Abstract
Background and Aims:
Post-operative pain is a major concern for day care surgeries like laparoscopic cholecystectomy. This study aimed to compare the efficacy of ultrasound guided abdominal field blocks (USAFB) with port site infiltrations for post-operative analgesia in terms of quality of pain relief, opioid consumption and patient satisfaction for day care surgeries
Methods:
Eighty patients presenting for laparoscopic cholecystectomy were randomly allocated to two groups either to receive port-site infiltration of local anaesthetic (n = 40, Group A) or USAFB (n = 40, Group B group). Numeric rating scores (NRS) were measured postoperatively to primarily assess the pain severity and opioid requirements. Data were analysed using Chi-Square test/Fisher's exact test for categorical data and Mann–Whitney test/unpaired t-test for quantitative data.
Results:
The study group (Group B) had significantly reduced NRS and opioid consumption over 24 h. The overall fentanyl consumption in patients receiving port infiltrations was approximately twice (200 100 μg) as compared to patients in USAFB group (120 74 μg) (P < 0.0001). Maximum fentanyl consumption was 400 μg (Group A) and 262 μg (Group B) over 24 h and the minimum requirement was 50 μg and zero, respectively.
Conclusion:
Superior post-operative analgesia was observed with USAFB which may help in minimising opioid-related adverse effects and facilitating faster recovery.
Key words: Laparoscopic cholecystectomy, post-operative analgesia, rectus sheath block, subcostal transversus abdominis block
INTRODUCTION
Pain is one of the most common medical causes of delayed discharge (17–40%) after ambulatory surgery.[1,2] Substantial component of the pain experienced by patients postoperatively is derived from the anterior abdominal wall incisions. Nerve blocks of anterior abdominal wall provide effective analgesia.
Subcostal transversus abdominis (STA) block and trasnsversus abdominis plane (TAP) block are currently described as effective techniques for providing supraumbilical and infraumbilical analgesia, respectively. However, the spread of local anaesthetic injected into these planes is limited by the lateral border of the rectus sheath, limiting their analgesic effects in midline, and require modification of port sites.[3,4] Addition of posterior rectus abdominis sheath block provides adequate analgesia covering thoracic dermatomes (T5 – T10) in midline, without modification of conventional port sites.
The present study compared ultrasound-guided abdominal field blocks (USAFB), i.e., combined bilateral posterior rectus sheath block and right STA plane block with widely practiced traditional port infiltrations, for post-operative pain relief and opioid consumption.
METHODS
The study was sponsored by the hospital and approved by the Local Research Ethics Committee. It was undertaken over a period of 1 year from May 2014 to April 2015. Written informed consent was taken from eighty patients (18–70 years) of American Society of Anesthesiologists physical status I/II listed for elective laparoscopic cholecystectomy and enrolled in the study. Exclusion criteria included allergies to local anaesthetic agents, skin infections preceding the block, pre-operative chronic opioid dependence, surgeries converted to open cholecystectomy, pregnancy and refusal by patient.
A standard balanced general anaesthetic regime was employed, consisting of propofol (2 mg/kg), fentanyl (1.5 μg/kg) and vecuronium 0.1 mg/kg. Top up of fentanyl 0.5 μg/kg was administered as and when required depending on the variability in heart rate and blood pressure if it was >20% from base line. Intra-operative non-opioid analgesia included intravenous (IV) paracetamol (1 g) and diclofenac (75 mg IV) which were administered to all the patients. Anaesthesia was maintained with oxygen, nitrous oxide and sevoflurane with the circle absorber system.
Patients were randomised using computer-generated random numbers into two groups to receive either local anaesthetic infiltration of the laparoscopy port sites (n = 40, Group A/standard group) or USAFBs (n = 40, Group B/study group) using a total dose of 30 ml of ropivacaine 0.2% with sterile technique.
In group A, pre-incisional port-site infiltration was performed by the same surgeon every time, after the induction of anaesthesia and local anaesthetic was divided equally between port sites.
