ABSTRACT
Purpose: Transforming growth factor beta 1 (TGF-β1) is a cytokine involved in a variety of processes, such as differentiation of fibroblasts into myofibroblasts. TGF-β1 has also been shown to delay the internalization of the neurokinin-1 receptor (NK-1 R) after its activation by its ligand, the neuropeptide substance P (SP). NK-1 R comprises two naturally occurring variants, a full-length and a truncated form, triggering different cellular responses. SP has been shown to affect important events in the cornea – such as stimulating epithelial cell proliferation – processes that are involved in corneal wound healing and thus in maintaining the transparency of the corneal stroma. An impaired signaling through NK-1 R could thus impact the visual quality. We hypothesize that TGF-β1 modulates the expression pattern of NK-1 R in human corneal stroma cells, keratocytes. The purpose of this study was to test that hypothesis.
Methods: Cultures of primary keratocytes were set up with cells derived from healthy human corneas, obtained from donated transplantation graft leftovers, and characterized by immunocytochemistry and Western blot. Immunocytochemistry for TGF-β receptors and NK-1 R was performed. Gene expression was assessed with real-time polymerase chain reaction (qPCR).
Results: Expression of TGF-β receptors was confirmed in keratocytes in vitro. Treating the cells with TGF-β1 significantly reduced the gene expression of NK-1 R. Furthermore, immunocytochemistry for NK-1 R demonstrated that it is specifically the expression of the full-length isotype of the receptor that is reduced after treatment with TGF-β1, which was also confirmed with qPCR using a specific probe for the full-length receptor.
Conclusions: TGF-β1 down-regulates the gene expression of the full-length variant of NK-1 R in human keratocytes, which might impact its signaling pathway and thus explain the known delay in internalization after activation by SP seen with TGF-β1 treatment.
KEYWORDS: Cornea, cytokines, neuropeptides, stroma, substance P
Introduction
Eye trauma that results in corneal ulceration is an important cause of visual impairment and is estimated to lead to 1.5–2 million new cases of unilateral blindness every year. 1 Maintaining a transparent cornea is of utmost importance after injury, infection, or penetrating transplant surgery, all of which can otherwise lead to formation of opaque corneal tissue, i.e., scarring. The corneal stromal transparency is based on tightly regulated and highly organized collagen fibers. 2 Certain proteoglycans, such as lumican 3 and keratocan, 4 are responsible for maintaining the highly controlled collagen organization. These proteoglycans are normally produced by keratocytes, the primary resident cells of the stroma, which also produce the extracellular matrix (ECM) surrounding them. After an injury, keratocytes can be activated by cytokines in the tear fluid, 5 or by factors produced by the corneal epithelium, 6 like transforming growth factor Beta (TGF-β).
TGF-β is a cytokine comprising three isoforms in mammals, TGF-β1, TGF-β2, and TGF-β3, displaying different and sometimes opposing effects during corneal wound healing. 7 TGF-β1 and TGF-β3 bind to the high affinity type II receptor TGFBR2. This association subsequently triggers the dimerization of TGFBR2 with the low affinity type I receptor TGFBR1.8, 9 TGF-β2 requires the intervention of the type III receptor betaglycan to present the ligand to TGFBR2.10, 11 TGF-β is a cytokine which is restricted to the epithelium in healthy corneas but which upon injury is released into the stroma, 12 where it is involved in cell proliferation, migration, and differentiation. 13 TGF-β1 triggers transformation of quiescent keratocytes into myofibroblasts, contractile cells expressing alpha smooth muscle actin (αSMA) and producing ECM components including collagen. 14 The newly produced collagen fibrils are disorganized and result in corneal haze which often disappears after myofibroblasts apoptosis.
TGF-β has also been shown to delay internalization of the neurokinin-1 receptor (NK-1 R) after its activation by its ligand, the neuropeptide substance P (SP), in T lymphocytes. 15 NK-1 R is encoded by the TACR1 gene which gives rise to two variants, a full-length variant comprising 407 amino acids, and a truncated form resulting from a splicing, lacking the last 96 amino acids of the intracellular C-terminal region. 16 Contrary to the truncated receptor, the full-length variant has a high affinity for SP and induces a rapid activation of the extracellular-signal-regulated kinases (ERK). 17
Both SP and NK-1 R are expressed by human corneal epithelial cells and keratocytes in the stroma. 18 SP is an 11 amino acid pro-inflammatory neuropeptide encoded by the preprotachykinin-A (PPT-A) gene and is expressed by neuronal as well as non-neuronal cell. 18 – 20 It enhances cells proliferation and reduces or delays apoptosis in vitro and in vivo 21 in an Akt-dependent manner.22, 23 In an alkali burn rabbit model, exogenously administered SP enhanced the healing of the corneal epithelium and reduced the bleeding. 24 Furthermore, SP regulates the expression of some matrix components such as collagen type I and III in explants of ligaments. 25 An impaired signaling through NK-1 R could thus disturb the corneal ECM and impact visual quality.
In the present work, we studied whether TGF-β1 could impact the expression pattern of the NK-1 R in human keratocytes in vitro.
Materials and methods
Isolation and primary culture of human keratocytes
Healthy human corneal material was obtained from the corneal biobank at the University Hospital of Umeå, either as whole corneas that were not used for transplantation by lack of matched recipient, or from transplantation graft leftovers from surgery. The tissue, which is tested according to Swedish law for infectious transmitted diseases like HIV etc, is derived from deceased individuals who had chosen, when alive, to donate their corneas post-mortem for transplantation and research. In case of suspicion of disease or abnormal cell count by the biobank, the corneas were discarded. Both central and peripheral cells were used in the experiments. Healthy donors used in this study comprise a male/female ratio of 1/6 with average age of 65.0 and 66.3 (range: 37–90), respectively. The study was vetted by the Regional Ethical Review Board in Umeå (2010-373-31M) without objections. The study was performed according to the principles of the Declaration of Helsinki.
Samples were scraped using a sterile scalpel to remove any remaining epithelial or endothelial cells before being washed in sterile Hanks’ Balanced Salt Solution (HBSS; Invitrogen™, CA, USA, # 14170). The remaining stroma was then minced with a scalpel and digested in collagenase (Clostridopeptidase A, Sigma-Aldrich®, Stockholm, Sweden, # C-1030) diluted in Dulbecco’s Modified Eagle Medium: Nutrient Mixture F-12 (DMEM/F-12, Fisher Scientific, Göteborg, Sweden, # 21331-046) to a concentration of 2 mg/ml for 30 min at 37°C. The digestion product was then transferred to a culture dish containing DMEM/F-12 supplemented with 2% fetal bovine serum (FBS; Invitrogen™, CA, USA, # 16000), 1% penicillin-streptomycin (Invitrogen™, CA, USA, # 15410), and 0.2% L-Glutamine (Invitrogen™, CA, USA, # 25030) and cultured at 37°C in a humidified atmosphere supplemented with 5% CO2. Medium was replaced every second to third day until the cells reached confluence. Confluent cells were detached with 0.05% trypsin–EDTA (Invitrogen™, CA, USA, # 25300) and split in a 1:3 ratio. Cells from passage three to four were used for experiments.
