Abstract
BACKGROUND
Preemptive kidney transplantation (preKT) is associated with higher patient survival, improved quality of life, and lower costs. However, only a minority of patients receives preKT. The aim of this study was to examine changes over the past decade in rates of preKT, focusing on living donor kidney transplantation (LDKT) and specifically recipients who underwent kidney transplantation within one year of initiating dialysis.
METHODS
Using United Network of Organ Sharing data, we examined retrospectively all kidney transplant candidates (n=369,103) and recipients (n=141,254) from 2003-2012 in the United States focusing on LDKT (n=47,108). Predictors of preKT were examined, and patient and graft survival were compared for preKT, pre-transplant dialysis < 1 year, and pre-transplant dialysis ≥ 1 year recipients.
RESULTS
PreKT occurred in only 17% of recipients overall and 31% of LDKT recipients. Medicare patients (OR=0.29, 95%CI=0.28-0.31), diabetics (OR=0.75, 95%CI=0.69-0.80), and minorities (Hispanics OR=0.62, 95%CI=0.57-0.68 & African-Americans OR=0.58, 95%CI=0.53-0.63) were less likely to receive preKT. Dialysis < 1 year recipients comprised 30% of non-preemptive LDKT. Dialysis < 1 year recipients had similar patient survival to preKT (5 yr: preKT 94%, dialysis < 1 yr 94%, & dialysis ≥ 1 yr 89%, p<0.01), but decreased death-censored graft survival (5 yr: preKT 93%, dialysis < 1 yr 89%, & dialysis ≥ 1 yr 89%, p<0.01).
CONCLUSIONS
PreKT remains an unrealized goal for the majority of recipients. Medicare patients, diabetics, and minorities are less likely to receive preKT. Almost one-third of non-preemptive LDKT recipients dialyze < 1 year, highlighting an important target for improvement.
INTRODUCTION
Compared with dialysis, kidney transplantation leads to higher patient survival, improved quality of life, and lower costs [1-4]. In reality, the majority of patients with end-stage renal disease (ESRD) undergo both therapies, receiving several years of dialysis prior to kidney transplantation. However, limiting dialysis time is an important goal as increased time on dialysis before transplant is associated with decreased patient and graft survival [5-8].
Given the shortage of deceased donor organs, a period of pre-transplant dialysis is likely obligatory for most deceased donor kidney candidates. However, it is concerning that the majority of recipients of living donor kidneys also undergo dialysis pre-transplantation. Even with living donor kidney transplantation (LDKT), historically fewer than one-quarter of recipients undergo preemptive kidney transplantation (preKT)[7, 9, 10].
In the past several years, interest in preKT has increased[4, 11, 12]. The report from the National Kidney Foundation conference in 2007 suggested several tactics for increasing preKT[11]. However, it is unclear if increased awareness has led to changes in practice.
The aim of the current study was to examine recent (2003 to 2012) rates of preKT in the United States, with a particular emphasis on recipients of living donor kidneys. In addition to studying changes in the rates of preKT, we were interested in any changes in demographics for preKT versus non-preemptive KT in this time frame. Finally, we focused on recipients of living donor kidney transplants who underwent only a short course of dialysis (<1 year) to begin to understand the demographics of non-preemptive recipients who might be more likely to avoid dialysis.
MATERIALS AND METHODS
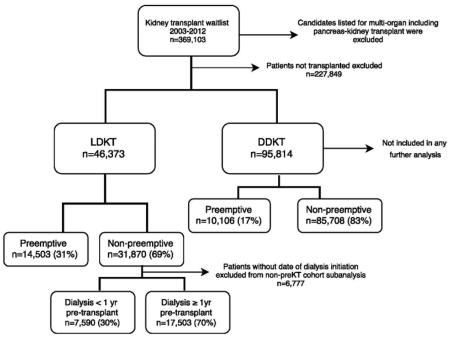
Using national data from the United Network of Organ Sharing (UNOS), we examined retrospectively all adult and pediatric kidney transplant recipients who were transplanted from June 2003 to September 2012. This cohort included 47,018 patients who received a living donor kidney transplant (LDKT) and 95,814 patients who received a deceased donor kidney transplant (DDKT). Recipients of multi-organ transplants (including kidney-pancreas transplants) were excluded. The majority of our analyses focused on LDKT recipients as we believe LDKT provides the greatest potential for increasing the rate of preKT.
We analyzed the rates of preKT over the study time period. We examined duration of dialysis prior to transplant in non-preemptive transplant recipients and divided these patients into < 1 year of dialysis and ≥ 1 year of dialysis subgroups. We compared donor and recipient characteristics for preKT, dialysis < 1 year, and ≥ 1 year of dialysis pre-transplantation subgroups including age at transplant, gender, race, renal diagnosis, primary payer at time of transplant, ABO blood type, recipient sensitization according to calculated panel reactive antibody (cPRA) at time of transplant. We compared patient survival and overall and death-censored graft survival for living donor preKT, dialysis < 1 year, and ≥ 1 year of dialysis pre-transplantation.
Statistical analyses and missing data
Categorical variables were compared using chi-square statistics, and t-test statistics were used to compare continuous variables. Missing data for recipient demographics is reported in the tables and was combined with “other” for variables with a large number of small frequency responses such as renal diagnoses and race. Time on dialysis prior to transplant was not available for 19% of non-preemptive transplant recipients. Mandatory reporting of cPRA was not instituted until 2007, and was thus only available for 53% of patients, specifically those listed from 2007-2012.