A total of four ports-supraumbillical, subxiphoid and two ports in the right subcostal area at mid-clavicular and anterior axillary line were made.
In group B, the blocks were performed under ultrasound guidance (GE Vivid E™) by the same investigator every time. Linear array ultrasound probe with a 6–13 MHz frequency was used. After the induction of anaesthesia, the skin was disinfected with 10% chlorhexidine. Posterior rectus sheath block was administered by placing ultrasonography (USG) transducer 2 cm below the xiphisternum in transverse position. A 90-mm, 22-G Quincke spinal needle was inserted in-plane and advanced until the tip rested on the posterior rectus sheath. After negative aspiration, 2 mL of saline was injected to verify needle tip location. When the correct needle position was achieved, 5 mL of 0.2% ropivacaine was injected bilaterally on each side [Figure 1a].
Figure 1.
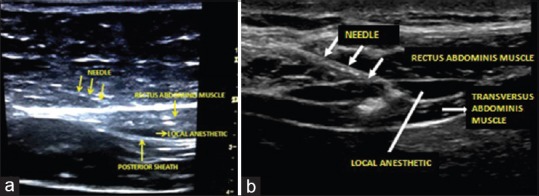
Ultrasonography image of rectus sheath block (a) and subcostal transversus abdominis block (b)
For right STA block, the USG probe was placed in the midline of the abdomen 2 cm below the xiphisternum and moved right laterally along the subcostal margin to the anterior axillary line. The transversus abdominis muscle was identified lying beneath and extending lateral to the rectus abdominis muscle. A 90-mm, 22-guage Quincke's spinal needle was then guided, in-plane, to a point just inferior to the right costal margin at the anterior axillary line such that the tip was between the transversus abdominis and internal oblique muscle within the neurovascular fascial plane. After careful aspiration to exclude vascular puncture, a test dose of 2 mL normal saline was injected to confirm needle tip placement and determine resistance to flow. Following aspiration, 20 mL of 0.2% ropivacaine was deposited within the plane [Figure 1b].
Following adequate and complete recovery, patients were transferred to the post-anaesthesia care unit. Both groups received post-operative analgesia using a patient-controlled analgesia (PCA) device which provided 20 μg bolus of fentanyl without a basic infusion rate with 15 min lock-out time. The correct use of the device was explained during the patient's informed consent in the pre-operative period. The patients stayed for 2 h in the recovery room and were then transferred and followed up in their rooms.
Patients were monitored for pain scores, fentanyl consumption, and adverse effects like nausea vomiting and vital parameters including heart rate, NIBP, SPO2 and respiratory rate at 1, 2, 4, 6, 12 and 24 h in the ward. Pain was assessed by Numeric Rating Scale (NRS), a 10 cm long scale on which 0 is taken as no pain and 10 being worst possible pain. NRS <3 was taken as satisfactory pain relief. At the end of 24 hrs, patients were asked to rank the quality of pain relief on a four point Patient satisfaction scale, where 1 – Excellent, 2 – Very good, 3 – Satisfactory, 4 -Poor.
Data were assessed by a member of the research team blinded to the group allocation. The data were analysed using software Statistical package for the Social Sciences (version 16, SPSS Inc., Chicago, IL, USA). The variables were expressed as mean and standard deviation Chi-square test/Fisher's exact test for categorical data and Mann–Whitney test/unpaired t-test for quantitative data were used respectively.
The sample size was calculated, assuming a 40% reduction in opioid use in patients receiving anterior abdominal wall blocks to provide 90% power at a statistical significance level of 5%. The 40% assumed reduction was based upon prior studies which showed 45–70% reductions in post-operative opioid requirement following anterior abdominal wall block.[5,6,7] The sample size after this calculation came out to be 37 in each group. To minimise any effects related to data loss, we recruited forty patients in each group, assuming a 10% dropout rate.