Immunocytochemistry
104 cells per well were seeded in eight well chamber slides (BD Falcon™, Bedford, MA, USA, # 354118) overnight before being fixed in 2% paraformaldehyde (PFA) diluted in 0.1 M phosphate buffered saline (PBS) (pH 7.4) for 10 min. Fixed cells were washed repeatedly in PBS, then blocked with 1:20 diluted normal serum (Table 1) corresponding to the host type of the secondary antibody for 15 min. After carefully disposing of the serum, cells were incubated with the primary antibody (Table 2) overnight at 4°C. Washing and blocking were repeated and secondary antibody (Table 3) was added for 30 min at 37°C. Cells were washed and mounted in Vectashield mounting medium for fluorescence (Vector Laboratories, Burlingam, CA, USA, # H-1500). A control well was also prepared for each secondary antibody by replacing the primary antibody with PBS. A Zeiss Axioskop 2 plus microscope equipped with epifluorescence and an Olympus DP70 digital camera were used for analysis.
Table 1.
Normal serum used for immunocytochemistry.
| Serum | Code | Source | Dilution |
|---|---|---|---|
| Donkey | 017-000-121 | Jackson I.R., West Grove, PA, USA | 1:20 |
| Rabbit | X0902 | Dako, Glostrup, Denmark | 1:20 |
| Swine | 014-000-121 | Jackson I.R., West Grove, PA, USA | 1:20 |
Table 2.
Primary antibodies used for immunocytochemistry.
| Antigen | Code | Source | Type | Dilution |
|---|---|---|---|---|
| CD34 | sc-9095 | Santa Cruz Biotechnology®, Dallas, TX, USA | Rabbit | 1:50 |
| ALDH | ab52492 | Abcam®, Cambridge, UK | Rabbit | 1:100 |
| Keratocan | sc-66941 | Santa Cruz Biotechnology®, Dallas, TX, USA | Rabbit | 1:100 |
| Lumican | sc-166871 | Santa Cruz Biotechnology®, Dallas, TX, USA | Mouse | 1:50 |
| TGFBR1 | ab155258 | Abcam®, Cambridge, UK | Rabbit | 1:100 |
| TGFBR2 | ab78419 | Abcam®, Cambridge, UK | Mouse | 1:50 |
| TGFBR3 | ab166705 | Abcam®, Cambridge, UK | Mouse | 1:100 |
| NK-1R interal loop | sc-5220 | Santa Cruz Biotechnology®, Dallas, TX, USA | Goat | 1:100 |
| NK-1R C-terminal | s8305 | Sigma St Louis, MO, USA | Rabbit | 1:100 |
Table 3.
Secondary antibodies used for immunocytochemistry.
| Secondary antibody | Code | Source | Dilution |
|---|---|---|---|
| Alexa Fluor 488, donkey anti-goat | A-11055 | Invitrogen, Carlsbad, CA, USA | 1:300 |
| TRITC-conjugated rabbit anti-mouse | R0270 | Dako, Glostrup, Denmark | 1:20 |
| TRITC-conjugated swine anti-rabbit | R0156 | Dako, Glostrup, Denmark | 1:40 |
Stimulation of human keratocytes
2.5 × 105 cells per well in six well plates (Sarstedt, Newton, NC, USA, # 83.1839) were cultivated and kept in serum-reduced medium (containing 0.1% of FBS) for 24 h. Then, cells received either 10 ng/ml of GW 788388 (Tocris bioscience, Bristol, UK, # 3264), a selective inhibitor of TGF-β1 receptor, or the equivalent volume of medium. 20 min later, cells received either 10 ng/ml of human recombinant TGF-β1 (R&D system, Abingdon, UK, # P01137) or the equivalent volume of medium. Cells were then incubated 24, 48, or 72 h at 37°C in a humidified atmosphere supplemented with 5% CO2 before being lysed.
RNA extraction and quantitative real-time polymerase chain reaction (qPCR)
Isolation of total RNA was performed with an RNeasy kit (Qiagen, Sollentuna, Sweden, # 74106) following the manufacturer’s instructions on 2.5 × 105 cells per well. Isolated RNA was converted to cDNA using a high capacity cDNA reverse transcription kit (Applied Biosystems®, # 4368813) following the manufacturer’s instruction. Conversion was performed on an Eppendorf Mastercycler EP Gradient S (VWR International, Spånga, Stockholm, Sweden), using the following protocol: 10 min at 25°C followed by 120 min at 37°C and 5 min at 85°C. Quantitative PCR (qPRC) was performed using TaqMan fast universal PCR master mix (Life technology, Stockholm, Sweden, # 4352042) and NK-1 R probes, of which the first (TACR1, Life technology, Stockholm, Sweden, # Hs00185530-m1) targets both forms of the receptor and the second (TACR1, Life technology, Stockholm, Sweden, # Hs01025732-m1) is specific for the full-length variant, αSMA probe (ACTA2, Life technology, Stockholm, Sweden, # Hs00909449_m1), and housekeeping gene probes, human beta actin (ACTB) (Life technology, Stockholm, Sweden, # 4352935) and 18 s rRNA Oligo Mix (Life technology, Stockholm, Sweden, # 4332641). Samples were run on a ViiA 7 Real-Time PCR System (Life technology, Stockholm, Sweden) using the following settings: 20 s of denaturation at 95°C followed by 40 cycles of 95°C for 1 s and 60°C for 20 s. All samples were run in technical duplicates and the mean value was used for analyses.
Protein extraction and western blot
2.5 × 105 cells per well in six well plates (Sarstedt, Newton, NC, USA, # 83.1839) were cultivated in DMEM/F-12 medium containing either 0.1% or 2% FBS. After 24 h of culture, cells were washed in ice-cold PBS and lysed in Radio-immunoprecipitation assay buffer supplemented with 0.5% of protease inhibitor cocktail (Sigma, St. Louis, MO, USA # P1860). Samples were then diluted in Laemmli Sample buffer supplemented with 2-mercaptoethanol. Proteins were separated by gel electrophoresis under denaturing conditions and transferred to PVDF membranes. Blots were incubated with primary antibodies (Table 4) overnight at 4°C. Blots were then washed and incubated with horseradish peroxydase-conjugated secondary antibodies (Table 5) for 1 h at room temperature, washed again, and incubated with chemiluminescent reagents (Amersham Pharmacia Biotech INC, Piscataway, NJ, USA, # RPN2232). Images were taken by Odyssey® Fc imaging system (LI-COR, Lincoln, NE, USA). Densitometry was performed using Image J analysis software (NIH).
Table 4.
Primary antibodies used for Western blot.
| Antigen | Code | Source | Type | Dilution |
|---|---|---|---|---|
| CD34 | sc-9095 | Santa Cruz Biotechnology®, Dallas, TX, USA | Rabbit | 1:200 |
| ALDH | ab52492 | Abcam®, Cambridge, UK | Rabbit | 1:500 |
| Keratocan | sc-66941 | Santa Cruz Biotechnology®, Dallas, TX, USA | Rabbit | 1:200 |
| Lumican | sc-166871 | Santa Cruz Biotechnology®, Dallas, TX, USA | Mouse | 1:200 |
| Procollagen I | ab155258 | Santa Cruz Biotechnology®, Dallas, TX, USA | Goat | 1:100 |
Table 5.
