Abstract
The distribution of Aedes albopictus in Africa has thus far been known to be restricted to coastal Sub-Saharan countries. This report describes the first record of the tiger mosquito in habitats located in Mali, at a significant distance from the coastal areas of the continent. Aedes. albopictus was observed over several years in increasing frequency in Mopti in Central Mali and later in the capital city Bamako, both adjacent to the Niger River. These findings suggest further dissemination of Ae. albopictus could be facilitated by river transport of goods and commodities which harbor larvae and eggs of this species. If correct, the distribution of Ae. albopictus is expected to extend to areas located upstream of the Niger River and its tributaries.
Keywords: Aedes albopictus, Mali, Niger River
Graphical abstract
The first occurrence, and possible river dissemination, of Aedes albopictus in inland Africa is documented from Mali along the Niger River
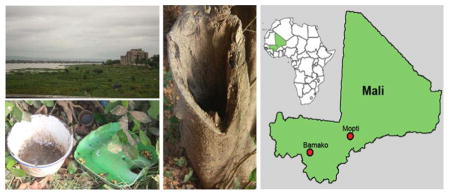
Aedes albopictus collection locations
The vector and nuisance mosquito Aedes albopictus (Skuse), common name tiger mosquito, was formerly restricted to South-East Asia. Throughout the second half of the 20th century it rapidly spread to many parts of the world, with initial sightings on islands of the Western Pacific (Belkin, 1962), followed by invasion of the Americas (Reiter and Sprenger, 1987), Europe (Sabatini et al., 1990, Dalla Pozza and Majori 1992, Mitchell 1995, Knudsen et al. 1996, Romi et al., 1999) and the Middle East (Haddad et al., 2007). The first recorded sighting of Ae. albopictus on the African continent was reported in the early nineties in the Republic of South Africa (Cornel and Hunt, 1991). The following year, the distribution of the tiger mosquito extended to Nigeria (Savage et al., 1992), and thereafter it increased rapidly to the coastal regions of Western and Central Africa (Fontenille and Toto, 2001; Toto et al., 2003). This vast and rapid global dispersion of Ae. albopictus is directly linked to intercontinental trade of used tires, which constitute important larval habitats of Aedes mosquitoes (Reiter and Spenger, 1987; Cornel and Hunt, 1991).
Besides being a highly problematic day-biting nuisance mosquito, Ae. albopictus is also highly anthropophilic and a competent vector for at least 22 arboviruses, notably chikungunya and the four serotypes of dengue (reviewed by Gratz, 2004). Furthermore, though the Zika virus has yet to be isolated from wild type Ae. albopictus, Wong and colleagues (2013) were able to establish infections in the laboratory proving that this mosquito is a susceptible Zika vector. The spread of Ae. albopictus in Africa is thus very troubling, as arbovirus epidemics are becoming increasingly prevalent in many regions of the continent. In 2004, an outbreak of chikungunya was reported in Kenya (Chretien et al., 2007), and a short time later in Cameroon and Gabon (Peyrefitte et al., 2007; Peyrefitte et al., 2008), Central African Republic (Diallo et al., 2010), Cote d’Ivoire (Konan et al., 2013) and the Republic of Congo (Mombouli et al. 2013; Moyen et al., 2014). A dengue virus (DENV) serosurvey conducted in Mali in 2006 showed that 93% (87/93) of the samples obtained from the Institut National de Recherche en Sante Publique in Bamako were positive for anti-DENV IgG antibodies, with some samples neutralized monotypically against DENV-1 and DENV-2, while others neutralized broadly against multiple DENV in plaque-reduction neutralization tests (Phoutrides et al., 2011). The further spread of Ae. albopictus in Africa can potentially enhance the dissemination of vector-borne arboviruses like chikungunya and dengue, increasing the frequency of outbreaks and the impact on human health in countries which are already susceptible to a multitude of arthropod-borne diseases.
Our first detection of Ae. albopticus in Mali was in 2008, when a single female of the species was captured with a CDC trap which was positioned for 24 hours in a fishing village located on the northern outskirts of Mopti. On that occasion, further attempts to locate additional specimens of the tiger mosquito in traps or landing on human bait proved unsuccessful, indicating the species was yet to be stably established at that time. In 2010, we again discovered Ae. albopictus in CDC traps and landing on human hosts around the area of Mopti, while conducting other mosquito studies (Müller et al., 2010). In surveys conducted in October 2014 we hand nets were used to collect the tiger mosquito landing on human hosts in shady areas along the river located within the city of Mopti, confirming that this species is well established in this area. In these surveys, 10 specimens were identified in the laboratory by cross-referencing with pictorial species identification keys (Rueda 2004).
Additional sightings of Ae. albopictus were made in Bamako. A small survey was conducted over two days in November 2012 in several neighborhoods, in which mosquitoes attempting to land on human bait were collected using entomological hand nets in the late afternoons. In this instance, we collected 125 Ae. albopictus in shaded areas near the Niger River. The occurrence of the tiger mosquito decreased with increasing distance from the river. About 1 km from the river, the dominant day-feeding mosquito was Ae. Aegypti; no specimens of Ae. albopictus were observed beyond this distance.