RESULTS
Both the groups were comparable with respect to patient demographics and duration of surgery [Table 1]. Lower pain numeric rating scores (NRS) in patients receiving abdominal field blocks were accompanied by lower analgesic requirements postoperatively [Figure 2].
Table 1.
Personal characteristic of study subjects and duration of surgery
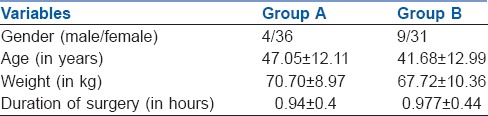
Figure 2.
Variation in numeric rating score over 24 h post-surgery
The average fentanyl consumption remained higher for port infiltration (Group A) (223.60 ± 101.96 μg) and was approximately twice of Group B (120.22 ± 74.93 μg) with a statistically significant difference between the two groups (P < 0.05, independent t-test) [Figure 3]. Maximum fentanyl consumption was 400 μg (Group A) and 262 μg (Group B) over 24 h, and the minimum requirements of fentanyl were 50 μg (Group A) and zero (Group B), respectively [Figure 4]. In the Group B, 20% patients received <50 μg fentanyl, whereas there were none with such minimal requirement in the Group A. Better oxygen saturation was observed in the USAFB group, though it was confined within normal limits (P < 0.05 at 6,12 and 24 h). Patient satisfaction scores correlated well with analgesia and opioid consumption [Figure 5].
Figure 3.
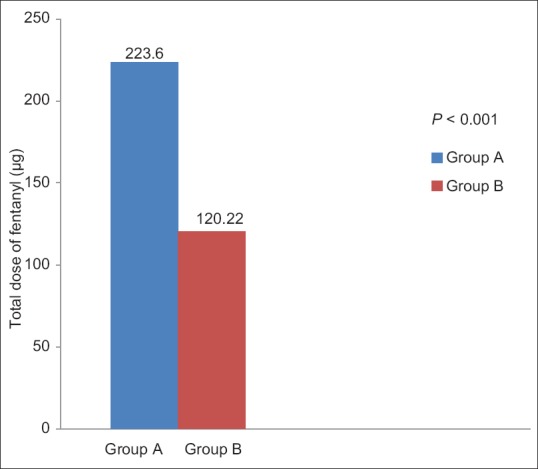
Total fentanyl consumption in 24 h
Figure 4.
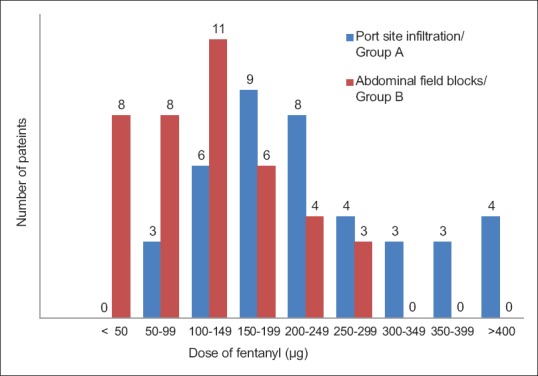
Fentanyl consumption, with distribution at every 50 μg interval
Figure 5.
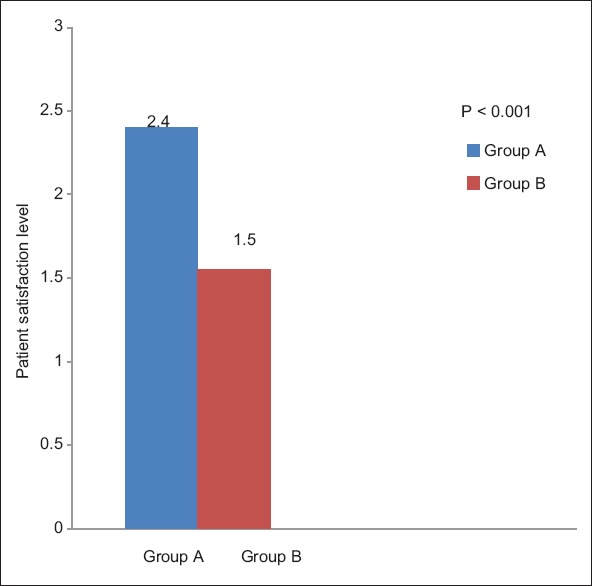
Patient satisfaction level
DISCUSSION
Inadequate pain relief, apart from being unethical, may result in increased morbidity and mortality.[8] Opioid-sparing, balanced analgesia provides enhanced pain relief and faster recovery compared with opioids or local anaesthetics alone.
