta-1 protein in vitro and in vivo
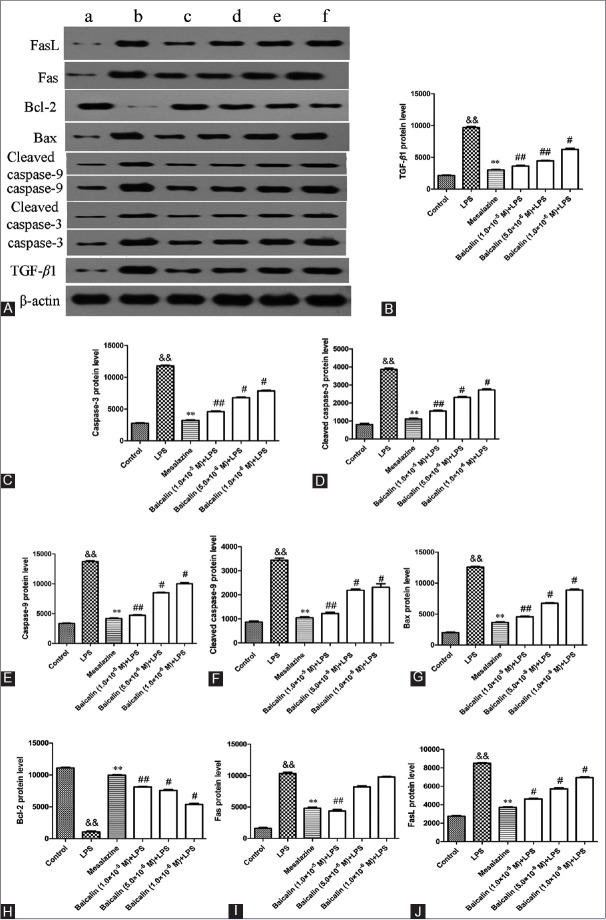
By immunohistochemical assay in Figure 7A, we observed that TNBS dramatically elevated TGF-β1 protein expression in the colon of UC rats. Positive drug mesalazine (100 mg/kg) and baicalin (30 mg/kg, 60 mg/kg, 120 mg/kg) could decrease TGF-β1 protein expression to a lower level than model rats. As shown in Figure 8A and B, LPS markedly increased the expression of TGF-β1 protein in vitro than that in BSA group (9677.35 ± 282.68 vs. 2125.10 ± 124.58, P < 0.01). However, a significant decrease on the expression of TGF-β1 protein could be observed by the treatment with 10 μM mesalazine (P < 0.01, vs. LPS). Interestingly, this increase could also be significantly downregulated after treatment with baicalin at doses of 1.0 × 10−5 M, 5.0 × 10−6 M, and 1.0 × 10−6 M (P < 0.05, P < 0.01).
Figure 7.
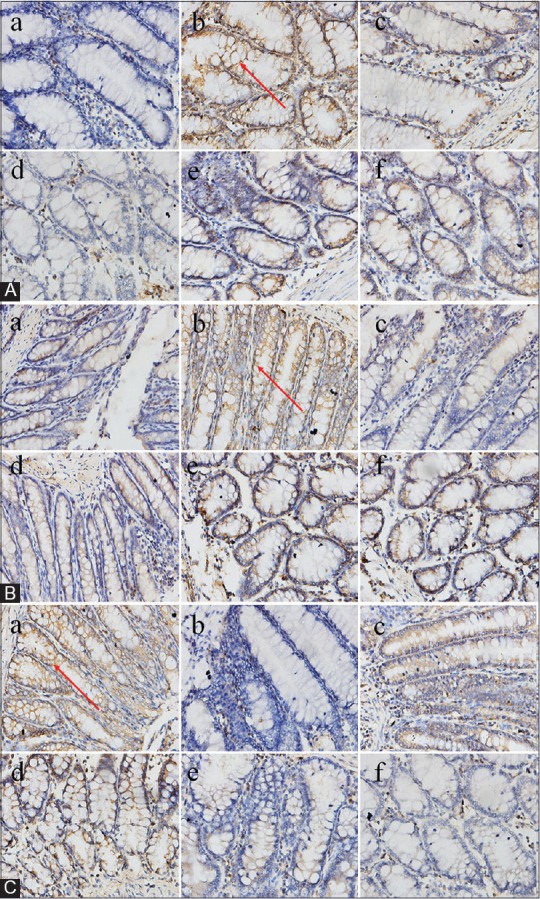
Effect of baicalin on 2,4,6-trinitrobenzene sulfonic acid-induced related protein expression in colon tissues by immunohistochemistry assay. Red arrows show the protein expression. (A) transforming growth factor beta-1 protein expression, (B) Bax protein expression, and (c) Bcl-2 protein expression. a: Control blank group; b: Model group (2,4,6-trinitrobenzene sulfonic acid); c: mesalazine group (100 mg/kg); d: High-dose baicalin (120 mg/kg); e: Medium-dose baicalin (60 mg/kg) and f: Low-dose baicalin (30 mg/kg) (for interpretation of the references to color in this figure legend, the reader is referred to the web version of this article)
Figure 8.
