Abstract
Introduction
Budesonide is a synthetic corticosteroid characterized by enhanced topical potency and limited systemic bioavailability. Its use in ulcerative colitis (UC) was limited to rectal preparations until recently when the new oral budesonide formulation incorporating the multi-matrix system technology was introduced. The purpose of this review is to evaluate the current role of oral and rectal budesonide in managing UC patients
Areas Covered
In this paper, we described the chemical structure and pharmacologic characteristics of the different oral and rectal budesonide preparations, provided a summary of the published trials that evaluated the efficacy and safety of budesonide in UC, and discussed the current status of its use in this population
Expert Opinion
Budesonide is effective in inducing remission in a subset of patients with mild-moderate UC. Nevertheless, the current evidence suggests inferiority of oral budesonide to 5-aminosalisylates (5-ASA) and systemic steroids, whereas rectal applications are comparable to other rectal steroid preparations but still inferior to rectal 5-ASA. In clinical practice, several issues need clarification including, its exact position in the line of induction agents; the role of combining budesonide and 5-ASAs; the role of combining oral and rectal budesonide; and the role of budesonide in maintenance therapy.
Keywords: Ulcerative colitis, inflammatory bowel disease, budesonide, steroids
1. Introduction
Ulcerative colitis (UC) is a form of inflammatory bowel disease (IBD) characterized by diffuse mucosal inflammation that invariably involves the rectum and extends proximally to variable lengths in the colon. UC is a chronic disease with a remitting-relapsing nature and hence, the goal of therapy is to induce remission and to prevent relapses. Systemic corticosteroids were amongst the first therapies used in the treatment of UC. Their efficacy in inducing remission is well-established from the earlier clinical trials in 1950s and 1960s with clinical response rates approaching 80% in some studies.1–3 Those results are further supported by the clinical experience over the last half century.4–7 The impact of corticosteroids on the immune response is carried through their interaction with the intracytoplasmic glucocorticoid receptors. This interaction results in downregulation of several proinflammatory cytokines and subsequent inhibition of inflammatory cells proliferation and recruitment.8,9 However, the powerful anti-inflammatory effect of corticosteroids is counterbalanced by the long list of well-recognized complications associated with systemic corticosteroids. Furthermore, corticosteroids have not been shown to reduce the risk of disease relapse when used as a maintenance therapy.1,10 Thus, the only indication for the use of systemic corticosteroids in UC is to induce remission in moderate to severe cases.6 To ameliorate or prevent steroid associated side effects, a “second generation” of topically acting corticosteroids characterized by higher potency and lower systemic bioavailability was developed.11 Budesonide is considered the prototype of the topically acting corticosteroids and the most extensively studied form in IBD. It is recommended as first line therapy for induction of remission in Crohn’s disease (CD) patients with mild-moderate disease, particularly those with disease distribution involving the distal ileum and/or right colon.12,13 Nevertheless, its role in UC patients is not as established. The purpose of this report is to evaluate the current status of budesonide use in UC and to discuss some of the concerns associated with its use in clinical practice.
2. Overview of the market
The estimated annual incidence of UC is 24.3 per 100,000 person-years in Europe and 19.2 per 100,000 person-years in North America.14 It affects approximately 500,000 individuals in USA.15 Until recently, therapeutic options for mild-moderate UC were limited to oral and rectal 5-aminosalisylates (5-ASA) preparations and rectal corticosteroids preparation. Patients who fail those therapies are frequently treated with systemic corticosteroid to induce remission and may require escalation to immunomodulator and biologic therapies to maintain remission. The oral extended release formulation as well as rectal application of budesonide was recently approved by the US Food and Drug Administration (FDA) for induction of remission in patients with mild to moderate UC, which expanded the therapeutic options for this subpopulation.16,17
3. What is Budesonide?
Budesonide is a synthetic, non-halogenated corticosteroid that is structurally related to 16α-hydroxyprednisolone. It includes asymmetric 16α, 17α- acetyl groups resulting in a 1:1 mixture of two epimers labeled as 22R and 22S [figure 1].18,19 Both epimers are biologically active with similar terminal half-life of 2.7 ± 0.6 h. However, the 22R epimer is 2–3 times more potent than its counterpart and has higher distribution volume and clearance.18 This chemical structure accounts for the favorable characteristics of budesonide including its increased affinity to corticosteroid receptors and enhanced topical potency, which approaches 5 times that of the prednisone.19,20 It also allows for the rapid clearance of the drug through an extensive first-pass metabolism in the liver with a resultant low systemic bioavailability minimizing its systemic effects.20
Figure 1. Molecular structure of Budesonide and its two metabolites.
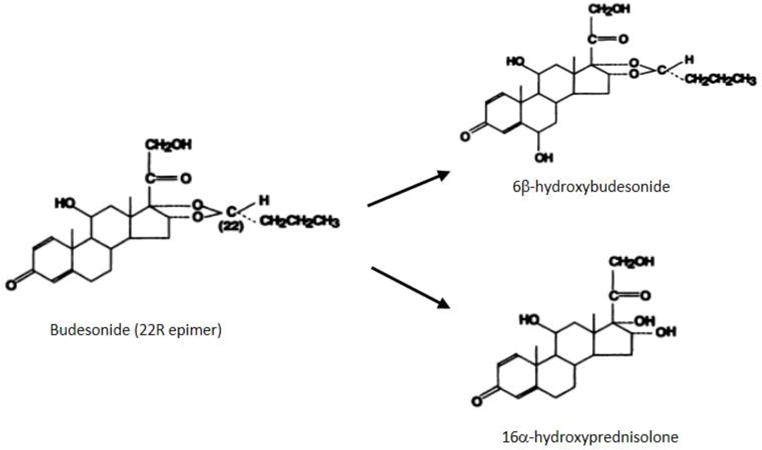
Figure adapted with permission from: G Jönsson, A Aström, and P Andersson, Budesonide is metabolized by cytochrome P450 3A (CYP3A) enzymes in human liver, Drug Metab Dispos January 1995 23:137-14218
In its native form, budesonide is rapidly absorbed in the proximal gastrointestinal tract and cleared through the liver. This poses a challenge for its use in UC as for topically acting steroids, drug delivery to the site of inflammation is critical. Several drug delivery mechanisms have been incorporated to allow targeted budesonide release and currently three oral formulations of budesonide are commercially available [table 1]:
PH-dependent-release formulation designed to deliver the drug at PH ≥ 6.4 (Budenofalk® Dr. Falk pharm, Freiburg Germany),
PH-dependent and time-dependent controlled- release formulation designed to dissolve at PH ≥ 5.5 (Entocort® AstraZeneca, Lund Sweden),
Multimatrix (MMX) formulation consistent of tablets with three matrix layers designed to release budesonide homogenously throughout the ascending, transverse and descending colon (Uceris® Santarus, Inc. CA. USA)
Table 1.