Post-operative pain associated with laparoscopic cholecystectomy is less intense and lasts a shorter time than that seen with open surgery. As there is less functional impairment and pain, patients can be discharged and return to their normal daily activities earlier.[9] As laparoscopic cholecystectomy is being performed on an outpatient basis, USG guided nerve block is a good alternative to opioids and central neuraxial block.
Pain reaches a peak within the first few hours following the operation but diminishes during the next 2 or 3 days.[9,10] Mobilisation particularly aggravates the pain. Inadequately-treated acute pain after laparoscopic cholecystectomy may lead to chronic pain, i.e., post-laparoscopic cholecystectomy syndrome.[11] The use of long acting analgesic agents before surgery can avert the establishment of a sensitised state in the peripheral nervous system, thereby greatly diminishing the degree and persistence of post-operative pain culminating into chronic pain state.
Pain after laparoscopic cholecystectomy can be divided into three components: Visceral, parietal and referred shoulder tip pain. The main sources of pain include pain arising from incision sites (50–70%), pneumoperitoneum (20-30%) and ‘post-cholecystectomy wound’ (10-20%) within the liver causing visceral pain. Pneumoperitoneum has both local and systemic effects; local effects due to peritoneal and diaphragmatic stretching, acidosis and ischaemia and systemic effects due to hypercarbia causing sympathetic nervous system excitation with an amplification of local tissue inflammatory response.[12,13] The somatic pain is more important than visceral pain in the first 24–48 h postoperatively and during this period, the most common location of the pain is the right upper quadrant and the port sites, but the benefit of local anaesthetics is clear.[14]
The present study aims to compare the analgesic efficacy of US-guided regional blocks with port site local anaesthetic infiltration technique for post-operative pain relief, opioid consumption and patient satisfaction following elective laparoscopic cholecystectomy. Abdominal field blocks in this study comprise of right STA with bilateral posterior rectus sheath block. STA block provides effective analgesia to anterolateral upper abdominal wall, but its spread is limited by the lateral border of the rectus sheath, limiting its analgesic effect in midline and necessitating modification of port sites. Addition of posterior rectus sheath block provides adequate analgesia covering T5 -T10 dermatomes in midline, producing complementary results in terms of analgesic effects covering anatomical area of all the port sites without modifying their location.
Port site infiltration involves the injection of local anaesthetics subcutaneously into the incisional site. This blocks Aδ and C fibres in periportal fascia, the muscle and parietal peritoneum. The effect of port-site infiltration is small, lasting only for 2-3 h and of doubtful clinical relevance.[2] Analgesia with regional blocks lasts for 36-48 h, which might be due to the slow clearance of local anaesthetics in the transversus abdominis plane where relatively fewer blood vessels are located.[15] Reduced vascularity also reduces the risk of systemic toxicity from the local anaesthetics.
Studies done previously reported efficacy of TAP block[6] and STA block[7] in laparoscopic cholecystectomy; however, both studies necessitated adjustment of the port site positions to facilitate the anatomical distribution of the block. TAP block with modification of port sites[6,16,17] or a modified TAP block called STA[7,18] provides effective post-operative analgesia.
Not much literature is available on the analgesic efficacy of abdominal field blocks, a combination of right STA block with bilateral rectus sheath block in patients undergoing laparoscopic cholecystectomy without any modification of conventional port sites.