Patient and (overall and death-censored) graft survival were compared with Kaplan-Meier analysis and log-rank tests. Additionally, Cox proportional hazards models were used to compare survival after adjusting for recipient factors (age at transplant, gender, race, ABO, etiology of renal disease [diabetes, HTN, GN, and PCKD], primary payer [medicare vs private insurance], BMI, and cPRA), living donor factors (age, gender, race, and BMI), and transplant factors (CIT and HLA mismatch). Factors included in survival models were chosen a priori based on clinical significance. Recipient characteristics that were statistically different between cohorts according to preemptive status were included in adjustment to address potential confounding. Multivariate logistic regression was utilized to identify recipient predictors of receiving preKT. Hazard ratios (HR) and odds ratios (OR) with 95% confidence intervals (CI) are reported. Schoenfeld residuals tests were utilized to confirm the proportional hazards assumption. Goodness of fit was assessed according to chi-squared statistics for survival models including global test statistics for nested models and according to the Cox-Snell pseudo R-squared statistics for multivariate logistic regression. A two-sided p-value < 0.01 was considered significant given the large sample size of this analysis. All analyses were performed with STATA software (version 11.2, College Station, TX). This study was approved as exempt by the Mayo Clinic Institutional Review Board as it involves de-identified publicly available data.
RESULTS
Overall Kidney Transplant Rates
From 6/2003 to 9/2012, 141,254 kidney transplants were performed in the United States. The majority were from deceased donors (94,881 deceased donors versus 46,373 living donors). The majority of patients underwent both dialysis and transplantation, with only 17% (24,609/141,254) transplanted preemptively over the study period. The rate of preKT was higher for living donor recipients than deceased donor recipients (31%, 14,503/46,373 versus 11%, 10,106/94,881, p<0.0001). However, given the large number of deceased donor kidney transplants, 41% (10,106/24,609) of all preemptive transplants were from deceased donors.
Dialysis time prior to transplantation
Dialysis time prior to transplantation varied according to donor type (Table 1). Seventy-nine percent of DDKT recipients dialyzed for more than 2 years while only 6% dialyzed less than 1 year. In contrast, only 38% of LDKT recipients dialyzed more than 2 years, while 32% dialyzed for 1-2 years and 30% dialyzed less than 1 year.
Table 1.
Proportion of preemptive kidney transplant according to donor type and dialysis time prior to transplant in non-preemptive transplants.
| Population | Total | Preemptive | Non-preemptive | Pre-transplant dialysis time in non-preemptive KT* | ||
|---|---|---|---|---|---|---|
| < 1 year | 1-2 years | > 2 years | ||||
| All transplant recipients | 141,254 | 17% (24,609) | 83% (116,645) | 13% (11,857) | 19% (18,282) | 68% (64,409) |
| LDKT recipients | 46,373 | 31% (14,503) | 69% (31,870) | 30% (7,590) | 32% (7,929) | 38% (9,574) |
| DDKT recipients | 94,881 | 11% (10,106) | 89% (84,775) | 6% (4,267) | 15% (10,352) | 79% (54,833) |
Dialysis start date missing in 19% of patients in this analysis and thus dialysis time on transplant not available for reporting in all patients included in this analysis.
Changes in preemptive transplant rates over time
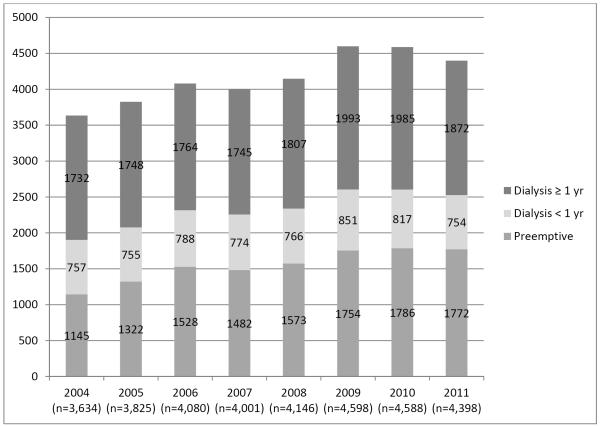
In the past decade, the proportions of preKT have increased marginally from 12% in 2003 to 18% in 2012. This was primarily related to an increase in living donor preKT from 23% in 2003 to 34% in 2012 (Figure 1). During this time, the overall number of LDKT increased by 25%. The number of patients dialyzing <1 year prior to LDKT was stable at approximately 750 per year.
Figure 1.
Annual trends in preemptive, dialysis < 1 year, and dialysis ≥ 1 year prior to living donor kidney transplantation.
* Data for 2003 and 2012 are not displayed as only partialyear data was available for analysis.
Preemptive LDKT: Recipient characteristics
LDKT recipient characteristics for preKT, dialysis < 1 year, and dialysis ≥1 year pre-transplantation are presented in Table 2, and a univariate analysis of predictors of living donor preKT is presented in Table 3. Private insurance and Caucasian race were two factors associated with preKT. In LDKT recipients with private insurance, 40% (11,003/27,083) were preKT. Compared to patients with private insurance, 16% (2,673/16,592) LDKT recipients with Medicare had a preKT corresponding to an adjusted OR=0.29 (95%CI=0.28-0.31) of receiving a preKT. African-Americans had an OR=0.58 (95%CI=0.53-0.63) and Hispanic/Latinos an OR=0.62 (95%CI=0.57-0.68) compared to Caucasians. Additionally, increased education level was associated with increased rates of preKT (OR=1.16 per increased level, 95% CI=0.13-0.18).