Pharmacokinetic characteristic of the oral and rectal budesonide formulations*
| Characteristics | PH-release formulation (Budenofalk®) | Controlled-release formulation (Entocort®) | MMX release formulation (Uceris®) | Budesonide Enema 2mg/100ml | Budesonide Foam 2mg/25ml |
|---|---|---|---|---|---|
| Formulation | Gelatin capsules containing budesonide pellets coated with methacrylic polymers (L, S, LS, and RS Eudragit®) | Gelatin capsules containing pellets with inner sugar core, surrounded by layer of budesonide in ethylcellulose and coated with an acrylic-based resin (L100-55 Eudragit®) | Tablets coated with polymethacrylate film and composed of an inner lipophilic matrix layer in which the budesonide is dispersed, an outer hydrophilic layer, and a third amphiphilic layer promoting the inner matrix wettability | The enema is reconstituted by adding a dispersible budesonide tablet to the enema solution, which contains sodium chloride, methylparaben, propylparaben and water purified | Budesonide is formulated as an emulsion filled into an aluminum canister with an aerosol propellant |
| Target PH | PH ≥ 6.4 | PH ≥ 5.5 | PH ≥ 7 | N/A | N/A |
| Site of release and distribution | Ileum and ascending colon | Jejunum | Ascending, transverse and descending colon | Proximal Spread up to the splenic flexure | Proximal Spread up to 11–40 cm |
| Cmax (ng/mL) | 2.5 (1.2) | 1.6 (0.6) | 1.3 (1) | 0.9 (0.5) | 0.8 (0.5) |
| Tmax (h) | 8.5 (1.1) | 5 (2–6) | 15 (6–24) | 1.3 (0.6) | 3.1 (1.4) |
| AUC (ng h/mL) | 13.4 (2.6) | 14.1 (6.4) | 15.2 (10) | 4.1 (2.1) | 5.2 (2.4) |
| T lag | 1.3 (0.8) | 1 (1–4) | 6 (4–7) | N/A | N/A |
The pharmacokinetics information for the oral formulations are based on assessment in healthy volunteered after a single dose of 9 mg. The pharmacokinetics information for the rectal formulations are based on studies conducted on patients with left-sided UC.21,28–34
Data presented as mean (SD) or mean (range)
Abbreviations: AUC: area under the plasma drug concentration time curve, Cmax: peak plasma concentration, Tmax: time to peak plasma concentration, Tlag: time to detect drug concentration in the plasma
In addition, for proctitis and left-sided colitis, rectal formulations of budesonide are available for topical use as an enema (Entocort®) or foam (Budenofalk® and Uceris®).
4. Pharmacokinetics
After oral administration, budesonide is released starting in the proximal jejunum (Entocort®), ileum (Budenofalk®), or homogenously throughout the ascending, transverse and descending colon (Uceris®).21–23 Once released, the apical enterocyte drug transporter, P-glycoprotein, facilitates its absorption to be rapidly metabolized via the cytochrome P450 isoenzymes CYP3A4 and CYP3A5 expressed in the liver and, to a lesser extent, in the intestinal epithelial cells.24,25 The products of budesonide metabolism, 16α-hydroxyprednisolone and 6β-hydroxybudesonide have negligible corticosteroid activity compared to their parent compound and do not contribute to its therapeutic effect [figure 1]. They are primarily cleared through the kidneys but small fraction is conjugated and excreted in the bile.24,26 About 90% of the orally administered budesonide undergo first-pass metabolism resulting in a low systemic bioavailability of 10–15%.26 Furthermore, most of the systemically available budesonide is bound to plasma proteins (88%).27 Apart from the differences in the site and rate of drug release, the different oral budesonide formulations share similar pharmacokinetic characteristics [table 1].21,28–34 Likewise, the two rectal formulations share similar pharmacokinetics, although the foam is characterized by less proximal spread and takes longer time to reach peak plasma concentration [table 1].
Several factors have been shown to impact the clearance and systemic bioavailability of budesonide through interference with its metabolism. For instance, the presence of liver cirrhosis was associated with 2.5-fold increase in systemic bioavailability of the controlled-release budesonide formulation.29 Likewise, concomitant use of ketoconazole or grapefruit juice, both act as inhibitors of cytochrome P450 isoenzymes, resulted in significant increase of budesonide systemic bioavailability.35,36 In addition, altered gastrointestinal motility or PH may interfere with budesonide release after oral administration. For example, the time to detect drug concentration in the plasma (Tlag) and the time to achieve maximum concentration (Tmax) were significantly increased in healthy volunteers given a dose of budesonide-MMX after a high-fat, high-calorie meal compared to those who received the drug while fasting.22 Post-prandial gastric emptying delay was suggested as a potential cause for the reduced absorption rate. Administering budesonide with food may also enhance its clearance given post-prandial increase in splanchnic circulation blood flow. Of note, neither the patient’s age nor the gender had an impact on budesonide metabolism and clearance.29
5. Clinical Efficacy
5.1. Oral budesonide for induction of remission in UC
Earlier studies did not provide adequate evidence to support the use of oral budesonide in patients with UC and traditionally, only rectal budesonide preparations were considered as a potential therapy option in this population.6 In a systemic review of Cochrane database published in 2010, three clinical trials addressing the role of oral budesonide in UC met the criteria for the review and were critically assessed (Löfberg 1996, Gross 2011, and D’Haens 2010) [table 2].37 The three studies varied in regard of the comparator medication (prednisolone in one study, mesalamine in the second and placebo in the third), budesonide formulation used, and the assessed primary outcomes.38–40 This review concluded that the evidence is not adequate to recommend the clinical use of oral budesonide for the induction of remission in active UC. Furthermore, mesalamine was superior to budesonide (ph ≥ 6.4-dependent release formulation; Budenofalk®) in this population. However, several recent trials using budesonide-MMX formulation showed more encouraging results reviving the interest in utilizing this compound in UC patients.41–43
Table 2.