In our study, we found that the NRS was less in patients receiving abdominal field blocks when compared to patients receiving port site infiltration. Rescue analgesia with intravenous bolus dose of 20 μg fentanyl was administered at NRS >3. The overall fentanyl consumption in Group A was approximately twice (223.60 ± 101.96 μg) as compared to Group B (120.22 ± 74.93 μg). The difference was found to be statistically significant (P < 0.0001). Statistically significant difference was found in oxygen saturation of two groups, though it was confined within normal limits. It was more in Group B patients at 6, 12 and 24 h (P < 0.05). This probably reflects better respiratory efforts in patients with low pain scores. No other adverse effects were observed in both the groups. The overall patient satisfaction score was much higher with Group B compared to the Group A (P < 0.05).
The advantages with abdominal field blocks involving STA and rectus sheath block are thus primarily improved patient comfort and reduction in concomitant opioid use and its adverse effects such as nausea, vomiting, sedation and respiratory depression.
The present study also has certain limitations. As proposed in the initial study plan, the analgesic supplementation was to be provided when the NRS was >3. Since patients had been provided with intravenous PCA, it was difficult to standardise the time to first analgesic dose administration as patients often ended up demanding the dose of analgesic based on their ability to tolerate pain. Other major disadvantage of STA block is the inability to block visceral pain, which can be substantial, both intra- and post-operatively.[19,20] The other major limitation is dermatomal limitation of the block.[20] STA block provides reliable analgesia covering T6 – T10 dermatomes.
CONCLUSION
STA block in addition with rectus abdominis sheath block is a good alternative for providing perioperative analgesia for upper abdominal surgery such as laparoscopic cholecystectomy and can cover the conventional anatomical port sites as well.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Lau H, Brooks DC. Predictive factors for unanticipated admissions after ambulatory laparoscopic cholecystectomy. Arch Surg. 2001;136:1150–3. doi: 10.1001/archsurg.136.10.1150. [DOI] [PubMed] [Google Scholar]
- 2.Bisgaard T. Analgesic treatment after laparoscopic cholecystectomy: A critical assessment of the evidence. Anesthesiology. 2006;104:835–46. doi: 10.1097/00000542-200604000-00030. [DOI] [PubMed] [Google Scholar]
- 3.Jankovic Z. Transversus abdominis plane block: The Holy Grail of anaesthesia for (lower) abdominal surgery. Period Biol. 2009;111:203–8. [Google Scholar]
- 4.Hebbard P. Subcostal transversus abdominis plane block under ultrasound guidance. Anesth Analg. 2008;106:674–5. doi: 10.1213/ane.0b013e318161a88f. [DOI] [PubMed] [Google Scholar]
- 5.Niraj G, Searle A, Mathews M, Misra V, Baban M, Kiani S, et al. Analgesic efficacy of ultrasound-guided transversus abdominis plane block in patients undergoing open appendicectomy. Br J Anaesth. 2009;103:601–5. doi: 10.1093/bja/aep175. [DOI] [PubMed] [Google Scholar]
- 6.El-Dawlatly AA, Turkistani A, Kettner SC, Machata AM, Delvi MB, Thallaj A, et al. Ultrasound-guided transversus abdominis plane block: Description of a new technique and comparison with conventional systemic analgesia during laparoscopic cholecystectomy. Br J Anaesth. 2009;102:763–7. doi: 10.1093/bja/aep067. [DOI] [PubMed] [Google Scholar]