Western blot analysis for oxidant stress and apoptosis-related protein expression in RAW264.7 cells. (A) RAW264.7 cells were treated with lipopolysaccharide in the presence or absence of mesalazine or baicalin, and protein expressions were analyzed by western blotting analysis. a: 1 μg/ml BSA; b: 1 μg/mL lipopolysaccharide; c: Mesalazine + lipopolysaccharide; d: Baicalin (1.0 × 10−5 M) + lipopolysaccharide; e: Baicalin (5.0 × 10−6 M) + lipopolysaccharide; f: Baicalin (1.0 × 10−6 M) + lipopolysaccharide. (B) transforming growth factor beta-1 protein level; (C) caspase-3 protein level; (D) cleaved caspase-3 protein level; (E) caspase-9 protein level; (F) cleaved caspase-9 protein level; (G) Bax protein level; (H) Bcl-2 protein level; (I) Fas protein level; (J) FasL protein level. Data are expressed as means ± standard deviation, n = 3. &&P < 0.01, lipopolysaccharide versus BSA group; **P < 0.01, mesalazine versus lipopolysaccharide group; #P < 0.05, ##P < 0.01, baicalin versus lipopolysaccharide group
Effect of baicalin on the expressions of serial proteins involved in intestinal epithelial cell apoptosis
As shown in Figure 8A and C–F, the expressions of caspase-3, cleaved caspase-3, caspase-9, and cleaved caspase-9 protein were markedly increased in model group in comparison with BSA group. Administration of mesalazine effectively suppressed these protein expressions. As expected, the four protein levels in baicalin group were also significantly decreased compared with that in model group.
The results of immunohistochemistry assay and Western blot analysis showed that the expression of Bax protein in model group was distinctly elevated in comparison with control blank group whereas the expression of Bcl-2 protein was significantly decreased [Figure 7B and C; Figure 8A, G, and H]. However, baicalin treatment significantly diminished the increasing of Bax protein expression in a concentration-dependent manner. Baicalin at 1.0 × 10−5 M exhibited the most impressive inhibiting effect in reducing the protein expression of Bax (P < 0.01). In addition, baicalin could concentration-dependently upregulate the protein level of Bcl-2 (P < 0.05, P < 0.01).
As shown in Figure 8A, I, and J, the expressions of Fas and FasL protein in BSA group were very low. The two protein expressions in LPS-stimulated RAW264.7 cells were significantly higher than that in BSA group (P < 0.01). As expected, mesalazine, positive drug, could effectively suppress the expressions of Fas and FasL protein (P < 0.01). Interestingly, the two protein expressions would also be downregulated by baicalin treatment in a concentration-dependent manner.
Our study suggested that one mechanism underlying the protective effect of baicalin involved in the adjusting of several proteins was associated with cell apoptosis in experimental colitis.
Baicalin-reduced reactive oxygen species generation
To evaluate whether the generation of ROS was related to UC, ROS assay was carried out in rat's colon. As shown in Figure 9, the level of ROS in model group was significantly increased compared with control group (P < 0.01). On the contrary, baicalin of different doses downregulated the generation of ROS in UC rats. From the above results, we could see that baicalin alleviated the colonic mucosal injuries induced by TNBS in a concentration-dependent manner. Therefore, we preliminarily speculated that the remission of colitis symptoms of rats by baicalin was associated with the down-regulation of ROS.
Figure 9.