Secondary antibodies used for Western blot.
| Secondary antibody | Code | Source | Dilution |
|---|---|---|---|
| Anti-rabbit IgG HRP-linked | 7074 | Cell Signaling, Danvers, MA, USA | 1:2000 |
| Anti-mouse IgG HRP-linked | 7076 | Cell Signaling, Danvers, MA, USA | 1:2000 |
| Anti-goat IgG HRP-linked | Sc-2020 | Santa Cruz Biotechnology®, Dallas, TX, USA | 1:2000 |
Statistical analysis
Data represent means ± standard deviation (SD) of three replicates. All experiments were performed at least three times with successfully repeated results. GraphPad Prism 5 software was used for data analysis. Statistical significance was determined using one-way ANOVA followed by Bonferroni post-hoc comparison test for the qPCR (more than two groups) or t-test for the Western blot densitometry analysis (two groups). Statistical significance was predetermined at p < 0.05.
Results
Characterization of isolated cells from donor cornea
In this study, cells were cultured in medium supplemented with 2% FBS, then switched to a medium containing a low amount of FBS, 0.1%, 24 h prior to stimulations. However, keratocytes are considered to be quiescent cells in the cornea and FBS is known to activate them into fibroblasts. Cells were therefore studied for the presence of keratocyte markers after 24 h of culture in medium supplemented with 2% or 0.1% FBS by immunocytochemistry and Western blot. CD34 is now considered as a well-established marker of keratocytes in vivo and its expression decreases upon cell activation. 26 Our cells showed a weak but positive staining for CD34, with only a few cells positively stained in 0.1% FBS (Figure 1A), but all cells in the 2% FBS condition (Figure 1B). Western blot analysis confirmed the expression of CD34 in both culture conditions, however densitometry analysis did not show any significant difference between the two conditions (Figure 1I). Aldehyde dehydrogenase (ALDH) is a crystallin expressed by keratocytes to help maintain corneal transparency, and its expression decreases when keratocytes turn into repair keratocytes or fibroblasts. 27 In this study, no ALDH was observed by immunocytochemistry in cells cultured in 0.1% FBS (Figure 1C) and very few cells displayed a weak positive staining for ALDH in the 2% FBS culture condition (Figure 1D). Bands were however visible on Western blot analysis for both conditions, with a significantly higher expression in cells cultured in medium supplemented with 2% FBS (Figure 1J). Keratocan and lumican are the two most commonly used markers of keratocytes and their expression decreases as well as the cells get activated.28, 29 All cells stained strongly positive for keratocan in 0.1% (Figure 1E) and 2% (Figure 1F) FBS conditions and Western blot densitometry analysis showed a significantly higher expression of keratocan in cells cultured in 2% FBS than in cells cultured in 0.1% FBS (Figure 1K). The opposite was observed for lumican, for which expression was significantly higher in cells cultured in 0.1% FBS as compared to cells cultured in 2% FBS (Figure 1L). No positive staining for lumican was observed by immunocytochemistry (Figure 1G and H) though strong bands were seen by Western blot. As keratocytes are cells that produce the corneal ECM, cells capacity to produce collagen was assessed by Western blot through expression of pro collagen I. A strong band could be observed in both cell culture conditions (Figure 1M), confirming that the cells produce ECM. Pro collagen I expression was significantly higher in cells cultured in 2% FBS condition than cells cultured in medium supplemented with 0.1% FBS.
Figure 1.
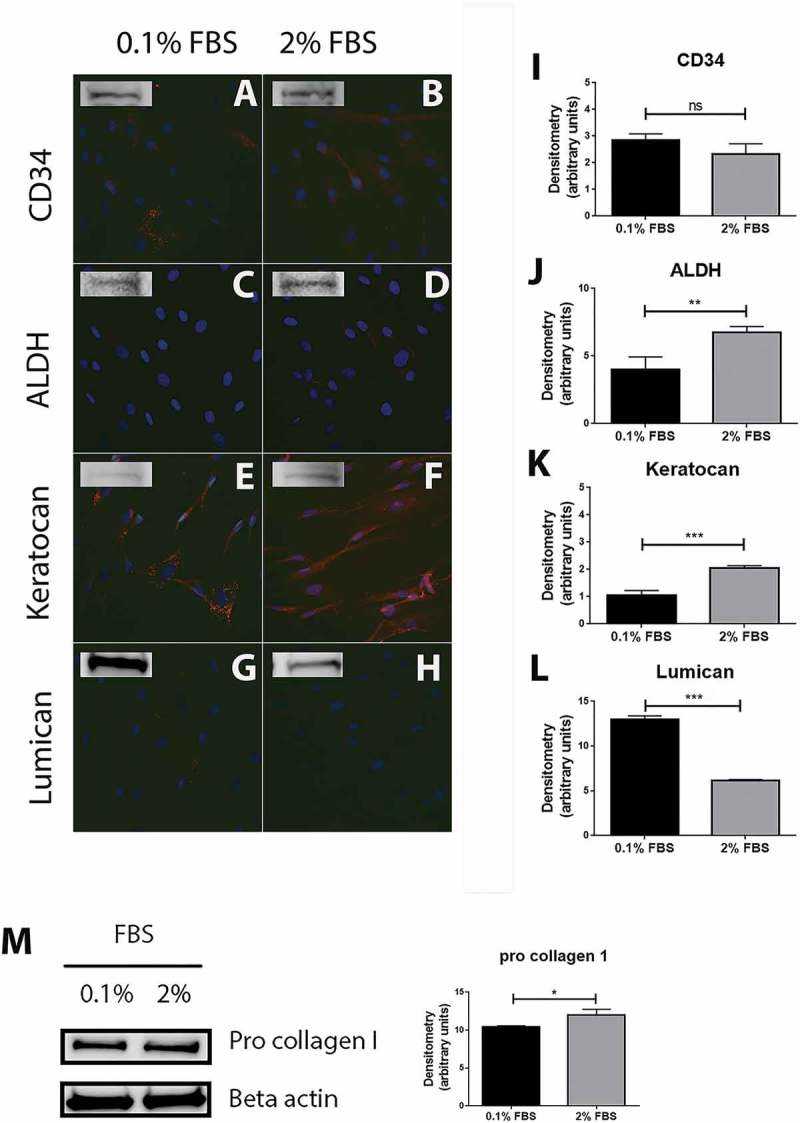
Characterization of cells extracted from healthy human cornea. Immunocytochemistry of the cells extracted from healthy human cornea after 24 h of culture in DMEM medium supplemented with either 0.1% or 2% fetal bovine serum (FBS) for 24 h shows that several cells were weakly stained for CD34 in 0.1% FBS condition (A) whereas the majority of cells showed a weak but positive staining in 2% FBS (B). ALDH (C and D) and lumican (G and H) stainings showed almost no positive reactions in both culture conditions. Keratocan was highly expressed in both 0.1% FBS (E) and 2% FBS (F) culture conditions. Western blot experiments were also performed on the cells and showed that all the markers were expressed by the cells in both culture conditions. Densitometry analysis further revealed no statistically significant (ns) difference in expression of CD34 between the cells cultured in 0.1% and 2% FBS (I). ALDH (J) and keratocan (K) had a significantly higher expression in cells cultured in 2% FBS than in 0.1% FBS (**p < 0.01 and ***p < 0.001, respectively). On the contrary, cells expressed significantly more lumican (L) in the 0.1% FBS than in 2% FBS culture conditions (***p < 0.001). In addition, cells strongly expressed pro collagen I in both culture conditions, with a significantly higher expression in medium supplemented with 2% FBS (*p < 0.05) (M). Values are means ± SD.