Summary of the randomized clinical trials evaluating oral budesonide in patients with ulcerative colitis
| Study/design | Study population | N | Budesonide formulation | Comparator group(s) | Duration | Primary outcome |
|---|---|---|---|---|---|---|
| Löfberg et al [1996]38 MC, DB, DD, R |
Mild-moderate UC [endoscopic inflammation score ≥ 2 with symptoms of bloody stool and increased frequency ≥ 3] | 72 | Controlled-release budesonide [10mg × 4 wks then 8 mg ×5 wks] | Oral prednisolone [40 mg × 2 wks followed by 5 mg reduction every week] | 9 weeks | Improvement in the endoscopic inflammation Score Main results: Mean decrease in endoscopic inflammation score was similar in both groups (1.2 and 1.36, respectively) |
| Gross et al [2011]39 MC, DB, DD, R |
Mild-moderate UC [CAI ≥ 6 and endoscopic index ≥4] | 343 | PH-dependent release budesonide (Budenofalk®) 9 mg | Oral mesalazine (Salofalk®) 3 g | 8 weeks | Clinical remission at 8 weeks defined by CAI<=4 and rectal bleeding score of 0 Main results: Primary outcome was achieved in 39.5% and 54.8% of patients treated with budesonide and mesalazine, respectively |
| D’Haens et al [2010]40 MC, DB, DD, R |
Moderate left-sided UC | 32 | Budesonide-MMX® 9 mg | Placebo | 8 weeks | Clinical remission at 8 weeks defined as CAI ≤ 4 and/or reduction in CAI score by 50% Main results: Primary endpoint achieved in 47.1% and 33.3% in the budesonide and placebo groups, respectively (NS) |
| Sandborn et al (CORE I) [2012]41 MC, DB, R |
Mild to moderate active UC (UCDAI score 4–10) | 509 | Budesonide-MMX® 9 mg | Budesonide-MMX® 6 mg Mesalamine (Asacol®) 2.4 g Placebo |
8 weeks | Combined clinical and endoscopic remission Main results: Primary endpoint achieved in 17.9%, 13.2%, and 12.1% in budesonide 9 mg, 6 mg, and mesalamine group, respectively, compared to 7.4% in placebo. The difference was only significant for budesonide 9 mg [P=0.01] |
| Travis et al (CORE II) [2014]42 MC, DB, R |
Mild to moderate active UC (UCDAI score 4–10) | 512 | Budesonide-MMX® 9 mg | Budesonide-MMX® 6 mg Entocort® 9 mg Placebo |
8 weeks | Combined clinical and endoscopic remission Maisa results: Primary endpoint achieved in 17.4%, 8.3%, and 12.6 % in budesonide 9 mg, 6 mg, and Entocort group, respectively, compared to 4.5% in placebo. The difference was only significant for budesonide 9 mg [P=0.005] |
| Rubin et al [2014]43* MC, DB, R |
Mild to moderate active UC (UCDAI score 4–10) not adequately controlled by oral mesalamine therapy at dose ≥2.4 g/day for ≥ 6 weeks prior to entry | 510 | Budesonide-MMX® 9 mg | Placebo | 8 weeks | Combined clinical and endoscopic remission Main results: Primary endpoint achieved in 13% in the budesonide group vs. 7.5% in placebo [p=0.048] |
| Sandborn et al [2012]46* MC, DB, R |
patients in CORE I and CORE II who were in remission at the end of the induction phase (8 weeks) | 122 | Budesonide-MMX® 6 mg | Placebo | 12 months | Proportion of patients in clinical remission after 1, 3, 6, 9, 12 months and/or end of the study Main results: No significant difference was noted in regard of the primary endpoint. The probability of clinical relapse was reduced in budesonide group (40.9% vs. 59.7%, respectively, and nd median time for relapse was longer in the budesonide-treated patients |
MC: multicenter, DB: double-blind, DD: double-dummy, R: randomized, CAI: colitis activity index, UCDAI: ulcerative colitis disease activity index
Available in abstract format only
The initial pilot study by D’Haens et al. (included in the above mentioned Cochrane review) did not show significant difference in rates of clinical remission between budesonide-MMX 9mg and placebo (47.1% vs. 33.3%, respectively. P-value 0.14).40 This study included a small number of patients (n=36) and the disease distribution was limited to left-sided colitis. Subsequently, two larger, identically designed phase III clinical trials, CORE I and CORE II were conducted.41,42 In CORE I, 509 patients with active mild-moderate UC were randomized to four arms; budesonide-MMX 9mg/day, budesonide-MMX 6mg/day, mesalamine 2.4 g/day (Asacol® Warner Chilcott plc. Dublin, Ireland), or placebo. The primary outcome was combined clinical and endoscopic remission at 8 weeks, which was achieved in 17.9%, 13.2%, and 12.1% of patients treated with budesonide-MMX 9mg, budesonide-MMX 6mg, and mesalamine, respectively, compared to 7.4% in the placebo group. The therapeutic advantage compared to placebo was only significant for budesonide- MMX 9 mg (P=0.0143). Interestingly, there was no significant difference in rates of remission between the budesonide-MMX groups and the mesalamine group. This is contrary to the results from the earlier trial by Gross et al., which compared the efficacy of (PH-dependent release) budesonide (9mg/day) to oral mesalamine (3g/day) and concluded that budesonide is inferior to mesalamine.39 The two studies used different primary outcome (clinical remission in Gross et al study vs. combined endoscopic and clinical remission in CORE I) and different mesalamine dosages (3 g vs 2.4g), both factors and the different budesonide formulations may have contributed to the inconsistent results. Furthermore, the CORE I trial was not sufficiently powered to detect differences between the active compactor groups. A similar study design was applied in CORE II except for the use of controlled-release budesonide (Entocort®) as the third comparator group instead of mesalamine.42 A total of 511 patients with mild-moderate active UC were randomized in this study. The rates of combined clinical and endoscopic remission were 17.4%, 8.3%, 12.6%, and 4.5% for the budesonide-MMX 9mg, budesonide-MMX 6mg, Entocort, and placebo groups, respectively. The therapeutic advantage compared to placebo was only significant for budesonide-MMX 9 mg (p-value 0.005). The remission rate amongst patients treated with budesonide-MMX 9mg was comparable to those who received Entocort. However, similar to CORE I, this study was not powered to detect differences between the active comparator groups. Combining the efficacy data from COREI and COREII, budesonide-MMX 9mg was associated with 17.7% remission rate (clinical and endoscopic) compared to 6.2% for placebo [OR 3.3, 95% CI 1.7–6.4 with number needed to treat (NNT) of 8.7] [figure 2].44 An additional study by Rubin et al assessed the efficacy of budesonide-MMX 9 mg in patients who had inadequate response to oral 5-ASA compounds. The results from this trial are currently available in an abstract format only. In this study, a total of 510 patients with inadequate response to therapeutic dose of oral 5-ASA compounds, were randomized to receive budesonide-MMX 9 mg or placebo for 8 weeks.43 The primary endpoint was combined endoscopic and clinical remission at 8 weeks, which was achieved in 13% of the budesonide-treated patients vs. 7.5% for placebo (p= 0.049) [figure 2].