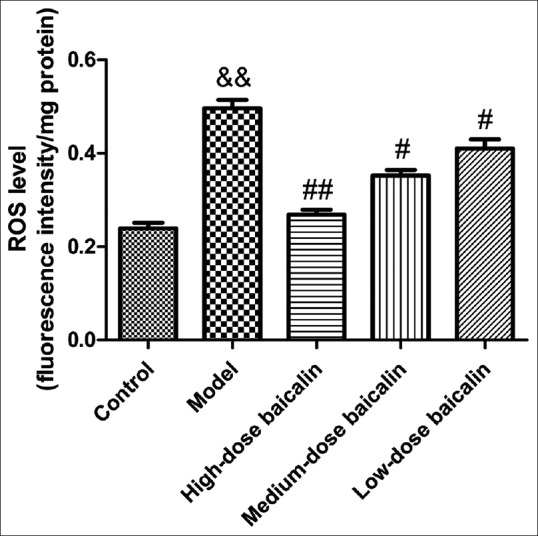
Effect of baicalin on reactive oxygen species generation in the colon of ulcerative colitis rat. Data are expressed as means ± standard deviation, n = 3. &&P < 0.01, model versus control group; #P < 0.05, ##P < 0.01, baicalin versus model group
DISCUSSION
Several experimental and clinical studies have shown that ROS was associated with the pathogenesis of UC.[26,27,28] Superoxide radicals produced by activated leukocytes or xanthine oxidase pathway and prostaglandin products, or leukotrienes formed by the oxidation of arachidonic acid, constituted the main source of ROS in inflamed mucosa. These toxic products affected cellular membrane stability and promote lipid peroxidation.[29] Many studies have shown that CAT was able to strengthen the oxidation resistance as the main biochemical target.[30,31] It could also maintain the low steady-state concentration of ROS.[32] The biochemical function of GSH-PX was to reduce lipid hydroperoxides to their corresponding alcohols and to reduce free hydrogen peroxide to water.[33] SOD was an important antioxidant enzyme in mitochondria against oxidative stress.[34] MDA, as a decomposition product of lipid hydroperoxides, was an indicator of oxidative damage to cells and tissues and in vitro reacts with hydrogen peroxide to form undetermined degradation products.[35] Contradictory results were present in literatures with respect to plasma lipid peroxidation in UC patients. Durak et al.[36] observed that there was no significant difference of GSH-PX activity between patients with UC and controls. However, we found that the levels of GSH-PX were significantly increased both in LPS-stimulated RAW264.7 cells and TNBS-induced rats, which was consistent with a study by Beno et al.[37] Baicalin possessed antioxidant ability both in vivo and in vitro.[19] Thus, the capacity of baicalin to upregulate the intrinsic antioxidant enzyme expressions may be deemed an important noninvasive method of combating oxidative stress in experimental colitis. Although the biological responses to baicalin have been well researched, its application in different disease circumstances has not yet been fully elucidated. Therefore, the identification of the antioxidant properties of baicalin in the context of LPS- or TNBS-induced oxidative stress may be considered as an important revelation of this study.
The pathogenesis of UC was also related to the abnormal apoptosis. Plenty of evidences suggested that the number of apoptotic epithelial cells increased with the development of UC, which may cause a change of the epithelial barrier function, resulting in pathogenic microorganism infiltration.[36,37] Different mechanisms were involved in the induction of apoptosis. One pathway was mediated by proapoptotic signals at the mitochondria level including members of Bcl-2 family and caspase-9. Bcl-2 was anti-apoptotic whereas Bax was pro-apoptotic. The imbalance between Bcl-2 and Bax could lead to apoptosis. The interplay between cell surface death receptor Fas and its specific ligand FasL also contributed to the effect of baicalin. The interaction resulted in the constitution of the death-inducing signaling complex and activated downstream effector caspase-3.[38] Zheng et al.[19] had demonstrated that baicalin could increase the Bcl-2 protein expression and decrease caspase-3 protein expression, which were attenuated by AG-490 in PC12 cells. Lin et al.[39] also confirmed that baicalin could attenuate renal ischemia-reperfusion injury by inhibiting mitochondria-mediated apoptosis: Decreasing caspase-3 activity and increasing the Bcl-2/Bax ratio. Liu et al.[40] reported that baicalin can reduce expressions of Fas and FasL protein in HIBD rats, inhibit apoptosis of nerve cells, achieving the protective effect on HIBD rat nerves. In the present study, baicalin upregulated Bcl-2 protein expression and decreased the expression of Bax protein in UC rats, inducing the subsequent translocation of the marked activation of the caspase-cascade system, and concentration-dependently downregulated the expressions of Fas and FasL protein.