Immature stages of Ae. albopictus were also collected by dipping and a cooking baster. Larvae were collected and reared to maturity for subsequent identification as described above. Aedes albopictus larvae were found exclusively in container-type habitats found in an area spanning several hundreds of meters parallel to the Niger River. These included plastic buckets (2 out of 17 surveyed buckets), plastic bags (3 out of 50), dried-fruit containers (1 out of 29), tires (3 out of 6), marooned small boats (1 out of 8) and in water accumulated within active boats used for fishing (2 out of 5), sand transport (1 out of 7), and other goods/human transport (2 out of 28). Samples obtained at greater distances from the river contained only 39% Ae. aegypti and 61% Culex quinquefasciatus juveniles.
These findings are the first observations of Ae. albopictus in land-locked Mali, at sites located more than 1000 km from any coastal regions. Ae. albopictus was found exclusively in habitats with close proximity to the Niger River, often in or near patches of dense vegetation, as opposed to Ae. aegypti which was found at both vegetated and urban sites. The high proportion of Ae. albopictus collected at six locations, 68.75%, (versus 31.25% Ae. aegypti) indicates Ae. albopictus populations are well established at densely vegetated sites adjacent to the Niger River.
As mentioned above, the dissemination of Ae. albopictus in Africa has been attributed to the thriving local trade of second hand tires (Cornel and Hunt, 1991). Mosquitoes may have arrived through several different routes to African harbors by importing used tires directly from North America, Europe and East Asia. It is feasible that transportation of such commodities has facilitated the dispersion of mosquitoes further inland. In this context, it is noteworthy that the majority of the breeding sites observed in Bamako were in close proximity of the river, mostly in containers used for shipping transport, or in puddles in leaking wooden boats. Our findings of large Ae. albopictus populations along the Niger river in Bamako (regular observations from 2008 until 2015) and several records from Mopti obtained through CDC trap catches and observations on human hosts (unpublished data of the authors, 2008, 2014) suggest that this species is further disseminated through boat traffic along the river. The records from Bamako and Mopti, located approximately 1000 km and 1600 km from the coast, respectively, are thus far the furthest sites Ae. albopictus has been discovered from the seashore. If Ae. albopictus is indeed disseminated through boat traffic, it seems highly likely this species will soon be found along the entire Niger River system, and accordingly, Guinea Conakry and Niger could be the subsequent countries invaded. The results from Mali suggest a different mechanism of dissemination, possibly linked to river transport, although further research is required to prove whether this is the primary mode of dispersion that has caused this species to penetrate the interior of the continent following its arrival to the African shores.
Highlights.
The distribution of Ae. albopictus has rapidly increased over the last decades
Formerly restricted to coastal Africa, we have sighted the species in inland Mali
The species seems to be exclusively located in habitats proximal the Niger River
Larvae on small boats suggests the species may be disseminated through river traffic
Findings suggest the species may soon spread through the entire Niger River system
Acknowledgments
Research reported in this publication was supported by the National Institute of Allergy And Infectious Diseases of the National Institutes of Health under Award Number R01AI100968. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.”
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Belkin JN. The mosquitoes of the South Pacific (Diptera, Culicidae) 1 and 2. Berkeley and Los Angeles: Univ. of California Press; 1962. [Google Scholar]
- Chretien JP, Anyamba A, Bedno S. Drought-associated chikungunya emergence along coastal East Africa. Am J Trop Med Hyg. 2007;76:405–407. [PubMed] [Google Scholar]
- Cornel AJ, Hunt RH. Aedes albopictus in Africa. First records of live specimens in imported tires in Cape Town. J Am Mosq Control Assoc. 1991;7:107–108. [PubMed] [Google Scholar]
- Dalla Pozza G, Majori G. First record of Aedes albopictus establishment in Italy. J Am Mosq Control Assoc. 1992;8:318–320. [PubMed] [Google Scholar]
- Diallo M, Laganier R, Nangouma A. First record of Aedes albopictus in Central African Republic. Trop Med Int Health. 2010;15:1185–1189. doi: 10.1111/j.1365-3156.2010.02594.x. [DOI] [PubMed] [Google Scholar]