Table 2.
Recipient characteristics of preemptive compared with non-preemptive living donor kidney transplant recipients.
| LDKT recipient characteristics | Preemptive n=14,503 |
Dialysis < 1yr n=7,590 |
Dialysis ≥ 1yr n=17,503 |
p-value |
|---|---|---|---|---|
| Age (years: mean ± SD) | 47 (±15) | 43 (±16) | 46 (± 15) | <0.01 |
| Gender (% female) | 41% (5,937) | 37% (2,819) | 39% (6,761) | <0.01 |
| Race/Ethnicity: White | 77% (11,130) | 67% (5,102) | 52% (9,151) | <0.01 |
| African-American | 9% (1,340) | 12% (903) | 21% (3,655) | |
| Hispanic/Latino | 9% (1,384) | 15% (1,152) | 20% (3,518) | |
| Other/missing | 4% (649) | 6% (433) | 7% (1,179) | |
| Sensitization: cPRA <20% | 50% (7,185) | 44% (3,302) | 42% (7,282) | <0.01 |
| cPRA 20-79% | 6% (858) | 5% (378) | 11% (1,186) | |
| cPRA ≥80% | 1% (202) | 2% (124) | 3% (588) | |
| missing* | 43% (6,258) | 50% (3,786) | 48% (8,447) | |
| Blood type: O | 43% (6,188) | 44% (3,342) | 47% (8,271) | <0.01 |
| A | 40% (5,865) | 40% (2,995) | 35% (6,088) | |
| B | 13% (1,826) | 12% (943) | 15% (2,555) | |
| AB | 4% (624) | 4% (310) | 3% (589) | |
| Diagnosis: Diabetes | 16% (2,289) | 22% (1,651) | 24% (4,203) | <0.01 |
| Hypertension | 13% (1,939) | 16% (1,185) | 22% (3,839) | |
| PCKD | 19% (2,701) | 7% (536) | 5% (944) | |
| Glomerulonephritis | 28% (4,111) | 31% (2,383) | 27% (4,772) | |
| Other/missing | 24% (3,463) | 24% (1,835) | 21% (3,745) | |
| Pediatric patient (age<18) | 4% (576) | 4% (333) | 2% (401) | <0.01 |
| Payer/insurance: Private | 76% (11,003) | 64% (4,809) | 38% (6,688) | <0.01 |
| Medicare | 18% (2,673) | 31% (2,348) | 56% (9,721) | |
| Other/missing | 6% (827) | 6% (433) | 6% (1,094) | |
| Prior transplant | 8% (1,211) | 10% (790) | 10% (1,744) | <0.01 |
Mandatory reporting of cPRA was not instituted until 2007, and thus only available on patients listed from 2007-2012.
Table 3.
Predictors of preemptive transplantation in adult LDKT recipients.
| Recipient characteristic (LDKT n=33,847) | Odds Ratio | 95% confidence interval | |
|---|---|---|---|
| Age 50-59 (ref: age<50) | 1.13 | 1.06 | 1.20 |
| Age 60-69 (ref: age<50) | 1.45 | 1.35 | 1.56 |
| Age >70 (ref: age<50) | 2.54 | 2.21 | 2.91 |
| Gender (ref: female) | 0.87 | 0.82 | 0.91 |
| African-American (ref:white) | 0.58 | 0.53 | 0.63 |
| Hispanic/Latino (ref:white) | 0.62 | 0.58 | 0.68 |
| Blood type A (ref: O) | 1.09 | 1.03 | 1.16 |
| Blood type B (ref: O) | 0.98 | 0.91 | 1.07 |
| Blood type AB (ref: O) | 1.19 | 1.04 | 1.36 |
| Diabetes (ref: GN) | 0.75 | 0.69 | 0.80 |
| Hypertension (ref: GN) | 0.85 | 0.79 | 0.92 |
| PCKD (ref: GN) | 1.98 | 1.84 | 2.14 |
| Education (per increased level) | 1.16 | 1.13 | 1.18 |
| Medicare (ref: private insurance) | 0.29 | 0.28 | 0.31 |
| cPRA 20-80% (ref: cPRA <20%) | 0.83 | 0.74 | 0.92 |
| cPRA >80% (ref: cPRA <20%) | 0.54 | 0.43 | 0.68 |
| cPRA missing (ref: cPRA <20%) | 0.82 | 0.78 | 0.87 |
The rate of preKT also varied with the cause of renal failure: 65% (2707/4181) of recipients with polycystic kidney disease (PCKD), 37% (4111/11,276) of recipients with glomerulonephritis, and 28% (2289/8143) of patients with diabetes underwent preKT. PreKT also varied by age group, with 34% (746/2179) of recipients <20 years old receiving preKT compared to 23% (1,156/5,096) of 20-29 year old, 27% (2,075/7,561) of 30-39 year old, 33% (10,515/31,500) of 40-79 years old, and 29% (10/34) of LDKT recipients >79 years old. For older patients, odds of preKT with living donors were increased which was highest in the recipients > 70 years old (OR=2.54; 95% CI=2.21-3.91) likely reflecting an understanding by transplant clinicians of the dangers of waiting for a kidney and prolonged dialysis in older candidates and thus a higher selection rate of patients who present early with a donor.