Growing evidence indicated that TGF-β was highly expressed in patients or rats of UC, and it usually played a pivotal role in the modulation of the intestinal immune system.[41] TGF-β possessed both pro- and anti-inflammatory effects. It functions as a biological switch, antagonizing or modifying the action of other cytokines or growth factors. The diverse effects of TGF-β on proliferation, differentiation and function all depended on the ligand binding cell type. Chuang et al.[42] investigated that baicalin could upregulate TGF-β gene expression on RAW264.7 cells in a concentration-dependent manner whereas Lo et al.[43] found that baicalin significantly inhibited LPS-stimulated TGF-β expression in the lung tissue. TGF-β 1, one of the main subtypes of TGF-β, acted as a negative regulator of caspase-8 and thereby inhibited Fas-mediated apoptosis in mucosal inflammation, which was indispensable for wound healing and tissue repair.[44,45] There was also a study implying that successful treatment of UC-related mucosal injury resulted in a decrease of TGF-β 1 level in plasma and intestinal mucosa.[46] Our findings indicated that baicalin could weaken colon damage via downregulating TGF-β 1 protein expression.
CONCLUSION
The present results demonstrated that baicalin concentration-dependently ameliorated the severity of experimental colitis. The possible mechanisms of baicalin in protecting UC were associated with the inhibition of oxidative stress and modulation of serial proteins involved in IEC apoptosis. In addition, numerous studies from animal studies as well as clinical trial have shown that few toxic effects or side effects were found with treatment of baicalin. Therefore, baicalin might be an alternative drug for the prevention and treatment of UC.
Financial support and sponsorship
The study was supported by grants from the Outstanding Doctoral Thesis Support Project of Guangdong Province (85514045), the Technical Research and Development Project of Shenzhen (JCYJ20150403101028164 and JCYJ20150403102020231) and the Medical Research Foundation of Guangdong Province (B2013347).
Conflicts of interest
There are no conflicts of interest.
ABOUT AUTHORS

Li-sheng Wang
Li-sheng Wang is an associate professor at the Department of Gastrointestinal Studies, Jinan University of Medical Sciences, Shenzhen Municipal People's Hospital. His research interest is in the area of inflammatory bowel disease.

Jian-yao Wang
Jian-yao Wang is a doctor at the Department of Pediatric Surgery of Shen Zhen Children's Hospital, China. His research interest is in the area of pediatric surgery. The portrait photographs of the two leading authors are as follows:
REFERENCES
- 1.Zhao HM, Huang XY, Zhou F, Tong WT, Wan PT, Huang MF, et al. Si Shen Wan inhibits mRNA expression of apoptosis-related molecules in p38 MAPK signal pathway in mice with colitis. Evid Based Complement Alternat Med 2013. 2013 doi: 10.1155/2013/432097. 432097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saleh M, Trinchieri G. Innate immune mechanisms of colitis and colitis-associated colorectal cancer. Nat Rev Immunol. 2011;11:9–20. doi: 10.1038/nri2891. [DOI] [PubMed] [Google Scholar]