TGF-β1 down-regulates NK-1 R gene expression in keratocytes
Human keratocytes were studied for the presence of TGF-β receptors. Immunocytochemistry showed that TGFBR1, TGFBR2, and TGFBR3 were highly expressed by all cells with a slightly higher expression of TGFBR1 and TGFBR3 in cells cultured in medium supplemented with 2% FBS (Figure 2B and F) as compared to cells cultured in 0.1% FBS medium (Figure 2A and E). Culture condition did not impact TGFBR2 expression (Figure 2C and D). To determine the effect of TFG-β receptor activation on keratocytes, cells were stimulated with a recombinant human TGF-β1 for 24, 48, or 72 h. mRNA levels of αSMA, a marker of corneal fibroblast activation into myofibroblasts, 30 and of NK-1 R were subsequently determined by qPCR. TGF-β1 significantly increased the expression of αSMA (Figure 2G) after 24 h, which was expected, as TGF-β is known to induce myofibroblasts. This verifies the stimulation efficiency. Furthermore, qPCR showed that TGF-β1 stimulation significantly decreased the gene expression of NK-1 R (Figure 2H). This decrease was observed at all three time points studied. However, pre-incubation of the cells with GW 788388, an inhibitor of TGF receptor I, did not significantly attenuate the NK-1 R gene expression decrease induced by TGF-β1 stimulation.
Figure 2.
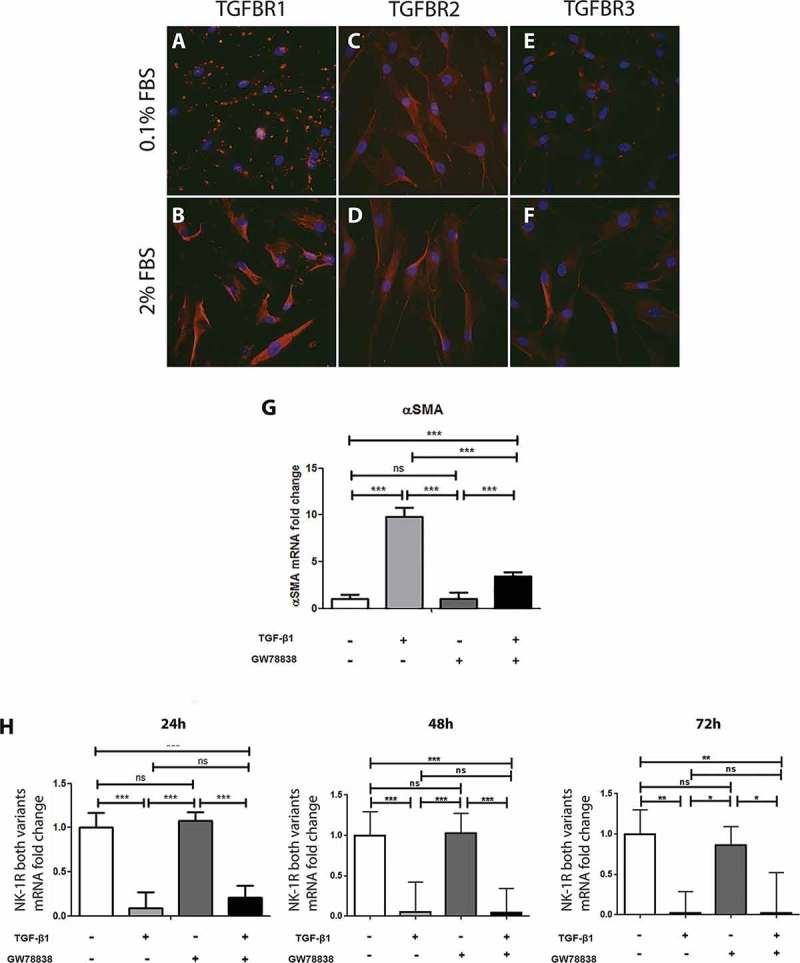
TGF-β stimulation of human keratocytes in vitro. Immunocytochemistry of human keratocytes in vitro revealed the presence of TGF-β receptor I (TGFBR1; red) in all cells cultured in medium supplemented with 0.1% FBS (A) and 2% FBS (B). TGF-β receptor II (TGFBR2; red) was also strongly positive in all cells in the 0.1% (C) and 2% (D) FBS culture conditions. TGF-β receptor III (TGFBR3) showed a more intense staining in the 2% FBS culture (F) than in the 0.1% FBS condition (E). All slides were double stained with DAPI (blue) to identify cell nuclei. ((G) and (H)) Cells were treated with TGF-β1 (10 ng/ml), the TGF receptor I inhibitor GW788388 (10 ng/ml) or both, 24, 48, or 72 h prior to RNA extraction and conversion to cDNA. qPCR showed an increase of αSMA mRNA levels (G) in TGF-β1 treated cells compared to untreated cells 24 h after stimulation. GW 788388 significantly reduced the effect of TGF-β1. For the NK-1 receptor, mRNA levels decreased significantly in TGF-β1 treated cells as compared to untreated (***p < 0.001 at 24 and 48 h, **p < 0.01 at 72 h). GW 788388 slightly reduced the effect of TGF-β1 at 24 h but not significantly (ns). Values are means ± SD.
TGF-β1 specifically down-regulates the expression of the full-length isoform of NK-1 R
To further elucidate whether it is one particular or both isoforms of NK-1 R that are down-regulated by TGF-β1, cells were stimulated with TGF-β1 for 24 h, then fixed and stained with two antibodies targeting different regions of the receptor. One antibody (# sc-5220) recognizes an inner-loop, thus identifying both isoforms. The other one (# s8305) targets the C-terminal region and is therefore specific of the full-length NK-1 R. In untreated keratocytes, both the internal loop (Figure 3A) and the C-terminal region (Figure 3C) were immunopositive. Overlap image (Figure 2E) revealed that both the full-length receptor (recognized by both antibodies) and the truncated receptor (recognized by only one of the antibodies) were expressed. On the TGF-β1 treated keratocytes, however, the truncated receptor was still expressed (Figure 3B) but the full-length form showed almost no positive reaction (Figure 3D), which is also seen in the overlap figure (Figure 3F) in which no more structures identified by both antibodies are visible. TGF-β1 thus seems to down-regulate only the full-length receptor, without impacting the truncated isoform of NK-1 R. The specific down-regulation of the full-length receptor was furthermore confirmed by qPCR using a probe (# Hs01025732-m1) that is specific to the mRNA of the full-length isotype (Figure 3G). Results also showed that the full-length isotype decreased expression was maintained 48 h after stimulation, and still showed the same trend, although not statistically significant, at 72 h.