Over time, there were improvements in preKT rates among minorities, with the largest increase being in Hispanic/Latino patients. Twenty-four percent (405/1,705) of African-American LDKT recipients in 2010-2012 underwent preKT compared with 20% (333/1,708) in 2003-2005 (p<0.01). For Hispanic/Latino LDKT recipients, preKT increased from 19% (299/1,607) in 2003-2005 to 26% (488/1,900) in 2010-2012 (p<0.01). Additionally, there was some improvement in the rate of preKT in adult Medicare LDKT recipients during the period studied, with 20% (917/4,590) of Medicare patients receiving preKT in 2010-2012 compared with 15% (601/3,985) in 2003-2005 (p<0.01) (Table 4).
Table 4.
LDKT demographics for preKT, dialysis < 1 yr, and dialysis ≥ 1 yr for the early and late time periods.
| Recipient Characteristic | 2003-2005 | 2010-2012 | p-value | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Preemptive | Dialysis < 1 yr | Dialysis ≥1 yr | Preemptive | Dialysis < 1 yr | Dialysis ≥ 1 yr | ||
|
| |||||||
| Total LDKT recipients | n=3,358 | n=2,283 | n=5,061 | n=4,808 | n=2,128 | n=5,133 | |
| Age (yrs) | 46 ± 15 | 42± 15 | 45 ± 15 | 49 ± 15 | 44± 17 | 47 ± 15 | <0.001*, <0.001+, <0.001# |
| Gender (% female) | 44% (1,477) | 39% (899) | 41% (2,076) | 40% (1,899) | 35% (749) | 38% (1,927) | <0.001*, 0.004+, <0.001# |
| Race: Caucasian | 77% (2,575) | 68% (1,556) | 52% (2,651) | 77% (3,697) | 67% (1,416) | 52% (2,682) | 0.04*, <0.001+, 0.8# |
| African-American | 10% (333) | 13% (298) | 21% (1,077) | 8% (405) | 10% (213) | 21% (1,087) | |
| Hispanic/Latino | 9% (299) | 13% (304) | 20% (1,004) | 10% (488) | 17% (363) | 20% (1,049) | |
| Other/missing | 5% (151) | 5% (125) | 7% (329) | 5% (218) | 6% (136) | 6% (315) | |
| Payer: Private insurance | 76% (2,553) | 64% (1,470) | 40% (2,006) | 76% (3,638) | 63% (1,331) | 36% (1,850) | 0.15*, 0.8+, <0.001# |
| Medicare | 18% (601) | 35% (808) | 54% (2,712) | 19% (917) | 32% (686) | 58% (2,987) | |
| Other/missing | 6% (204) | 6% (141) | 6% (343) | 5% (253) | 5% (111) | 6% (296) | |
| Diagnosis: Diabetes | 15% (510) | 22% (491) | 23% (1,169) | 15% (737) | 23% (490) | 25% (1,258) | 0.005*, 0.2+, 0.3# |
| Hypertension | 12% (415) | 15% (332) | 22% (1,098) | 14% (654) | 16% (330) | 21% (1,102) | |
| PCKD | 17% (586) | 7% (163) | 5% (271) | 20% (952) | 6% (122) | 6% (288) | |
| GN | 29% (973) | 32% (731) | 27% (1,388) | 28% (1,343) | 31% (668) | 27% (1,402) | |
| Other/missing | 26% (874) | 25% (566) | 22% (1,135) | 23% (1,122) | 24% (518) | 21% (1,083) | |
p-value for statistical difference between values for preemptive patients in early vs late time periods.
p-value for statistical difference between values for dialysis < 1 yr patients in early vs late time periods.
p-value for statistical difference between values for dialysis > 1 yr patients in early vs late time periods.
The degree of sensitization had an impact on the rate of preKT. In patients with a cPRA >80%, 22% (202/914) underwent preKT, compared with 35% (858/2,422) of recipients with a cPRA of 20-80% and 40% (7,185/17,769) of recipients with a cPRA <20% (Table 2).
Pre-transplant dialysis < 1 year recipients
We hypothesized those patients who dialyze only a short time prior to transplantation might undergo preKT if approached differently. Overall, dialysis < 1 year recipients generally were similar to preKT recipients with respect to age, blood group, cPRA, and incidence of diabetes, hypertension, and glomerulonephritis (Table 2). The percentage of dialysis < 1 year recipients that were Caucasian was lower (67%) than preKT (77%), but higher than those dialyzing ≥1 year (52%). While rates of preKT appeared to be similar between African-Americans and Hispanic/Latinos, there was a slightly higher rate of dialysis < 1 year in Hispanic/Latinos recipients. Among all recipients who underwent non-preemptive LDKT, 25% (1,152/4,670) were dialysis < 1 year recipients in Hispanic/Latinos compared with 20% (903/4,558) in African-Americans.
Insurance status appeared to be an important factor not only in avoiding dialysis, but also in the duration of dialysis prior to LDKT. Medicare was the primary payer in 18% of preKT, 31% of dialysis < 1 year recipients, and 56% of patients dialyzing >1 year compared with private insurance in 76% of preKT, 64% of dialysis < 1 year, and 38% of dialysis ≥ 1 year recipients (Table 2).