- 3.Hamouda HE, Zakaria SS, Ismail SA, Khedr MA, Mayah WW. p53 antibodies, metallothioneins, and oxidative stress markers in chronic ulcerative colitis with dysplasia. World J Gastroenterol. 2011;17:2417–23. doi: 10.3748/wjg.v17.i19.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin MH, Cheng CH, Chen KC, Lee WT, Wang YF, Xiao CQ, et al. Induction of ROS-independent JNK-activation-mediated apoptosis by a novel coumarin-derivative, DMAC, in human colon cancer cells. Chem Biol Interact. 2014;218:42–9. doi: 10.1016/j.cbi.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 5.Cao Y, Ruan Y, Shen T, Huang X, Li M, Yu W, et al. Astragalus polysaccharide suppresses doxorubicin-induced cardiotoxicity by regulating the PI3k/Akt and p38MAPK pathways. Oxid Med Cell Longev 2014. 2014 doi: 10.1155/2014/674219. 674219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tüzün A, Erdil A, Inal V, Aydin A, Bagci S, Yesilova Z, et al. Oxidative stress and antioxidant capacity in patients with inflammatory bowel disease. Clin Biochem. 2002;35:569–72. doi: 10.1016/s0009-9120(02)00361-2. [DOI] [PubMed] [Google Scholar]
- 7.Keshavarzian A, Banan A, Farhadi A, Komanduri S, Mutlu E, Zhang Y, et al. Increases in free radicals and cytoskeletal protein oxidation and nitration in the colon of patients with inflammatory bowel disease. Gut. 2003;52:720–8. doi: 10.1136/gut.52.5.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Custódio JB, Cardoso CM, Almeida LM. Thiol protecting agents and antioxidants inhibit the mitochondrial permeability transition promoted by etoposide: Implications in the prevention of etoposide-induced apoptosis. Chem Biol Interact. 2002;140:169–84. doi: 10.1016/s0009-2797(02)00020-0. [DOI] [PubMed] [Google Scholar]
- 9.Strober W, Fuss I, Mannon P. The fundamental basis of inflammatory bowel disease. J Clin Invest. 2007;117:514–21. doi: 10.1172/JCI30587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Waisundara VY, Hsu A, Huang D, Tan BK. Scutellaria baicalensis enhances the anti-diabetic activity of metformin in streptozotocin-induced diabetic Wistar rats. Am J Chin Med. 2008;36:517–40. doi: 10.1142/S0192415X08005953. [DOI] [PubMed] [Google Scholar]
- 11.Xing S, Wang M, Peng Y, Chen D, Li X. Simulated gastrointestinal tract metabolism and pharmacological activities of water extract of Scutellaria baicalensis roots. J Ethnopharmacol. 2014;152:183–9. doi: 10.1016/j.jep.2013.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tian X, Cheng ZY, He J, Jia LJ, Qiao HL. Concentration-dependent inhibitory effects of baicalin on the metabolism of dextromethorphan, a dual probe of CYP2D and CYP3A, in rats. Chem Biol Interact. 2013;203:522–9. doi: 10.1016/j.cbi.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 13.Waisundara VY, Siu SY, Hsu A, Huang D, Tan BK. Baicalin upregulates the genetic expression of antioxidant enzymes in Type-2 diabetic Goto-Kakizaki rats. Life Sci. 2011;88:1016–25. doi: 10.1016/j.lfs.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 14.Wen YF, Zhao JQ, Bhadauria M, Nirala SK. Baicalin prevents cadmium induced hepatic cytotoxicity, oxidative stress and histomorphometric alterations. Exp Toxicol Pathol. 2013;65:189–96. doi: 10.1016/j.etp.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 15.Cao Y, Mao X, Sun C, Zheng P, Gao J, Wang X, et al. Baicalin attenuates global cerebral ischemia/reperfusion injury in gerbils via anti-oxidative and anti-apoptotic pathways. Brain Res Bull. 2011;85:396–402. doi: 10.1016/j.brainresbull.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 16.Shen M, Wang L, Yang G, Gao L, Wang B, Guo X, et al. Baicalin protects the cardiomyocytes from ER stress-induced apoptosis: Inhibition of CHOP through induction of endothelial nitric oxide synthase. PLoS One. 2014;9:e88389. doi: 10.1371/journal.pone.0088389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu Y, Feng Y, Li H, Gao Z. Ferric citrate CYP2E1-independently promotes alcohol-induced apoptosis in HepG2 cells via oxidative/nitrative stress which is attenuated by pretreatment with baicalin. Food Chem Toxicol. 2012;50:3264–72. doi: 10.1016/j.fct.2012.05.061. [DOI] [PubMed] [Google Scholar]