Figure 2.
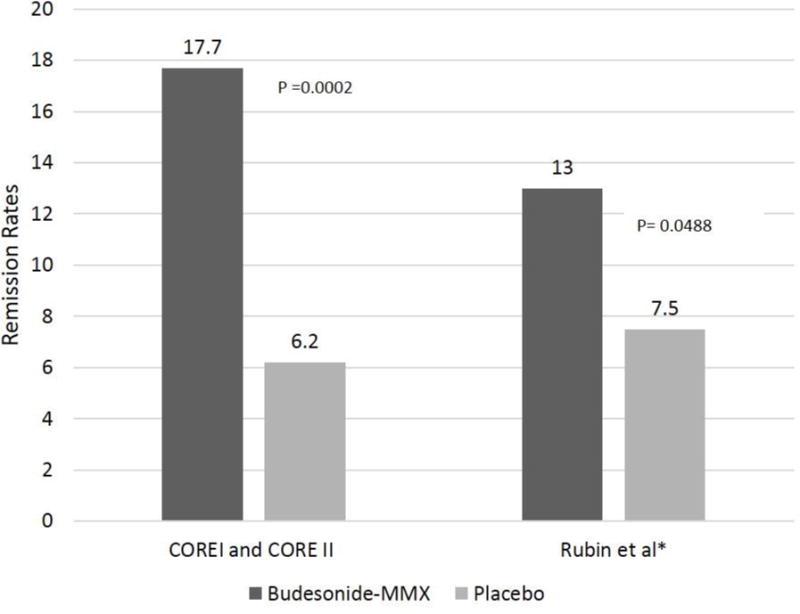
Rates of remission (clinical and endoscopic) reported from combined CORE I and CORE II trials and Rubin et al study
A pooled analysis of data from CORE I, CORE II, and the study by Rubin et al, was recently presented in the updated Cochrane review.45 Budesonide-MMX was noted to be significantly superior to placebo for inducing combined clinical and endoscopic remission, 15% vs. 7%, respectively [RR 2.25, 95% CI 1.50 to 3.39. NNT of 12.5]. Furthermore, subgroup analysis suggested higher efficacy in patients who were not considered to be mesalamine refractory [RR 2.89, 95%CI 1.59–5.25. NNT of 8.3] and those with left-sided disease only [RR 2.98, 95% CI 1.56–5.67. NNT of 7.1].
5.2. Oral budesonide for maintenance of remission in UC
Sandborn et al evaluated the efficacy of extended budesonide-MMX use in patients with UC who were in clinical and endoscopic remission at the end of the induction phase of CORE I and CORE II clinical trials.46 A total of 122 patients were randomized to receive budesonide-MMX 6 mg or placebo for 12 months. The primary outcome was the proportion of patients in clinical remission after 1, 3, 6, 9, 12 months and/or end of the study and the secondary outcome was time to relapse. No significant difference was noted between budesonide and placebo in regard of the primary outcome. However, in an intention to treat analysis, the probability of clinical relapse was reduced in the budesonide-treated group compared to placebo, 40.9% vs. 59.7%, respectively. In addition, the median time for relapse was longer in the budesonide-treated patients. The rates of adverse events were comparable between the 2 groups. Those results are currently published in an abstract form only. Hence, the details about of the patients’ characteristics, disease distribution and concomitant therapies are not available. In a smaller pilot study by Keller et al, patients with steroid-dependent UC were treated with oral (PH-dependent) budesonide 9mg for 6 months while attempting to taper the conventional corticosteroid.47 Of the 14 patients included in the study, a total of 11 (78.6%) were able to terminate the conventional corticosteroids within 3 months of starting the budesonide. Those results were not replicated in a larger studies or in the context of the current advances in UC medical therapies.
Overall, while it appears to be safe, there is no sufficient evidence to support the use of oral budesonide for maintenance of therapy in UC patients.
5.3. Rectal budesonide formulations in UC
The role of rectal budesonide formulations (enema and foam) in left-sided UC is more established. Several studies have been published since 1987 comparing rectal budesonide to placebo, conventional rectal steroid preparations (prednisolone, methylprednisolone, and hydrocortisone), and rectal 5-ASA compounds [table 3]. Compared to placebo, rectal budesonide has consistently shown superior efficacy in treating left-sided UC regardless of form used and the assessed endpoint in each particular study.48–51
Table 3.