Figure 3.
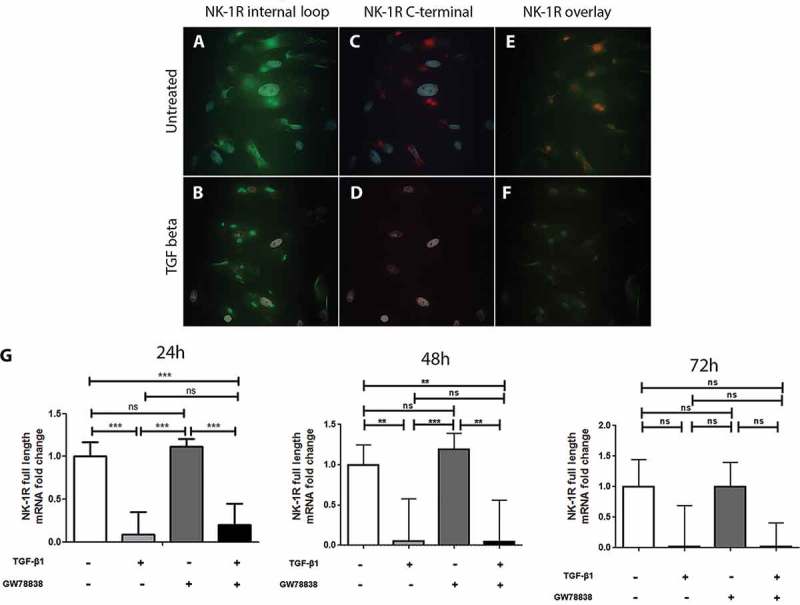
TGF-β1 down-regulates the expression of the full-length but not of the truncated form of NK-1 R in human keratocytes in vitro. Immunocytochemistry of the untreated ((A), (C), and, (E)) or TGF-β1 treated ((B), (D), and (F)) human keratocytes in vitro shows that untreated cells display positive reactions for the antibody targeting the inner-loop of NK-1 R (green) (A) as well as for the antibody targeting the C-terminal of the receptor (red) (C). Both antibodies overlap (yellow) but some green staining is still visible, indicating that both forms are expressed (E). The reactions of the inner-loop antibody are still present in the TGF-β1 treated cells (B), but the full-length receptor is not expressed anymore (D), as is confirmed by the overlapping image in which no yellow color can be seen (F). DAPI (blue) was applied to identify nuclei. (G) Quantitative real-time PCR, using a probe that specifically targets the full-length form of the NK-1 receptor further confirms the down-regulation of this isoform of the receptor after TGF-β1 treatment (***p < 0.001 at 24 h, **p < 0.01 at 48 h, not significantly (ns) at 72 h). The TGF receptor I inhibitor GW 788388 reduces the effect of TGF-β1 at 24 h, but this was not significant (ns). Values are means ± SD.
Discussion
Soon after an injury occurs, quiescent keratocytes get activated, stop expressing lumican and keratocan, start expressing stress fibers due to changes in the ECM tension, 31 and finally express αSMA, a marker of myofibroblasts.32, 33 The generated myofibroblasts produce less crystallins, which decrease the transparency of the corneal stroma, 34 and produce high amounts of ECM components, 14 leading to haze in some cases. 35 Key regulators of the wound healing response are likely to be constitutively produced in order to be quickly available, 36 like TGF-β which is produced as a precursor containing the latency-associated protein and the latent TGF-binding protein, anchoring it to the ECM until a proteolytic cleavage converts it into its mature form. 37 TGF-β binds to type IV collagen of the basement membranes of the epithelium with a high affinity 34 and it has been noted that the expression of fibrotic markers is only observed if Bowman’s layer is disrupted 37 thereby allowing TGF-β release in the stroma. Therefore, TGF-β plays a central role in corneal wound healing as it is responsible for myofibroblast transformation, and topical application of blocking antibodies to TGF-β prevents corneal fibrosis in a rabbit model. 38
In our study, we extracted stromal cells from healthy human corneas. As the culture medium used was supplemented with FBS, which is known to change keratocytes phenotype, 39 we investigated the expression of several keratocyte markers by immunocytochemistry and Western blot. CD34, a glycosylated transmembrane protein that is expressed by keratocytes in vivo, is lost when cells are cultured in vitro on plastic with serum-containing medium. 40 In our experiments, cells cultured in 0.1% FBS showed no positive staining for CD34, and cells in 2% FBS only showed a weak staining but on all cells. However, clear bands were observed in both culture conditions by Western blot. The difference is likely due to a less efficient antibody in immunofluorescence. The same was observed for ALDH and lumican, two other keratocyte markers that are decreased in activated cells,27, 41 with weak or absence of staining by immunofluorescence but bands were seen in Western blot. Keratocan, a crystallin expressed by keratocytes, showed a strong staining in both culture conditions as well as bands on Western blot. Surprisingly, keratocan had a significantly higher expression in cells cultured with more FBS. Cells also strongly expressed pro collagen I, showing their ability to produce ECM components. In summary, the extracted cells showed characteristic markers of keratocytes despite the presence of FBS in the medium, and it seems that switching to a concentration of 0.1% FBS 24 h before stimulation slightly increases the expression of two out of four markers. Most of the studies comparing the effect of FBS on cultured keratocytes used 10% versus 0% FBS,42– 45 five times more FBS than we used in our experiments. 46 This might explain why our cells still express some keratocyte markers despite the presence of serum, even though those levels are low.
In our study, we assessed the presence of TGF-β receptor I, II, and III at the cell surface of human keratocytes in vitro by immunocytochemistry. The cells were then stimulated with human recombinant TGF-β1 which resulted in an increase in αSMA mRNA levels. This result showed that stimulation was effective, as it is known that TGF-β activates fibroblasts into myofibroblast.47, 48 Myofibroblasts generation also correlated to a drop in NK-1 R gene expression, and this decrease remained up to 72 h after addition of TGF-β1. Upon TGF-β binding to the high affinity TGFBR2, the receptor transphosphorylates TGFBR1 and associates with it in a complex containing two type II and two type I receptors. TGFBRI subsequently phosphorylates downstream kinases in the cell. 49 – 51 Addition of TGFBRI blocker significantly attenuated TGF-β1 enhanced α-SMA expression in our study, as compared to TGF-β1 alone. However, it did not significantly prevent this decrease in NK-1 R expression. One explanation could be that the efficient dose of the blocker used to attenuate α-SMA expression is not sufficient to prevent NK-1 R expression decrease.