- 18.Liou SF, Hsu JH, Liang JC, Ke HJ, Chen IJ, Wu JR, et al. San-Huang-Xie-Xin-Tang protects cardiomyocytes against hypoxia/reoxygenation injury via inhibition of oxidative stress-induced apoptosis. J Nat Med. 2012;66:311–20. doi: 10.1007/s11418-011-0592-0. [DOI] [PubMed] [Google Scholar]
- 19.Zheng WX, Wang F, Cao XL, Pan HY, Liu XY, Hu XM, et al. Baicalin protects PC-12 cells from oxidative stress induced by hydrogen peroxide via anti-apoptotic effects. Brain Inj. 2014;28:227–34. doi: 10.3109/02699052.2013.860469. [DOI] [PubMed] [Google Scholar]
- 20.Cui L, Feng L, Zhang ZH, Jia XB. The anti-inflammation effect of baicalin on experimental colitis through inhibiting TLR4/NF-kappaB pathway activation. Int Immunopharmacol. 2014;23:294–303. doi: 10.1016/j.intimp.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 21.Giris M, Depboylu B, Dogru-Abbasoglu S, Erbil Y, Olgaç V, Alis H, et al. Effect of taurine on oxidative stress and apoptosis-related protein expression in trinitrobenzene sulphonic acid-induced colitis. Clin Exp Immunol. 2008;152:102–10. doi: 10.1111/j.1365-2249.2008.03599.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murthy SN, Cooper HS, Shim H, Shah RS, Ibrahim SA, Sedergran DJ. Treatment of dextran sulfate sodium-induced murine colitis by intracolonic cyclosporin. Dig Dis Sci. 1993;38:1722–34. doi: 10.1007/BF01303184. [DOI] [PubMed] [Google Scholar]
- 23.Jiang SZ, Yang ZB, Yang WR, Gao J, Liu FX, Broomhead J, et al. Effects of purified zearalenone on growth performance, organ size, serum metabolites, and oxidative stress in postweaning gilts. J Anim Sci. 2011;89:3008–15. doi: 10.2527/jas.2010-3658. [DOI] [PubMed] [Google Scholar]
- 24.Hafeman DG, Sunde RA, Hoekstra WG. Effect of dietary selenium on erythrocyte and liver glutathione peroxidase in the rat. J Nutr. 1974;104:580–7. doi: 10.1093/jn/104.5.580. [DOI] [PubMed] [Google Scholar]
- 25.Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974;47:469–74. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 26.Giris M, Erbil Y, Dogru-Abbasoglu S, Yanik BT, Alis H, Olgaç V, et al. The effect of heme oxygenase-1 induction by glutamine on TNBS-induced colitis. The effect of glutamine on TNBS colitis. Int J Colorectal Dis. 2007;22:591–9. doi: 10.1007/s00384-006-0238-y. [DOI] [PubMed] [Google Scholar]
- 27.Hagar HH, El-Medany A, El-Eter E, Arafa M. Ameliorative effect of pyrrolidinedithiocarbamate on acetic acid-induced colitis in rats. Eur J Pharmacol. 2007;554:69–77. doi: 10.1016/j.ejphar.2006.09.066. [DOI] [PubMed] [Google Scholar]
- 28.Lih-Brody L, Powell SR, Collier KP, Reddy GM, Cerchia R, Kahn E, et al. Increased oxidative stress and decreased antioxidant defenses in mucosa of inflammatory bowel disease. Dig Dis Sci. 1996;41:2078–86. doi: 10.1007/BF02093613. [DOI] [PubMed] [Google Scholar]
- 29.Pavlick KP, Laroux FS, Fuseler J, Wolf RE, Gray L, Hoffman J, et al. Role of reactive metabolites of oxygen and nitrogen in inflammatory bowel disease. Free Radic Biol Med. 2002;33:311–22. doi: 10.1016/s0891-5849(02)00853-5. [DOI] [PubMed] [Google Scholar]