Summary of the clinical trials evaluating rectal budesonide in patients with ulcerative colitis
| Study/design | Study population | N | Budesonide formulation | Comparator group(s) | Duration | Primary outcome/Results |
|---|---|---|---|---|---|---|
|
Danielsson et al [1992]48 MC, DB, R |
Left-sided colitis (up to the splenic flexure) | 40 | Enema 2mg/100ml QD | Placebo | 4 weeks | Endoscopic healing at 2 and 4 weeks Main results: Budesonide was associated with significant drop in endoscopic scores, histologic grading scores, and clinical symptoms |
|
Hanauer et al [1998]49 MC, DB, R |
Left-sided colitis (up to the splenic flexure) | 233 | Enema at 3 doses; 0.5, 2, and 8 mg/100ml QD | Placebo | 6 weeks | Improvement in sigmoidoscopic inflammation grade, total histopathologic score, and remission rates Main results: all primary outcomes were similarly better in the 2mg and 8 mg groups compared to the 0.5 mg and placebo group. 2mg was the lowest effective dose |
|
Lindgren et al [2002]52 MC, DB, R |
Left-sided colitis (up to the splenic flexure) Inductions: |
149 | Enema 2mg/100 ml-BID | Enema 2mg/100 ml-QD | 8 weeks | Rate of remission [endoscopic and clinical] Main results: Remission rate 54% in BID vs. 51% in QD (NS) |
| Maintenance: | 77 | Enema 2mg/100 ml twice/week | Placebo | 24 weeks | Rate of relapse Main results: Rate of relapse 41% budesonide vs. 51 placebo (NS) |
|
|
Sandborn et al [2015]50 2 identical MC, DB, R trials |
Proctitis or proctosigmoiditis | 546 | Foam 2mg/25ml BID for 2 weeks, then daily for 4 weeks | Placebo | 6 weeks | Combined clinical and endoscopic remission Main results from combined trials: primary outcome was achieved in 41.2% in budesonide vs. 24% in placebo [p <0.001] |
|
Naganuma et al [2015]51 MC, DB, R |
Proctitis or proctosigmoiditis | 165 | Foam 2mg/25ml BID | Budesonide foam 2mg/25ml QD Placebo |
6 weeks | Complete mucosal healing [endoscopic subscore 0] Main results: complete mucosal healing in BID budesonide was 46.4% compared to 23.6% for QD [p=0.009], and 5.6% for placebo [p<0.0001]. Rates of clinical remission and rates of endoscopic subscore ≤1 were similar in BID and QD dosing groups |
|
Gross et al [2006]53 MC, DB, DD, R |
Proctitis or proctosigmoiditis | 541 | Foam 2mg/25ml QD | Budesonide enema 2mg/100ml QD | 4 weeks | Clinical remission Main results: remission rates 60% and 66% for the foam and enema (NS). More patients preferred the foam (88%) |
|
Danielsson et al [1987]54 IB, R |
Active left-sided colitis (up to the splenic flexure) | 64 | Enema 2mg/100ml QD | Prednisolone disodium phosphate enema 31.25mg/100ml QD | 4 weeks | Endoscopic healing at 4 weeks Main results: 52% and 24% healed endoscopically on budesonide enema and prednisolone enemas, respectively [p=0.045] |
|
Danish budesonide Study Group [1991]55 DB. R |
Proctosigmoiditis (up to 25cm from anal verge) | 139 | Enema at 3 doses; 1, 2, and 4 mg/100ml QD | Prednisolone disodium phosphate enema 25mg/100ml QD | 2 weeks | Improvement in clinical symptoms, endoscopic and histologic grades Main results: improvement in clinical and endoscopic variables was noted in all treatment groups, but was lower in 1 mg group. |
|
Porro et al [1994]58 MC, IB, R |
Left-sided colitis (up to the splenic flexure) | 88 | Enema 2mg/100ml QD | Methylprednisolone hemisuccinate (MP) enema 20mg/100ml | 8 weeks (4 weeks-blind and 4 weeks open budesonide for partial responders) | Clinical remission at 4 weeks and 8 weeks Main results: clinical remission at 4 weeks was 39% vs. 36% in budesonide and MP groups, respectively. 37 patients received open label budesonide for 4 weeks, of which 65% achieved remission |
|
Trapila et al [1994]57 IB, R |
Proctitis | 72 | Enema 2mg/100ml QD | Hydrocortisone acetate foam 125 mg | 4 weeks | Clinical and endoscopic response Main results: Both treatment groups showed comparable improvement in endoscopic scores |
|
Löfberg et al [1994]56 MC, IB, R |
Left-sided colitis (up to the splenic flexure) | 100 | Enema 2.3ml/100 ml QD | Prednisolone disodium phosphate enema 31.25mg/100ml QD | 8 weeks | Clinical remission, endoscopic and histologic scores Main results: both treatment groups had similar rate of endoscopic and histologic improvement. Clinical remission at 8 weeks was 36% and 47% in budesonide and prednisolone groups, respectively (NS) |
|
Bar-Mier et al [2003]59 MC, open, R |
Proctosigmoiditis | 251 | Foam 2 mg/25ml QD | Hydrocortisone foam 100 mg QD | 8 weeks | Clinical remission Main results: clinical remission was 53% and 52% in budesonide and hydrocortisone groups, respectively (NS) |
|
Le’mann et al [1995]60 MC, IB, R |
Left-sided colitis (up to the splenic flexure) | 97 | Enema 2mg/100ml QD | 5-ASA enema 1g/100ml QD | 4 weeks | Endoscopic and histologic scores Main results: no difference in endoscopic and histologic scores between the groups. clinical remission rate was 38% in budesonide vs. 60% in 5-ASA group [p=0.03] |
|
Hartmann et al [2010]61 MC, open, R |
Left-sided colitis (up to the splenic flexure) | 237 | Enema 2mg/100ml QD | Mesalamine enema 4g/60ml QD | 8 weeks | Clinical activity index, endoscopic, histologic, and IBDQ score at 4 and 8 weeks Main results: clinical remission was lower in the budesonide group compared to mesalamine at 4 and 8 weeks (63.5% vs 77.2% and 64.4% vs. 77.4%, respectively [p<0.05]). No statistical difference in endoscopic, histologic and IBDQ scores between the groups, but a trend favoring mesalamine was noted. |
MC: multicenter, DB: double-blind, IB: investigator-blind, DD: double-dummy, R: randomized, NS: non-significant, BID: twice daily, QD, once daily