NK-1 R has two naturally occurring variants, a full-length form comprising 407 amino acid residues and a truncated form lacking 96 amino acids from the C-terminal domain. The full-length receptor is mostly expressed in the human brain whereas the truncated form is found in the central nervous system as well as in peripheral tissues. 52 SP binds to both isoforms, and such binding induces responses by both receptors although through different pathways. The full-length receptor activation leads to a transient increase in intracellular calcium, activation of nuclear factor kappa B (NFκB), production of interleukin 8 (IL-8), phosphorylation of protein kinase C delta (PKCδ), and fast activation of the ERK (1–2 min). Activation of the truncated isoform induces a decrease in IL-8, inhibition of PKCδ phosphorylation, and a later peak in ERK activation, around 20–30 min. 17 It has also previously been shown that exogenously added TGF-β delays NK-1 R internalization after SP binding. 15 Among the demonstrated effects of SP, some could be of importance during corneal wound healing, such as CD29+ cell recruitment, 24 cell proliferation, 53 anti-apoptotic agent,22, 23 cell migration, 54 and angiogenesis. 55
In our study, we investigated which forms of NK-1 R are expressed by human keratocytes and which forms are down-regulated following TGF-β1 stimulation. We found that both receptor variants were expressed by the cells, but only the full-length receptor was regulated by TGF-β1. Other studies have also shown differences in the regulation of NK-1 R isoforms during cell differentiation. 56 For example, mRNA expression of NK-1 R truncated form is higher in patients with high grade dysplasia than in patients with carcinoma. 57 Also, undifferentiated monocyte/macrophage THP-1 cells only express NK-1 R truncated form, but start expressing the full-length after phorbol myristate acetate (PMA)-differentiation. 58 These regulations imply that the cells can switch the expression of NK-1 R isoforms when they differentiate. In our experiments, TGF-β stimulated cells differentiated into αSMA-positive myofibroblasts, and then almost exclusively expressed the receptor’s truncated variant. The truncated NK-1R has a 10 times lower affinity to SP as compared to the full-length, and it is believed that the lack in the C-terminal domain results in the stimulation of the truncated isoform leading to a prolonged cellular responsiveness and reduced homologous desensitization, as compared to stimulation of the full-length receptor. 59 Further studies will be necessary to investigate what is the benefit for myofibroblasts to down-regulate the expression of the full-length and keep only the truncated NK-1 R.
To conclude, human keratocytes express both variants of the neurokinin-1 receptor. After a TGF-β1-mediated differentiation to myofibroblasts, the cells keep expressing the truncated variant of the receptor, but the full-length expression is highly decreased.
Acknowlegments
The authors thank Mr. Peter Boman for technical assistance and scientific advice. We also thank Dr. Maria Brohlin and Ms. Randi Elstad for help in providing the donated corneas from the biobank, as well as to all the OR-staff at the Ophthalmic Surgery Clinic, especially Dr. Berit Byström, of the University Hospital of Umeå for deliverance of graft leftovers.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Financial support was obtained by P.D. from the national Swedish Research Council (grant no. 521-2013-2612), the J.C. Kempe and Seth M. Kempe Memorial Foundations (for S.L.), the Swedish Society of Medicine, the Cronqvist foundation, the foundation Kronprinsessan Margaretas Arbetsnämnd för synskadade (KMA), the foundation Ögonfonden, and Västerbotten County Council (Spjutspetsmedel). Financial support was furthermore provided to P.D. through a regional agreement between Umeå University and Västerbotten County Council (ALF).
References
- Whitcher JP, Srinivasan M, Upadhyay MP. Corneal blindness: a global perspective. Bull World Health Organ. 2001;79(3):214–221. [PMC free article] [PubMed] [Google Scholar]
- Bron AJ. The architecture of the corneal stroma. Br J Ophthalmol. 2001 Apr 1;85(4):379–381. doi: 10.1136/bjo.85.4.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarti S, Magnuson T, Lass JH, Jepsen KJ, LaMantia C, Carroll H. Lumican regulates collagen fibril assembly: skin fragility and corneal opacity in the absence of lumican. J Cell Biol. 1998 Jun 1;141(5):1277–1286. doi: 10.1083/jcb.141.5.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C-Y, Birk DE, Hassell JR, Kane B, Kao WW-Y. Keratocan-deficient mice display alterations in corneal structure. J Biol Chem. 2003 Jun 13;278(24):21672–21677. doi: 10.1074/jbc.M301169200. [DOI] [PubMed] [Google Scholar]
- Tuominen IS, Tervo TM, Teppo AM, Valle TU, Grönhagen-Riska C, Vesaluoma MH. Human tear fluid PDGF-BB, TNF-alpha and TGF-beta1 vs corneal haze and regeneration of corneal epithelium and subbasal nerve plexus after PRK. Exp Eye Res. 2001 Jun;72(6):631–641. doi: 10.1006/exer.2001.0999. [DOI] [PubMed] [Google Scholar]
- Stramer BM, Zieske JD, Jung J-C, Austin JS, Fini ME. Molecular mechanisms controlling the fibrotic repair phenotype in cornea: implications for surgical outcomes. Invest Ophthalmol Vis Sci. 2003 Oct;44(10):4237–4246. doi: 10.1167/iovs.02-1188. [DOI] [PubMed] [Google Scholar]
- Carrington LM, Albon J, Anderson I, Kamma C, Boulton M. Differential regulation of key stages in early corneal wound healing by TGF-beta isoforms and their inhibitors. Invest Ophthalmol Vis Sci. 2006 May;47(5):1886–1894. doi: 10.1167/iovs.05-0635. [DOI] [PubMed] [Google Scholar]
- Groppe J, Hinck CS, Samavarchi-Tehrani P, Zubieta C, Schuermann JP, Taylor AB. Cooperative assembly of TGF-β superfamily signaling complexes is mediated by two disparate mechanisms and distinct modes of receptor binding. Mol Cell. 2008 Feb 1;29(2):157–168. doi: 10.1016/j.molcel.2007.11.039. [DOI] [PubMed] [Google Scholar]
- Hart PJ, Deep S, Taylor AB, Shu Z, Hinck CS, Hinck AP. Crystal structure of the human TβR2 ectodomain–TGF-β3 complex. Nat Struct Mol Biol. 2002 Mar;9(3):203–208. doi: 10.1038/nsb766. [DOI] [PubMed] [Google Scholar]
- Rodriguez C, Chen F, Weinberg RA, Lodish HF. Cooperative binding of transforming growth factor (TGF)-beta 2 to the types I and II TGF-beta receptors. J Biol Chem. 1995 Jul 7;270(27):15919–15922. doi: 10.1074/jbc.270.27.15919. [DOI] [PubMed] [Google Scholar]