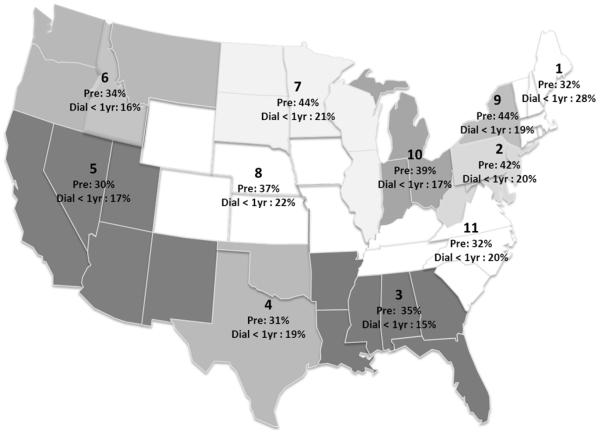
Regional Variation
There was significant variation in the proportion of preKT according to UNOS region (Figure 2), ranging from 30% in Region 5 to 44% in Regions 7 and 9 (p<0.001) during the study period. Regions with a high proportion of LDKT (Regions 7: 44% LDKT/KT and Region 9: 37% LDKT/KT) were the regions identified as having highest proportion of preKT for both LDKT (Figure 2) and all KT (data not shown). However, differences in rates of preKT are likely multifactorial, as Region 1 had one of the lowest rates of preKT (32% preKT for LDKT and 14% preKT for KT overall) despite having one the highest proportions of LDKT (38% LDKT/KT; range for all regions 24-44%) and one of the lowest median waitlist times (715 days for Region 1 compared with median of 1499 days and IQR of 1103-1902 days for all Regions). Some regions with lower rates of preKT had a higher incidence of dialysis < 1 year, such as Region 1 with 32% preKT and 28% dialysis < 1 year (Figure 2).
Figure 2.
Regional variation in rate of preemptive and dialysis < 1 yr living donor kidney transplant recipients.
We found no association between higher proportions of Medicare patients or minorities and Regions with high or low proportions of preKT. Interestingly, the 3 regions with highest proportion of preKT (Regions 2, 7, and 9) had the lowest proportion of diabetics (20%, 13%, and 11%, respectively), and the 4 regions with lowest proportion of preKT (1, 4, 5, and 11) had highest proportions of diabetics (31%, 43%, 50%, and 55%).
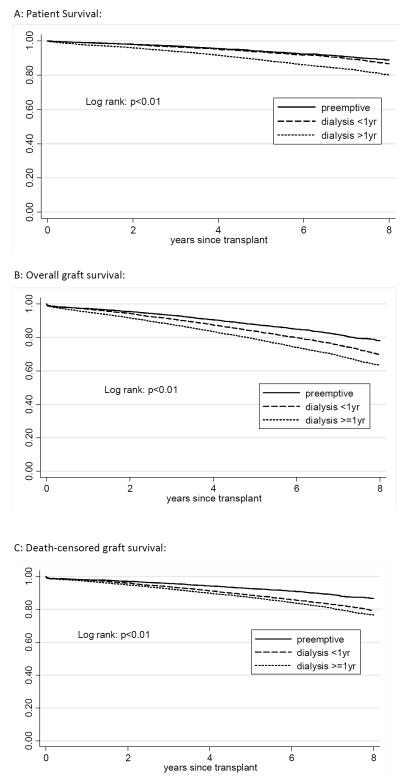
LDKT patient and graft survival
Dialysis <1yr recipients had similar patient survival to preKT with 5-year survival of 94% for preKT and dialysis <1yr compared with 89% for recipients who dialyzed ≥ 1 year (log rank: p<0.01). (Figure 3A) Overall graft survival was significantly different for each group (log rank: p<0.01) (Figure 3B) with 5-year survival of 87% for preKT, 83% for dialysis <1yr, and 79% for dialysis ≥1yr recipients. However when death-censored graft survival was examined separately, dialysis <1yr recipients had significantly inferior graft survival to preKT and similar to recipients who dialyzed ≥1 year (5-year death-censored graft survival: 93% for preKT and 89% for dialysis <1yr and ≥1 year recipients, log rank: p<0.01) (Figure 3C).
Figure 3.
Unadjusted patient, overall graft, and death-censored graft survival after living donor kidney transplant according to preemptive status.
Additionally, we compared survival for preKT, dialysis <1 year, and dialysis ≥ 1 year, adjusting for recipient factors (age at transplant, gender, race, ABO, etiology of renal disease [diabetes, HTN, GN, and PCKD], insurance [medicare vs private insurance], BMI, and cPRA), living donor factors (age, gender, race, and BMI), and transplant factors (CIT and HLA mismatch). For patient survival, preKT had a HR=0.55 (95%CI=0.48-0.64) and dialysis <1 year had a HR=0.65 (95%CI=0.55-0.76) when compared to the reference group of recipients who dialyzed ≥ 1 year. For death-censored graft survival, significant improvements in survival were seen for preKT and dialysis <1 year when compared to recipients who dialyzed ≥ 1 year (preKT: HR=0.61; 95%CI=0.53-0.71 and dialysis <1 year: HR=0.79; 95%CI=0.68-0.90).