The standard dose in the commercially available rectal budesonide preparations is 2 mg/application, which is the dose utilized in the majority of the clinical trials evaluating rectal budesonide in UC. Moreover, in a dose finding trial by Hanauer et al comparing the efficacy of 3 budesonide enema preparations (0.5mg/100ml, 2mg/100ml, and 8mg/100ml) to placebo, the 2 mg and 8 mg doses showed equivalent efficacies in improving endoscopic inflammation grades, total histopathology scores, and clinical remission rates.49 In addition, both doses were superior to the 0.5 mg budesonide dose and to the placebo suggesting that 2 mg is the lowest effective dose. In term of the dose intervals, two studies investigated whether BID dosing is superior to QD dosing in inducing remission.51,52 Lindgren et al compared the remission rates (clinical and endoscopic) in patients with active left-sided UC treated with either budesonide enema 2mg BID or QD for 8 weeks.52 The 2 groups had comparable remission rates (54% vs. 41% for the BID and QD dosing groups, respectively). More recently, Naganuma et al, investigated whether budesonide foam at BID dosing for 4 weeks is superior to QD dosing in patients with active proctitis or proctosigmoiditis.51 While the BID dosing was associated with higher rates of complete mucosal healing, defined as endoscopic subscore of 0 (46.4%, 23.6%, and 5.6%, for BID, QD and placebo groups, respectively), the two active therapy groups were comparable in achieving clinical remission and endoscopic subscore ≤ 1. As it remains unclear and highly debatable, whether complete mucosal healing offer significant advantage over endoscopic and clinical remission and based on the available evidence, QD dosing seems to be appropriate. Of note, in two recent trials assessing the efficacy of budesonide foam in active proctitis or proctosigmoiditis and resulted in recent FDA approval, the active therapy group received budesonide foam BID for 2 weeks and then daily for another 4 weeks.50 Therefore, this was the recommended dose in the FDA approval letter.17
Compared to budesonide enema, budesonide foam has lower volume per application and higher viscosity enhancing patient’s tolerability and retention. Gross et al compared the efficacy of budesonide enemas vs. budesonide foam in inducing clinical remission in patients with active proctitis and proctosigmoiditis.53 The two forms showed comparable efficacy (60% vs. 66% for the foam and enema, respectively) and both preparations were safe and neither caused significant drop in cortisol level. However, more patients preferred the foam preparation.
In comparison with conventional rectal steroid preparations, budesonide enema has been shown to have equivalent efficacy in treating active left-sided UC.54–59 Lastly, 2 studies compared the efficacy of budesonide enema to 5-ASA enema and showed that rectal 5-ASA is superior to rectal budesonide in inducing clinical remission in patients with left-sided UC.60,61
To date, only one study assessed the role of rectal budesonide as a maintenance therapy. In the abovementioned trial by Lindgren et al, patients who were in remission at the end of the induction period entered a maintenance phase and were randomized to receive either budesonide enema twice weekly or placebo for 24 weeks. Relapse rate at 24 weeks was comparable between the 2 groups (41% vs. 51% for the budesonide and placebo groups, respectively).52
6. Safety
Budesonide, in both oral and rectal formulations, has been repeatedly shown to have an excellent safety profile even with long term use. In the recent Cochrane review that evaluated the role of oral budesonide in UC, pooled analysis of three studies (CORE 1, CORE II, and Rubin et al- total of 971 participants) showed no statistically significant difference between budesonide-MMX 9mg and placebo in the proportion of patients who experienced at least one adverse event [RR 1.09, 95% CI 0.95–1.26].45 In addition, based on pooled data from CORE I and CORE II trials, the rate of serious adverse events was comparable between budesonide-MMX 9mg and placebo [RR 0.88, 95% CI 0.33–2.40].45 Furthermore, the rates of adverse events with long term budesonide use in the oral maintenance study by Sandborn et al and the rectal enema study by Lindgren et al were comparable to placebo.46,52 A dose-dependent reduction in plasma cortisol levels have been noted with both oral and rectal budesonide formulations. Nevertheless, the cortisol levels remained within the normal range in most of the studies and did not seem to have an impact on the rate of corticosteroid-related adverse events.34,45,50,62
7. Regulatory Affairs
-
-
Oral budesonide-MMX (Uceris®) received FDA approval on January 14, 2013 for the use in patients with active mild-moderate UC. The recommended dosage is 9 mg daily for up to 8 weeks.16
-
-
Budesonide rectal foam (Uceris®) received FDA approval on October 7, 2014 for the use in patients with active mild-moderate distal UC (up to 40 cm from the anal verge). The recommended dosage is 2 mg twice daily for 2 weeks, then 2 mg daily for 4 weeks.17
8. Conclusion
Budesonide, in its oral and rectal format, is effective for induction of remission in a subset of patients with mild-moderate UC. Thus far, oral budesonide has no proven superiority to oral 5-ASAs and is notably inferior to systemic steroids. Hence, the exact position in the line of induction regimens for patients with active UC remains unclear. The role of rectal budesonide is managing distal UC is more established with comparable efficacy to other rectal steroid preparations but yet suggested inferiority to rectal 5-ASA preparations. Based on the currently available data, budesonide (oral and rectal preparations) has no role in the maintenance of remission for UC patients.