- Fontenille D, Toto JC. Aedes (Stegomyia) albopictus (Skuse), a potential new dengue vector in southern Cameroon. Emerg Infect Dis. 2001;7:1066–1067. doi: 10.3201/eid0706.010631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratz NG. Critical review of the vector status of Aedes albopictus. Med Vet Entomol. 2004;40:595–596. doi: 10.1111/j.0269-283X.2004.00513.x. [DOI] [PubMed] [Google Scholar]
- Haddad N, Harbach RE, Chamat S, Bouharoun-Tayoun H. Presence of Aedes albopictus in Lebanon and Syria. J Am Mosq Control Assoc. 2007;23:226–228. doi: 10.2987/8756-971X(2007)23[226:POAAIL]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Knudsen AB, Romi R, Majori G. Occurrence and spread in Italy of Aedes albopictus, with implications for its introduction into other parts of Europe. J Am Mosq Control Assoc. 1996;12:177–183. [PubMed] [Google Scholar]
- Konan YL, Coulibaly ZI, Koné AB, Ekra KD, Doannio JM, Dosso M, Odénhouri-Koudou P. Species composition and population dynamics of Aedes mosquitoes, potential vectors of arboviruses, at the container terminal of the autonomous port of Abidjan, Côte d’Ivoire. Parasite. 2013;20:13. doi: 10.1051/parasite/2013013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell CJ. Geographic spread of Aedes albopictus and potential for involvement in arbovirus cycles in Mediterranean basin. J Vector Ecol. 1995;20:533–537. [Google Scholar]
- Mombouli JV, Bitsindou P, Elion DOA, Grolla A, Feldmann H, Niama FR, Parra HJ, Munster VJ. Chikungunya Virus Infection, Brazzaville, Republic of Congo. Emerg Infect Dis. 2013;19:1542–1543. doi: 10.3201/eid1909.130451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyen N, Thiberville SD, Pastorino B, Nougairede A, Thirion L, Mombouli JV, Dimi Y, Leparc-Goffart I, Capobianchi MR, Lepfoundzou AD, de Lamballerie X. First Reported Chikungunya Fever Outbreak in the Republic of Congo. PLoS One. 2014;9:e115938. doi: 10.1371/journal.pone.0115938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller GC, Beier JC, Traore SF, Toure MB, Traore MM, Bah S, Doumbia S, Schlein Y. Field experiments of Anopheles gambiae attraction to local fruits/seedpods and flowering plants in Mali to optimize strategies for malaria vector control in Africa using attractive toxic sugar bait methods. Malaria J. 2010;9:262. doi: 10.1186/1475-2875-9-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyrefitte CN, Bessaud M, Pastorino BA, Gravier P, Plumet S, Merle OL, Moltini I, Coppin E, Tock F, Daries W, Ollivier L, Pages F, Martin R, Boniface F, Tolou HJ, Grandadam M. Circulation of chikungunya virus in Gabon, 2006–2007. J Med Virol. 2008;80:430–433. doi: 10.1002/jmv.21090. [DOI] [PubMed] [Google Scholar]
- Peyrefitte CN, Rousset D, Pastorino BA, Pouillot R, Bessaud M, Tock F, Mansaray H, Merle OL, Pascual AM, Paupy C, Vessiere A, Imbert P, Tchendjou P, Durand JP, Tolou HJ, Grandadam M. Chikungunya virus, Cameroon: 2006. Emerg Infect Dis. 2007;13:768–771. doi: 10.3201/eid1305.061500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phoutrides EK, Coulibaly MB, George CM, Sacko A, Traore S, Bessoff K, Wiley MR, Kolivras KN, Adelman Z, Traore M, Hunsperger EA. Dengue virus seroprevalence among febrile patients in Bamako, Mali: results of a 2006 surveillance study. Vect Bor Zoo Dis. 2011;11:1479–85. doi: 10.1089/vbz.2011.0622. [DOI] [PubMed] [Google Scholar]
- Reiter P, Spenger D. The used tire trade: a mechanism for the worldwide dispersal of container breeding mosquitoes. J Am Mosq Control Assoc. 1987;3:494–501. [PubMed] [Google Scholar]
- Romi R, Di Luca M, Majori G. Current status of Aedes albopictus and Aedes atropalpus in Italy. J Am Mosq Control Assoc. 1999;15:425–427. [PubMed] [Google Scholar]
- Rueda LM. Pictorial keys for the identification of mosquitoes (Diptera: Culicidae) associated with dengue virus transmission. Zootaxa. 2004;589:1–60. [Google Scholar]
- Sabatini A, Raineri V, Trovato G, Coluzzi M. Aedes albopictus in Italy and possible diffusion of the species into the Mediterranean area. Parassitologia. 1990;32:301–304. [PubMed] [Google Scholar]
- Savage HM, Ezike VI, Nwankwo CAN, Spiegel R, Miller BR. First record of breeding populations of Aedes albopictus in continental Africa: implications for arboviral transmission. J Am Mosq Control Assoc. 1992;8:101–102. [PubMed] [Google Scholar]
- Toto JC, Abaga S, Carnevale P, Simard F. First report of the oriental mosquito Aedes albopictus on the west African island of Bioko, Equatorial Guinea. Med Vet Entomol. 2003;17:173–176. doi: 10.1046/j.1365-2915.2003.00447.x. [DOI] [PubMed] [Google Scholar]
- Wong PS, Li MZ, Chong CS, Ng LC, Tan CH. Aedes (Stegomyia) albopictus (Skuse): a potential vector of Zika virus in Singapore. PLoS Negl Trop Dis. 2013;7(8):e2348. doi: 10.1371/journal.pntd.0002348. [DOI] [PMC free article] [PubMed] [Google Scholar]