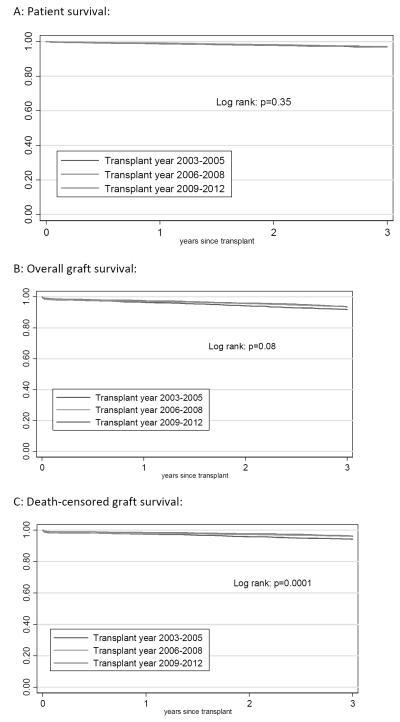
Survival for preemptive LDKT kidney transplant recipients was compared according year of transplant to determine if there were improvements over time. Death-censored graft survival did improve significantly for recipients from the later time period (2009-2012) when compared with the early time period (2003-2005) (log rank: p=0.0001) with 3 year death-censored graft survival of 94% for 2003-2005, 96% for 2006-2008, and 96% for 2009-2012 (Figure 4C). However, patient survival was not significantly different according to transplant year (log rank: p=0.35), and as such there were no significant improvements in overall graft survival according to transplant year (log rank: p=0.08) (Figure 4A and 4B).
Figure 4.
Patient and graft survival for preemptive LDKT transplant recipients according to year of transplant.
DISCUSSION
PreKT remains an unrealized goal for the majority of patients. More than a decade ago, the rate of preKT in the US was 13% overall and 22-25% for LDKT[7, 9]. In this study, we identified that the rate of preKT for the subsequent decade (2003-2012) was 17% overall and 31% for LDKT representing negligible change over the past decade. This small change is cause for concern given the large body of evidence demonstrating improved outcomes with preKT [5, 6, 9, 13] and national initiatives aimed at augmenting preKT[11]. Recent analysis of preemptive DDKT show that rates remain persistently low at 9%[14]. In this study, we focused primarily on patients who eventually underwent LDKT because we believe that this group might be able to undergo preKT if managed differently. The rate of living donor preKT increased from 23% in 2003 to 34% in 2012. However, the majority of patients who eventually received a LDKT still underwent a period of dialysis prior to transplantation—many for more than a year. Moreover, this slightly more than 10% increase in the proportion of preKT for LDKT was during a period of more than 25% growth in the rate of LDKT.
Additionally, we sought to characterize with increased granularity a group of patients who underwent only a brief course of dialysis (< 1 year). We showed that these patients represented one-third of the non-preemptive LDKT highlighting a novel but potentially meaningful target for initiatives directed at augmenting preKT.
Most concerning is the finding that non-medical conditions such the lack of private insurance and race/ethnicity seemed to play major roles in the inability to receive a preKT. Interestingly, these same barriers were identified in studies examining DDKT recipients[14-16]. Medicare as the primary payer for kidney transplantation in the US is a critical factor in promoting preKT. Medicare entitlement begins with initiation of dialysis or two months prior to kidney transplant once the transplant is performed. As such, there can be clear financial disincentives facing transplant centers due to the complexity of covering pre-transplant costs associated with the evaluation of potential living donors and uncertainties regarding coverage for those who ultimately are eligible for Medicaid or Medicare part A only[11]. We acknowledge that disparities in access to healthcare including access to transplant and especially early preKT are impacted by patient’s socioeconomic status based on an abundance of literature on this topic. Accordingly, we did find that patient education level was predictive of preKT. However, we believe that differences in preKT are likely multifactorial. Given that current Medicare policies are not currently structured to incentive preKT, we suggest that this may be a contributing factor to the low rate of preKT in Medicare recipients. The disparity in rates of preKT between Medicare and private insurance patients clearly points out the opportunity cost of current Medicare practices and policies, and given the frequency of Medicare as the primary payer for kidney transplant there is a tremendous opportunity to influence practice through carefully constructed policies aimed at augmenting earlier referral and transplant.
Also, we demonstrated that Hispanics have a higher proportion of dialysis < 1 year among non-preemptive LDKT recipients. Additionally, diabetics had a very low rate of preKT despite the fact that most of these recipients are known to have diabetes for many years and experience a slow progression of associated nephropathy. In contrast, recipients with PCKD who also typically experience a slow progression of renal failure had a high rate of preKT. Given the frequency of diabetes in the US population and evidence demonstrating a survival benefit for this group of patients with preKT[15, 17], efforts should be made to improve earlier referral of diabetics with progressive renal decline.
Additionally, the existence of regional variation could provide interesting avenues of insight into some barriers to augmenting preKT. We showed that these differences were more complicated than simple differences in the utilization of LDKT or median wait times as seen with the low rate of preKT in Region 1. When examining factors related to regional variation, we again saw a disparity faced by diabetics in receiving preKT despite a typically indolent disease course. Further study of this specific group of patients may yield improved insight into the barriers to preKT.
We believe improvements in preKT might be possible in Hispanics, diabetics, and Medicare recipients with improved education and emphasis on early referral to a transplant center. In 2002, the KDOQI guidelines set treatment metrics for the management of chronic kidney disease patients [11]. The guidelines focus primarily on preparing patients with ESRD for dialysis rather than for transplantation. While “fistula first” is a laudable goal, we suggest that there should be a similar emphasis on avoidance of dialysis when possible. Not only would this lead to improved quality of life and life expectancy, but also would result in cost savings. Medicare expenditures are estimated to be greater than $87,000 per year for hemodialysis compared with $33,000 per year after kidney transplant.[18] Similarly, two years after either initiation of dialysis or transplantation, preKT was associated with a 34% decrease in expenditures compared with patients who underwent 12 months of dialysis prior to transplantation.[11] The bulk of costs associated with dialysis are incurred in a 3 month time interval around the initiation of dialysis.[11] Additionally, mortality incidence peaks in the second month after initiation of dialysis.[18] This further highlights the potential tremendous impact of targeting increases in preKT for those patients who underwent a short duration of dialysis prior to transplant. Medicare patients are clearly one group of recipients who currently are disadvantaged from receiving preKT and would benefit from policy reform to address these inequities.