9. Expert Opinion/Conclusion
In the era of expanding biologic therapies, budesonide has emerged as an attractive therapeutic option with excellent safety profile for patients with mild to moderate UC. The efficacy of oral budesonide-MMX in inducing clinical and endoscopic remission has been shown in several large, well-designed, clinical trials.41–43 Furthermore, for patients with left-sided colitis, rectal budesonide preparations were superior to placebo and comparable to conventional rectal steroid preparations.48–52,54–59 Despite the proven efficacy of budesonide, 5-ASAs remain the first line option and the treatment of choice for patients with mild-moderate UC. This is mainly driven by the extensive evidence supporting the efficacy of 5-ASAs in this population and the earlier studies that revealed superiority of 5-ASAs when compared to budesonide in rectal and controlled-release oral preparations.39,60,61 Consequently, many experts have suggested positioning budesonide ahead of systemic steroids in patients with mild-moderate UC who had inadequate response to an appropriate dose of a 5-ASA agent.15,63–65 Nevertheless, several questions remained to be explored in order to better understand how to best incorporate this therapy in clinical practice. One in particular is whether there is the role of combining oral budesonide and oral 5-ASAs. Concomitant 5-ASAs were not allowed in the 2 pivotal budesonide-MMX trials (CORE I and CORE II).41,42 The study by Rubin et al required inadequate response to 5-ASA monotherapy as criteria for entering the study and budesonide-MMX was an added therapy.43 As mentioned above 13% of the patients randomized to receive budesonide-MMX were able to achieve clinical remission supporting the notion of using budesonide as an add-on therapy when 5-ASAs fail to achieve complete remission. However, the efficacy of budesonide-MMX appears to be lower in those who have failed 5-ASAs raising a question about a potential added benefit for upfront use of combined budesonide and 5-ASAs, which theoretically may impact the rate of remission and/or the time to achieve remission. Likewise, there are no data on combining rectal 5-ASAs and rectal budesonide for patients with left-sided colitis. A second question, which has not been addressed in any of the aforementioned studies, is whether there is an added benefit for the induction of remission in combining oral and rectal budesonide, similar to what has been shown with 5-ASAs.66 The role of budesonide in maintaining remission is also in question. The current data do not support the use of budesonide as a maintenance therapy in UC. In the maintenance study by Sandborn et al, there was no significant difference between budesonide-MMX 6 mg and placebo in regard of the primary outcome (clinical remission after 1, 3, 6, 9, 12 months and/or end of the study).46 However, the probability of clinical relapse was significantly lower and the time to relapse was longer in the budesonide group suggesting potential benefit. These results seem to be comparable to trials evaluating the ability of budesonide to maintain remission in CD, which revealed no difference between placebo and budesonide in maintaining remission after 12 months but in some trials a nominally longer time period to relapse in patients treated with budesonide.67 One more area to explore is whether a transient use of higher budesonide doses has an additional benefit for some patients. Trials comparing the efficacy of budesonide at different doses in inducing remission in patients with CD revealed similar efficacy of the various budesonide doses (6, 9, or 18mg/day) in patients with mild ileo/right colonic disease location, whereas higher doses of budesonide (Budenofalk®; 18 mg) increased the therapeutic response in patients with highly active disease (CDAI >300) or ileal disease with additional distal colonic manifestation.68 Thus a “budesonide taper” starting with 18 mg for e.g. 2 weeks before reducing the dose to 9 mg may ultimately yield better results with only a minor increase in steroid induced side effects. But this approach should be tested first in controlled trials with systemic steroids in the comparator arm, particularly as the potentially enhanced efficacy of the higher budesonide dose may be attributed in part to a higher degree of systemic effects.
While there was no direct comparison to systemic steroids, the efficacy of budesonide MMX to induce clinical remission appears to be clearly inferior to systemic steroids in inducing remission in patients with ulcerative colitis (NNT 12.5 vs. 2, respectively).69 Furthermore, at least in the US, prednisone and methylprednisolone are considerably cheaper compared to oral budesonide MMX [table 4].70 Thus a benefit/risk and cost evaluation in regard to the choice of steroid therapy for the individual patient should be performed before the start of therapy.
Table 4.
Price range of commonly used oral corticosteroids in IBD patients compared to budesonide-MMX (Uceris®) in the US
| Corticosteroids | Price (US dollars)* |
|---|---|
| Budesonide-MMX (Uceris®) 9mg (30 tablets) | $1600–1700 |
| Prednisone 20 mg (100 tablets) | $11–40 |
| Methylprednisolone 16mg (100 tablets) | $120–300 |
The prices vary based on the insurance carrier, pharmacy, and the individual State. The numbers provided in the table were obtained from the GoodRx website70
Acknowledgments
Source of funding: HH is supported by a grant of the National Health Institute (NIH) 1U01-DK092239-01
Footnotes
Disclosures: HH serves as a consultant for BMS and Janssen. MA: no disclosures.
References
- 1.TRUELOVE SC, WITTS LJ. Cortisone and corticotrophin in ulcerative colitis. Br Med J. 1959;1(5119):387–394. doi: 10.1136/bmj.1.5119.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.LENNARD-JONES JE, LONGMORE AJ, NEWELL AC, WILSON CW, JONES FA. An assessment of prednisone, salazopyrin, and topical hydrocortisone hemisuccinate used as out-patient treatment for ulcerative colitis. Gut. 1960;1:217–222. doi: 10.1136/gut.1.3.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.TRUELOVE SC, WATKINSON G, DRAPER G. Comparison of corticosteroid and sulphasalazine therapy in ulcerative colitis. Br Med J. 1962;2(5321):1708–1711. doi: 10.1136/bmj.2.5321.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Faubion WA, Jr, Loftus EV, Jr, Harmsen WS, Zinsmeister AR, Sandborn WJ. The natural history of corticosteroid therapy for inflammatory bowel disease: A population-based study. Gastroenterology. 2001;121(2):255–260. doi: 10.1053/gast.2001.26279. [DOI] [PubMed] [Google Scholar]