- 30.Abas R, Othman F, Thent ZC. Protective effect of Momordica charantia fruit extract on hyperglycaemia-induced cardiac fibrosis. Oxid Med Cell Longev 2014. 2014 doi: 10.1155/2014/429060. 429060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ku SK, Seo BI, Park JH, Park GY, Seo YB, Kim JS, et al. Effect of Lonicerae Flos extracts on reflux esophagitis with antioxidant activity. World J Gastroenterol. 2009;15:4799–805. doi: 10.3748/wjg.15.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zamocky M, Furtmüller PG, Obinger C. Evolution of catalases from bacteria to humans. Antioxid Redox Signal. 2008;10:1527–48. doi: 10.1089/ars.2008.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olsvik PA, Kristensen T, Waagbø R, Rosseland BO, Tollefsen KE, Baeverfjord G, et al. mRNA expression of antioxidant enzymes (SOD, CAT and GSH-Px) and lipid peroxidative stress in liver of Atlantic salmon (Salmo salar) exposed to hyperoxic water during smoltification. Comp Biochem Physiol C Toxicol Pharmacol. 2005;141:314–23. doi: 10.1016/j.cbpc.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 34.Hassan W, Rongyin G, Daoud A, Ding L, Wang L, Liu J, et al. Reduced oxidative stress contributes to the lipid lowering effects of isoquercitrin in free fatty acids induced hepatocytes. Oxid Med Cell Longev 2014. 2014 doi: 10.1155/2014/313602. 313602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bonnes-Taourel D, Guérin MC, Torreilles J. Is malonaldehyde a valuable indicator of lipid peroxidation? Biochem Pharmacol. 1992;44:985–8. doi: 10.1016/0006-2952(92)90132-3. [DOI] [PubMed] [Google Scholar]
- 36.Durak I, Yasa MH, Bektas A, Kaçmaz M, Cimen MY, Oztürk HS. Mucosal antioxidant defense is not impaired in ulcerative colitis. Hepatogastroenterology. 2000;47:1015–7. [PubMed] [Google Scholar]
- 37.Beno I, Staruchová M, Volkovová K. Ulcerative colitis: activity of antioxidant enzymes of the colonic mucosa. Presse Med. 1997;26:1474–7. [PubMed] [Google Scholar]
- 38.Sträter J, Wellisch I, Riedl S, Walczak H, Koretz K, Tandara A, et al. CD95 (APO-1/Fas)-mediated apoptosis in colon epithelial cells: A possible role in ulcerative colitis. Gastroenterology. 1997;113:160–7. doi: 10.1016/s0016-5085(97)70091-x. [DOI] [PubMed] [Google Scholar]
- 39.Lin M, Li L, Li L, Pokhrel G, Qi G, Rong R, et al. The protective effect of baicalin against renal ischemia-reperfusion injury through inhibition of inflammation and apoptosis. BMC Complement Altern Med. 2014;14:19. doi: 10.1186/1472-6882-14-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu XM, Feng Y, Li AM. Nerve protective effect of Baicalin on newborn HIBD rats. Asian Pac J Trop Med. 2014;7:806–10. doi: 10.1016/S1995-7645(14)60141-3. [DOI] [PubMed] [Google Scholar]
- 41.Ko JK, Auyeung KK. Inflammatory bowel disease: Etiology, pathogenesis and current therapy. Curr Pharm Des. 2014;20:1082–96. doi: 10.2174/13816128113199990416. [DOI] [PubMed] [Google Scholar]
- 42.Chuang HN, Wang JY, Chiu JH, Tsai TH, Yeh SF, Fu SL, et al. Enhancing effects of Scutellaria baicalensis and some of its constituents on TGF-beta1 gene expression in RAW 264.7 murine macrophage cell line. Planta Med. 2005;71:440–5. doi: 10.1055/s-2005-864140. [DOI] [PubMed] [Google Scholar]
- 43.Lo YC, Lin YL, Yu KL, Lai YH, Wu YC, Ann LM, et al. San-Huang-Xie-Xin-Tang attenuates inflammatory responses in lipopolysaccharide-exposed rat lungs. J Ethnopharmacol. 2005;101:68–74. doi: 10.1016/j.jep.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 44.Clavel T, Haller D. Bacteria- and host-derived mechanisms to control intestinal epithelial cell homeostasis: Implications for chronic inflammation. Inflamm Bowel Dis. 2007;13:1153–64. doi: 10.1002/ibd.20174. [DOI] [PubMed] [Google Scholar]
- 45.Irmler M, Thome M, Hahne M, Schneider P, Hofmann K, Steiner V, et al. Inhibition of death receptor signals by cellular FLIP. Nature. 1997;388:190–5. doi: 10.1038/40657. [DOI] [PubMed] [Google Scholar]
- 46.Wiercinska-Drapalo A, Flisiak R, Prokopowicz D. Effect of ulcerative colitis treatment on transforming growth factor beta (1) in plasma and rectal mucosa. Regul Pept. 2003;113:57–61. doi: 10.1016/s0167-0115(02)00300-2. [DOI] [PubMed] [Google Scholar]