- López-Casillas F, Payne HM, Andres JL, Massagué J. Betaglycan can act as a dual modulator of TGF-beta access to signaling receptors: mapping of ligand binding and GAG attachment sites. J Cell Biol. 1994 Feb 15;124(4):557–568. doi: 10.1083/jcb.124.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fini ME, Stramer BM. How the cornea heals: cornea-specific repair mechanisms affecting surgical outcomes. Cornea. 2005;24(8):S2–S11. doi: 10.1097/01.ico.0000178743.06340.2c. [DOI] [PubMed] [Google Scholar]
- Imanishi J, Kamiyama K, Iguchi I, Kita M, Sotozono C, Kinoshita S. Growth factors: importance in wound healing and maintenance of transparency of the cornea. Prog Retin Eye Res. 2000 Jan;19(1):113–129. doi: 10.1016/s1350-9462(99)00007-5. [DOI] [PubMed] [Google Scholar]
- Karamichos D, Guo XQ, Hutcheon AEK, Zieske JD. Human corneal fibrosis: an in vitro model. Invest Ophthalmol Vis Sci. 2010 Mar;51(3):1382–1388. doi: 10.1167/iovs.09-3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beinborn M, Blum A, Hang L, Setiawan T, Schroeder JC, Stoyanoff K. TGF-beta regulates T-cell neurokinin-1 receptor internalization and function. Proc Natl Acad Sci USA. 2010 Mar 2;107(9):4293–4298. doi: 10.1073/pnas.0905877107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong TM, Anderson SA, Yu H, Huang RR, Strader CD. Differential activation of intracellular effector by two isoforms of human neurokinin-1 receptor. Mol Pharmacol. 1992 Jan;41(1):24–30. [PubMed] [Google Scholar]
- Lai J-P, Lai S, Tuluc F, Tansky MF, Kilpatrick LE, Leeman SE. Differences in the length of the carboxyl terminus mediate functional properties of neurokinin-1 receptor. Proc Natl Acad Sci USA. 2008 Aug 26;105(34):12605–12610. doi: 10.1073/pnas.0806632105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Nakayasu K, Iwatsu M, Kanai A. Endogenous substance P in corneal epithelial cells and keratocytes. Jpn J Ophthalmol. 2002 Dec;46(6):616–620. doi: 10.1016/s0021-5155(02)00617-2. [DOI] [PubMed] [Google Scholar]
- Muñoz M, Coveñas R. Neurokinin-1 receptor: a new promising target in the treatment of cancer. Discov Med. 2010 Oct;10(53):305–313. [PubMed] [Google Scholar]
- Andersson G, Danielson P, Alfredson H, Forsgren S. Presence of substance P and the neurokinin-1 receptor in tenocytes of the human Achilles tendon. Regul Pept. 2008;150(1–3):81–87. doi: 10.1016/j.regpep.2008.02.005. Oct 9. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Barrett RP, McClellan SA, Zhang Y, Szliter EA, van Rooijen N. Substance P delays apoptosis, enhancing keratitis after Pseudomonas aeruginosa infection. Invest Ophthalmol Vis Sci. 2008 Oct;49(10):4458–4467. doi: 10.1167/iovs.08-1906. [DOI] [PubMed] [Google Scholar]
- Koon H-W, Zhao D, Zhan Y, Moyer MP, Pothoulakis C. Substance P mediates antiapoptotic responses in human colonocytes by Akt activation. Proc Natl Acad Sci USA. 2007 Feb 6;104(6):2013–2018. doi: 10.1073/pnas.0610664104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backman LJ, Danielson P. Akt-mediated anti-apoptotic effects of substance P in Anti-Fas-induced apoptosis of human tenocytes. J Cell Mol Med. 2013 Jun;17(6):723–733. doi: 10.1111/jcmm.12059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong HS, Lee J, Lee E, Kwon YS, Lee E, Ahn W. A new role of substance P as an injury-inducible messenger for mobilization of CD29(+) stromal-like cells. Nat Med. 2009 Apr;15(4):425–435. doi: 10.1038/nm.1909. [DOI] [PubMed] [Google Scholar]
- Salo P, Bray R, Seerattan R, Reno C, McDougall J, Hart DA. Neuropeptides regulate expression of matrix molecule, growth factor and inflammatory mediator mRNA in explants of normal and healing medial collateral ligament. Regul Pept. 2007;142(1–2):1–6. doi: 10.1016/j.regpep.2007.01.001. Jul 5. [DOI] [PubMed] [Google Scholar]
- Espana EM, Kawakita T, Liu C-Y, Tseng SCG. CD-34 expression by cultured human keratocytes is downregulated during myofibroblast differentiation induced by TGF-beta1. Invest Ophthalmol Vis Sci. 2004 Sep;45(9):2985–2991. doi: 10.1167/iovs.04-0201. [DOI] [PubMed] [Google Scholar]
- Pei Y, Reins RY, McDermott AM. Aldehyde dehydrogenase (ALDH) 3A1 expression by the human keratocyte and its repair phenotypes. Exp Eye Res. 2006 Nov;83(5):1063–1073. doi: 10.1016/j.exer.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Funderburgh JL, Funderburgh ML, Mann MM, Corpuz L, Roth MR. Proteoglycan expression during transforming growth factor beta-induced keratocyte-myofibroblast transdifferentiation. J Biol Chem. 2001 Nov 23;276(47):44173–44178. doi: 10.1074/jbc.M107596200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funderburgh JL, Mann MM, Funderburgh ML. Keratocyte phenotype mediates proteoglycan structure a role for fibroblasts in corneal fibrosis. J Biol Chem. 2003 Nov 14;278(46):45629–45637. doi: 10.1074/jbc.M303292200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizaki M, Zhu G, Haseba T, Shafer SS, Kao WW. Expression of collagen I, smooth muscle alpha-actin, and vimentin during the healing of alkali-burned and lacerated corneas. Invest Ophthalmol Vis Sci. 1993 Nov;34(12):3320–3328. [PubMed] [Google Scholar]
- Eckes B, Zweers MC, Zhang ZG, Hallinger R, Mauch C, Aumailley M. Mechanical tension and integrin alpha 2 beta 1 regulate fibroblast functions. J Investig Dermatol Symp Proc Soc Investig Dermatol Inc Eur Soc Dermatol Res. 2006 Sep;11(1):66–72. doi: 10.1038/sj.jidsymp.5650003. [DOI] [PubMed] [Google Scholar]
- Jester JV, Petroll WM, Barry PA, Cavanagh HD. Expression of alpha-smooth muscle (alpha-SM) actin during corneal stromal wound healing. Invest Ophthalmol Vis Sci. 1995 Apr;36(5):809–819. [PubMed] [Google Scholar]
- Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002 May;3(5):349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- Paralkar VM, Vukicevic S, Reddi AH. Transforming growth factor beta type 1 binds to collagen IV of basement membrane matrix: implications for development. Dev Biol. 1991 Feb;143(2):303–308. doi: 10.1016/0012-1606(91)90081-d. [DOI] [PubMed] [Google Scholar]
- Wilson SE. Corneal myofibroblast biology and pathobiology: generation, persistence, and transparency. 2012 doi: 10.1016/j.exer.2012.03.018. http://www.ncbi.nlm.nih.gov/pubmed/22542905 Exp Eye Res [Internet] Apr 20 [cited 2012 May 8] Available from. [DOI] [PMC free article] [PubMed]