- 7.Tolchard S, Davies R, Martindale S. Efficacy of the subcostal transversus abdominis plane block in laparoscopic cholecystectomy: Comparison with conventional port-site infiltration. J Anaesthesiol Clin Pharmacol. 2012;28:339–43. doi: 10.4103/0970-9185.98331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katz J, Jackson M, Kavanagh BP, Sandler AN. Acute pain after thoracic surgery predicts long-term post-thoracotomy pain. Clin J Pain. 1996;12:50–5. doi: 10.1097/00002508-199603000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Inan A, Sen M, Dener C. Local anesthesia use for laparoscopic cholecystectomy. World J Surg. 2004;28:741–4. doi: 10.1007/s00268-004-7350-3. [DOI] [PubMed] [Google Scholar]
- 10.Bisgaard T, Klarskow B, Kristiansen VB, Callesen T, Shulze S, Kehelet H, et al. Multiregional local anesthetic infiltration during laparoscopic cholecystectomy in patients receiving prophylactic multi-modal analgesia: A randomized double blinded placebo controlled study. Anesth Analg. 1999;89:1017. doi: 10.1097/00000539-199910000-00036. [DOI] [PubMed] [Google Scholar]
- 11.Bisgaard T, Rosenberg J, Kehlet H. From acute to chronic pain after laparoscopic cholecystectomy: A prospective follow-up analysis. Scand J Gastroenterol. 2005;40:1358–64. doi: 10.1080/00365520510023675. [DOI] [PubMed] [Google Scholar]
- 12.Joris J, Thiry E, Paris P, Weerts J, Lamy M. Pain after laparoscopic cholecystectomy: Characteristics and effect of intraperitoneal bupivacaine. Anesth Analg. 1995;81:379–84. doi: 10.1097/00000539-199508000-00029. [DOI] [PubMed] [Google Scholar]
- 13.Mouton WG, Bessell JR, Otten KT, Maddern GJ. Pain after laparoscopy. Surg Endosc. 1999;13:445–8. doi: 10.1007/s004649901011. [DOI] [PubMed] [Google Scholar]
- 14.Cantore F, Boni L, Di Giuseppe M, Giavarini L, Rovera F, Dionigi G. Pre-incision local infiltration with levobupivacaine reduces pain and analgesic consumption after laparoscopic cholecystectomy: A new device for day-case procedure. Int J Surg. 2008;6(Suppl 1):S89–92. doi: 10.1016/j.ijsu.2008.12.033. [DOI] [PubMed] [Google Scholar]
- 15.McDonnell JG, Curley G, O’Donnell B, Heffernan A, Power C, Laffey JG. The analgesic efficacy of transversus abdominis plane block after caesarean section: A prospective randomized controlled trial. Anesth Analg. 2008;106:186–91. doi: 10.1213/01.ane.0000290294.64090.f3. [DOI] [PubMed] [Google Scholar]
- 16.Zhao X, Tong Y, Ren H, Ding XB, Wang X, Zong JY, et al. Transversus abdominis plane block for postoperative analgesia after laparoscopic surgery: A systematic review and meta-analysis. Int J Clin Exp Med. 2014;7:2966–75. [PMC free article] [PubMed] [Google Scholar]
- 17.Yu N, Long X, Lujan-Hernandez JR, Succar J, Xin X, Wang X. Transversus abdominis-plane block versus local anesthetic wound infiltration in lower abdominal surgery: A systematic review and meta-analysis of randomized controlled trials. BMC Anesthesiol. 2014;14:121. doi: 10.1186/1471-2253-14-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ibrahim M, Shamaa HE. Efficacy of ultrasound-guided oblique subcostal transversus abdominis plane block after laparoscopic sleeve gastrectomy: A double blind, randomized, placebo controlled study. Egypt J Anaesth. 2014;30:285–92. [Google Scholar]
- 19.Niraj G, Kelkar A, Powell R. Ultrasound guided subcostal transversus abdominis plane block. Int J Ultrasound Appl Technol in Perioper Care. 2010;1:9–12. [Google Scholar]
- 20.Kerai S, Dabas N, Sehrawat L, Gupta N. Transversus abdominis plane block as sole anaesthetic technique for inguinal hernia repair in two patients having complex medical conditions. Indian J Anaesth. 2015;59:754–6. doi: 10.4103/0019-5049.170040. [DOI] [PMC free article] [PubMed] [Google Scholar]