One limitation of this analysis is the possibility of lead time bias in terms of the survival benefit seen with preemptive transplantation. Grams et al identified in their analysis higher eGFRs over time in the recipients of preemptive transplantation.[19] Prior studies have shown no improvement in graft survival with very early preKT (preKT at increased eGFR).[20, 21] We are not advocating for transplanting patients as early as possible while native renal function remains preserved, but rather assembling resources and structuring processes to allow for a “just in time” approach. We do believe the greatly improved patient survival seen in the recipients who dialyzed < 1 year (which more closely mirrored patient survival with preKT and was higher than the survival of those who dialyzed longer) does suggest a true patient benefit of earlier transplant and limiting time on dialysis. This patient survival benefit is likely the source of the allograft survival advantage seen with preKT as supported by prior evidence[22].
Additionally, given the limits of post-transplant complication data reported to UNOS, our analysis has only focused on potential survival benefits and cannot provide meaningful answers to other important concerns that have been raised in relation to preKT. Ojo et al. had previously shown that patients with pre-transplant peritoneal dialysis and those who underwent preKT had higher rates of graft thrombosis.[23] In our analysis, there were no statistically significant differences in graft thrombosis between groups according to preemptive status (preKT=11/333, 3% vs non-preKT 19/1266, 1.5%; p=0.11) or according to dialysis type (peritoneal vs. hemodialysis). This lack of significance could be due to insufficient power given the very small event rate; however, it is beyond the scope of this analysis to determine causality for this most unfortunate outcome. We would suggest that concerns about increased risks of graft thrombosis after preKT could be due to delays in detection of technical problems in patients who still produce urine masking early allograft function. As such at our center, we employ the routine use of postoperative ultrasound to assess adequacy of perfusion and vascular anatomy in immediate postoperative period. It is beyond the scope of this analysis to determine the etiology of graft thrombosis in these patients , whether preKT patients are truly at increased risks, or what preventive measure are needed to address these concerns. Institutional data will be needed to address these important questions.
A second limitation inherent to this type of analysis is that it is based on national registry data that lack granularity and may not include detailed data regarding other possible barriers to preKT. Some patients may encounter medically necessary delays prior to transplantation such as a period of observation to rule out cancer recurrence or to undergo psychiatric or substance abuse treatment, and such circumstances cannot be gleaned from registry data. A more detailed study is needed to determine the actual barriers to preKT. Prior efforts at understanding barriers to preKT have shown that almost half (45%) of patients began their evaluation for transplant prior to initiating dialysis and that 75% of these patients indicated a desire to undergo preKT rather than starting dialysis [10]. In addition, more than 60% of patients were followed by a nephrologist for more than 6 months prior to initiation of dialysis [10, 11]. Given this, targeting earlier initiation and completion of a transplant evaluation could provide a meaningful increase in preKT. This certainly is not the first evidence to suggest the importance of earlier referral and encourage its more widespread practice; however, as we have demonstrated, large changes have not occurred in terms of improving earlier referral. This might suggest the need for increased incentives such as pay for performance measures to augment referral. Currently, Medicare initiatives are aimed at mandating referral once patients are under the care of a dialysis center, but this obviously too late to target improving preemptive transplantation.
While this highlights the focus of physician education and prioritization in improving the rate of preKT, continuing to improve patient education about living donation remains an important objective as well. Weng et al found that more than 50% of patients initially expected to receive a DDKT.[10] Given waiting times exceeding five years in much of the US and longer in some regions, the ability to receive a timely DDKT is a naive goal.
We conclude that while there have been slight improvements in the rates of preKT over the past several years, rates overall remain quite low. The identification of diabetes, Medicare as primary payer, and race/ethnicity as recipient characteristics associated with lower rates of preKT in our study evaluating LDKT and those by others evaluating DDKT highlights how truly disadvantaged these patients are[14].
Further studies focused on the challenges facing Hispanics, diabetics, and Medicare recipients and evaluation of factors behind regional variation are needed to elucidate barriers to preKT. We suggest that changes in Medicare policies combined with early and better education of patients, potential donors, and referring physicians are necessary to ensure wider application of the preemptive kidney transplantation—the ideal treatment for ESRD.