- Wilson SE, Mohan RR, Mohan RR, Jr Ambrósio R, Hong J, Lee J. The corneal wound healing response: cytokine-mediated interaction of the epithelium, stroma, and inflammatory cells. Prog Retin Eye Res. 2001 Sep;20(5):625–637. doi: 10.1016/s1350-9462(01)00008-8. [DOI] [PubMed] [Google Scholar]
- Tandon A, Tovey JCK, Sharma A, Gupta R, Mohan RR. Role of transforming growth factor beta in corneal function, biology and pathology. Curr Mol Med. 2010 Aug;10(6):565–578. doi: 10.2174/1566524011009060565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jester JV, Barry-Lane PA, Petroll WM, Olsen DR, Cavanagh HD. Inhibition of corneal fibrosis by topical application of blocking antibodies to TGF beta in the rabbit. Cornea. 1997 Mar;16(2):177–187. [PubMed] [Google Scholar]
- Beales MP, Funderburgh JL, Jester JV, Hassell JR. Proteoglycan synthesis by bovine keratocytes and corneal fibroblasts: maintenance of the keratocyte phenotype in culture. Invest Ophthalmol Vis Sci. 1999 Jul;40(8):1658–1663. [PubMed] [Google Scholar]
- Espana EM, He H, Kawakita T, Di Pascuale MA, Raju VK, Liu C-Y. Human keratocytes cultured on amniotic membrane stroma preserve morphology and express keratocan. Invest Ophthalmol Vis Sci. 2003 Dec;44(12):5136–5141. doi: 10.1167/iovs.03-0484. [DOI] [PubMed] [Google Scholar]
- Funderburgh JL, Funderburgh ML, Mann MM, Corpuz L, Roth MR. Proteoglycan expression during transforming growth factor beta-induced keratocyte-myofibroblast transdifferentiation. J Biol Chem. 2001 Nov 23;276(47):44173–44178. doi: 10.1074/jbc.M107596200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borderie V, Sabolic V, Laroche L. Culture of human keratocytes. Influence of culture conditions and ultrastructural aspects. J Fr Ophtalmol. 1998 Feb;21(2):103–111. [PubMed] [Google Scholar]
- Borderie VM, Mourra N, Laroche L. Influence of fetal calf serum, fibroblast growth factors, and hepatocyte growth factor on three-dimensional cultures of human keratocytes in collagen gel matrix. Graefes Arch Clin Exp Ophthalmol Albrecht Von Graefes Arch Für Klin Exp Ophthalmol. 1999 Oct;237(10):861–869. doi: 10.1007/s004170050324. [DOI] [PubMed] [Google Scholar]
- Lakshman N, Kim A, Petroll WM. Characterization of corneal keratocyte morphology and mechanical activity within 3-D collagen matrices. Exp Eye Res. 2010 Feb;90(2):350–359. doi: 10.1016/j.exer.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petroll WM, Lakshman N, Ma L. Experimental models for investigating intra-stromal migration of corneal keratocytes, fibroblasts and myofibroblasts. J Funct Biomater. 2012 Mar 19;3(1):183–198. doi: 10.3390/jfb3010183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Słoniecka M, Le Roux S, Boman P, Byström B, Zhou Q, Danielson P. Expression profiles of neuropeptides, neurotransmitters, and their receptors in human keratocytes in vitro and in situ. PLoS One. 2015;10(7) doi: 10.1371/journal.pone.0134157. Jul 27. e0134157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachem MG, Sell KM, Melchior R, Kropf J, Eller T, Gressner AM. Tumor necrosis factor alpha (TNF alpha) and transforming growth factor beta 1 (TGF beta 1) stimulate fibronectin synthesis and the transdifferentiation of fat-storing cells in the rat liver into myofibroblasts. Virchows Arch B Cell Pathol Incl Mol Pathol. 1993;63(2):123–130. doi: 10.1007/BF02899251. [DOI] [PubMed] [Google Scholar]
- Jester JV, Barry-Lane PA, Cavanagh HD, Petroll WM. Induction of alpha-smooth muscle actin expression and myofibroblast transformation in cultured corneal keratocytes. Cornea. 1996 Sep;15(5):505–516. [PubMed] [Google Scholar]
- Wrana JL, Attisano L, Cárcamo J, Zentella A, Doody J, Laiho M. TGF beta signals through a heteromeric protein kinase receptor complex. Cell. 1992 Dec 11;71(6):1003–1014. doi: 10.1016/0092-8674(92)90395-s. [DOI] [PubMed] [Google Scholar]
- Wrana JL, Attisano L, Wieser R, Ventura F, Massagué J. Mechanism of activation of the TGF-β receptor. Nature. 1994 Aug 4;370(6488):341–347. doi: 10.1038/370341a0. [DOI] [PubMed] [Google Scholar]
- Zúñiga JE, Groppe JC, Cui Y, Hinck CS, Contreras-Shannon V, Pakhomova ON. Assembly of TβRI:TβRII:TGFβ ternary complex in vitro with receptor extracellular domains is cooperative and isoform-dependent. J Mol Biol. 2005 Dec 16;354(5):1052–1068. doi: 10.1016/j.jmb.2005.10.014. [DOI] [PubMed] [Google Scholar]
- Caberlotto L, Hurd YL, Murdock P, Wahlin JP, Melotto S, Corsi M. Neurokinin 1 receptor and relative abundance of the short and long isoforms in the human brain. Eur J Neurosci. 2003 May;17(9):1736–1746. doi: 10.1046/j.1460-9568.2003.02600.x. [DOI] [PubMed] [Google Scholar]
- Backman LJ, Fong G, Andersson G, Scott A, Danielson P. Substance P is a mechanoresponsive, autocrine regulator of human tenocyte proliferation. PloS One. 2011;6(11) doi: 10.1371/journal.pone.0027209. e27209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J, Feng F, Xu G, Zhang H, Hong L, Yang J. Elevated SP/NK-1R in esophageal carcinoma promotes esophageal carcinoma cell proliferation and migration. Gene. 2015 Apr 15;560(2):205–210. doi: 10.1016/j.gene.2015.02.002. [DOI] [PubMed] [Google Scholar]
- Kohara H, Tajima S, Yamamoto M, Tabata Y. Angiogenesis induced by controlled release of neuropeptide substance P. Biomaterials. 2010 Nov;31(33):8617–8625. doi: 10.1016/j.biomaterials.2010.07.079. [DOI] [PubMed] [Google Scholar]
- Akasaka Y, Abe K, Sato T, Inoue H. Regulation of neurokinin-1 receptor messenger RNA expression in synovial fibroblasts of patients with rheumatoid arthritis. Neuropeptides. 2005 Oct;39(5):467–474. doi: 10.1016/j.npep.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Gillespie E, Leeman SE, Watts LA, Coukos JA, O’Brien MJ, Cerda SR. Truncated neurokinin-1 receptor is increased in colonic epithelial cells from patients with colitis-associated cancer. Proc Natl Acad Sci USA. 2011 Oct 18;108(42):17420–17425. doi: 10.1073/pnas.1114275108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai J-P, Ho WZ, Kilpatrick LE, Wang X, Tuluc F, Korchak HM. Full-length and truncated neurokinin-1 receptor expression and function during monocyte/macrophage differentiation. Proc Natl Acad Sci USA. 2006 May 16;103(20):7771–7776. doi: 10.1073/pnas.0602563103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuluc F, Lai JP, Kilpatrick LE, Evans DL, Douglas SD. Neurokinin 1 receptor isoforms and the control of innate immunity. Trends Immunol. 2009 Jun;30(6):271–276. doi: 10.1016/j.it.2009.03.006. [DOI] [PubMed] [Google Scholar]