ABBREVIATIONS
- preKT
Preemptive kidney transplantation
- LDKT
Living donor kidney transplantation
- ESRD
End-stage renal disease
- UNOS
United Network of Organ Sharing
- DDKT
Deceased donor kidney transplant
- cPRA
Calculated panel reactive antibody
- HR
Hazard ratios
- OR
Odds ratios
- CI
Confidence intervals
Hazard ratios (HR) and odds ratios (OR) with 95% confidence intervals (CI)
Appendix A. Study cohort
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1.Port FK, et al. Comparison of survival probabilities for dialysis patients vs cadaveric renal transplant recipients. JAMA. 270(11):1339–43. [PubMed] [Google Scholar]
- 2.Schnuelle P, et al. Impact of renal cadaveric transplantation on survival in end-stage renal failure: evidence for reduced mortality risk compared with hemodialysis during long-term follow-up. Journal of the American Society of Nephrology. 9(11):2135–41. doi: 10.1681/ASN.V9112135. [DOI] [PubMed] [Google Scholar]
- 3.Wolfe RA, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. New England Journal of Medicine. 341(23):1725–30. doi: 10.1056/NEJM199912023412303. [DOI] [PubMed] [Google Scholar]
- 4.Friedewald JJ, Reese PP. The Kidney-First Initiative: What is the Current Status of Preemptive Transplantation? Advances in Chronic Kidney Disease. 2012;19(4):252–256. doi: 10.1053/j.ackd.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laupacis A, et al. A study of the quality of life and cost-utility of renal transplantation. Kidney International. 50(1):235–42. doi: 10.1038/ki.1996.307. [DOI] [PubMed] [Google Scholar]
- 6.Meier-Kriesche H-U, Kaplan B. Waiting time on dialysis as the strongest modifiable risk factor for renal transplant outcomes: a paired donor kidney analysis. Transplantation. 74(10):1377–81. doi: 10.1097/00007890-200211270-00005. [DOI] [PubMed] [Google Scholar]
- 7.Mange KC, Joffe MM, Feldman HI. Effect of the Use or Nonuse of Long-Term Dialysis on the Subsequent Survival of Renal Transplants from Living Donors. New England Journal of Medicine. 2001;344(10):726–731. doi: 10.1056/NEJM200103083441004. [DOI] [PubMed] [Google Scholar]
- 8.Mange KC, Joffe MM, Feldman HI. Dialysis prior to living donor kidney transplantation and rates of acute rejection. Nephrology Dialysis Transplantation. 2003;18(1):172–7. doi: 10.1093/ndt/18.1.172. [DOI] [PubMed] [Google Scholar]
- 9.Kasiske BL, et al. Preemptive kidney transplantation: the advantage and the advantaged. Journal of the American Society of Nephrology. 13(5):1358–64. doi: 10.1097/01.asn.0000013295.11876.c9. [DOI] [PubMed] [Google Scholar]
- 10.Weng FL, Mange KC. A comparison of persons who present for preemptive and nonpreemptive kidney transplantation. American Journal of Kidney Diseases. 42(5):1050–7. doi: 10.1016/j.ajkd.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 11.Abecassis M, et al. Kidney transplantation as primary therapy for end-stage renal disease: a National Kidney Foundation/Kidney Disease Outcomes Quality Initiative (NKF/KDOQITM) conference. Clinical Journal of The American Society of Nephrology: CJASN. 2008;3(2):471–80. doi: 10.2215/CJN.05021107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis CL. Preemptive transplantation and the transplant first initiative. Current Opinion in Nephrology and Hypertension. 2010;19:592–7. doi: 10.1097/MNH.0b013e32833e04f5. [DOI] [PubMed] [Google Scholar]
- 13.Innocenti GR, et al. Preemptive living donor kidney transplantation: do the benefits extend to all recipients? Transplantation. 83(2):144–9. doi: 10.1097/01.tp.0000250555.46539.65. [DOI] [PubMed] [Google Scholar]
- 14.Grams ME, et al. Preemptive deceased donor kidney transplantation: considerations of equity and utility. Clinical Journal of The American Society of Nephrology: CJASN. 2013;8(4):575–82. doi: 10.2215/CJN.05310512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Segev DL, et al. Age and comorbidities are effect modifiers of gender disparities in renal transplantation. Journal of the American Society of Nephrology. 2009;20(3):621–628. doi: 10.1681/ASN.2008060591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Held PJ, et al. Access to kidney transplantation. Has the United States eliminated income and racial differences. Archives of Internal Medicine. 1988;148(12):2594–2600. doi: 10.1001/archinte.148.12.2594. [DOI] [PubMed] [Google Scholar]
- 17.Becker BN, et al. Preemptive transplantation for patients with diabetes-related kidney disease. Archives of Internal Medicine. 2006;166(1):44–8. doi: 10.1001/archinte.166.1.44. [DOI] [PubMed] [Google Scholar]
- 18.System, U.R.D. USRDS 2013 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2013. pp. 281–290. [Google Scholar]
- 19.Grams ME, et al. Trends in the timing of Preemptive Kidney Transplantation. Journal of the American Society of Nephrology. 2011;22(9):1615–1620. doi: 10.1681/ASN.2011010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akkina SK, et al. Earlier is not necessarily better in preemptive kidney transplantation. American Journal of Transplantation. 2008;8(10):2071–6. doi: 10.1111/j.1600-6143.2008.02381.x. [DOI] [PubMed] [Google Scholar]
- 21.Ishani A, et al. The impact of residual renal function on graft and patient survival rates in recipients of preemptive renal transplants. American Journal of Kidney Diseases. 2003;42(6):1275–82. doi: 10.1053/j.ajkd.2003.08.030. [DOI] [PubMed] [Google Scholar]
- 22.Gill JS, et al. Why do preemptive kidney transplant recipients have an allograft survival advantage? Transplantation. 78(6):873–9. doi: 10.1097/01.tp.0000130204.80781.68. [DOI] [PubMed] [Google Scholar]
- 23.Ojo AO, et al. Dialysis modality and the risk of allograft thrombosis in adult renal transplant recipients. Kidney International. 1999;55(5):1952–60. doi: 10.1046/j.1523-1755.1999.00435.x. [DOI] [PubMed] [Google Scholar]