- 5.Katz JA. Treatment of inflammatory bowel disease with corticosteroids. Gastroenterol Clin North Am. 2004;33(2):171–89, vii. doi: 10.1016/j.gtc.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 6.Kornbluth A, Sachar DB, Practice Parameters Committee of the American College of Gastroenterology Ulcerative colitis practice guidelines in adults: American college of gastroenterology, practice parameters committee. Am J Gastroenterol. 2010;105(3):501–23. doi: 10.1038/ajg.2009.727. quiz 524. [DOI] [PubMed] [Google Scholar]
- 7.Ford AC, Bernstein CN, Khan KJ, et al. Glucocorticosteroid therapy in inflammatory bowel disease: Systematic review and meta-analysis. Am J Gastroenterol. 2011;106(4):590–9. doi: 10.1038/ajg.2011.70. quiz 600. [DOI] [PubMed] [Google Scholar]
- 8.Brattsand R, Linden M. Cytokine modulation by glucocorticoids: Mechanisms and actions in cellular studies. Aliment Pharmacol Ther. 1996;10(Suppl 2):81–90. doi: 10.1046/j.1365-2036.1996.22164025.x. discussion 91–2. [DOI] [PubMed] [Google Scholar]
- 9.Farrell RJ, Kelleher D. Glucocorticoid resistance in inflammatory bowel disease. J Endocrinol. 2003;178(3):339–346. doi: 10.1677/joe.0.1780339. [DOI] [PubMed] [Google Scholar]
- 10.LENNARD-JONES JE, MISIEWICZ JJ, CONNELL AM, BARON JH, JONES FA. Prednisone as maintenance treatment for ulcerative colitis in remission. Lancet. 1965;1(7378):188–189. doi: 10.1016/s0140-6736(65)90973-6. [DOI] [PubMed] [Google Scholar]
- 11.Nunes T, Barreiro-de Acosta M, Marin-Jimenez I, Nos P, Sans M. Oral locally active steroids in inflammatory bowel disease. J Crohns Colitis. 2013;7(3):183–191. doi: 10.1016/j.crohns.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 12.Lichtenstein GR, Hanauer SB, Sandborn WJ, Practice Parameters Committee of American College of Gastroenterology Management of crohn’s disease in adults. Am J Gastroenterol. 2009;104(2):465–83. doi: 10.1038/ajg.2008.168. quiz 464, 484. [DOI] [PubMed] [Google Scholar]
- 13.Rezaie A, Kuenzig ME, Benchimol EI, et al. Budesonide for induction of remission in crohn’s disease. Cochrane Database Syst Rev. 2015;6:CD000296. doi: 10.1002/14651858.CD000296.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ye Y, Pang Z, Chen W, Ju S, Zhou C. The epidemiology and risk factors of inflammatory bowel disease. Int J Clin Exp Med. 2015;8(12):22529–22542. [PMC free article] [PubMed] [Google Scholar]
- 15.Grinspan A, Kornbluth A. Positioning therapy for ulcerative colitis. Curr Gastroenterol Rep. 2015;17(8) doi: 10.1007/s11894-015-0454-0. 29-015-0454-0. [DOI] [PubMed] [Google Scholar]
- 16.U.S. Food and Drug Administration. Drug approval package: Uceris (budesonide) 9 mg tablets. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2013/203634_uceris_toc.cfm. Updated 2013. Accessed 4/14, 2016.
- 17.U.S. Food and Drug Administration. Uceris (budesonide) rectal foam. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/205613Orig1s000TOC.cfm. Updated 2015. Accessed 4/14/2016, 2016.
- 18.Ryrfeldt A, Edsbacker S, Pauwels R. Kinetics of the epimeric glucocorticoid budesonide. Clin Pharmacol Ther. 1984;35(4):525–530. doi: 10.1038/clpt.1984.71. [DOI] [PubMed] [Google Scholar]
- 19.Dahlberg E, Thalen A, Brattsand R, et al. Correlation between chemical structure, receptor binding, and biological activity of some novel, highly active, 16 alpha, 17 alpha-acetal-substituted glucocorticoids. Mol Pharmacol. 1984;25(1):70–78. [PubMed] [Google Scholar]
- 20.Hamedani R, Feldman RD, Feagan BG. Review article: Drug development in inflammatory bowel disease: Budesonide–a model of targeted therapy. Aliment Pharmacol Ther. 1997;11(Suppl 3):98–107. doi: 10.1111/j.1365-2036.1997.tb00814.x. discussion 107–8. [DOI] [PubMed] [Google Scholar]
- 21.Kolkman JJ, Mollmann HW, Mollmann AC, et al. Evaluation of oral budesonide in the treatment of active distal ulcerative colitis. Drugs Today (Barc) 2004;40(7):589–601. [PubMed] [Google Scholar]
- 22.Brunner M, Ziegler S, Di Stefano AF, et al. Gastrointestinal transit, release and plasma pharmacokinetics of a new oral budesonide formulation. Br J Clin Pharmacol. 2006;61(1):31–38. doi: 10.1111/j.1365-2125.2005.02517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klein S, Stein J, Dressman J. Site-specific delivery of anti-inflammatory drugs in the gastrointestinal tract: An in-vitro release model. J Pharm Pharmacol. 2005;57(6):709–719. doi: 10.1211/0022357056172. [DOI] [PubMed] [Google Scholar]
- 24.Jonsson G, Astrom A, Andersson P. Budesonide is metabolized by cytochrome P450 3A (CYP3A) enzymes in human liver. Drug Metab Dispos. 1995;23(1):137–142. [PubMed] [Google Scholar]
- 25.Dilger K, Schwab M, Fromm MF. Identification of budesonide and prednisone as substrates of the intestinal drug efflux pump P-glycoprotein. Inflamm Bowel Dis. 2004;10(5):578–583. doi: 10.1097/00054725-200409000-00012. [DOI] [PubMed] [Google Scholar]
- 26*.Ryrfeldt A, Andersson P, Edsbacker S, Tonnesson M, Davies D, Pauwels R. Pharmacokinetics and metabolism of budesonide, a selective glucocorticoid. Eur J Respir Dis Suppl. 1982;122:86–95. (Provides pharmacokinetics data on budesonide-MMX) [PubMed] [Google Scholar]
- 27.Silverman J, Otley A. Budesonide in the treatment of inflammatory bowel disease. Expert Rev Clin Immunol. 2011;7(4):419–428. doi: 10.1586/eci.11.34. [DOI] [PubMed] [Google Scholar]
- 28.Nicholls A, Harris-Collazo R, Huang M, Hardiman Y, Jones R, Moro L. Bioavailability profile of uceris MMX extended-release tablets compared with entocort EC capsules in healthy volunteers. J Int Med Res. 2013;41(2):386–394. doi: 10.1177/0300060513476588. [DOI] [PubMed] [Google Scholar]
- 29.Edsbacker S, Andersson T. Pharmacokinetics of budesonide (entocort EC) capsules for crohn’s disease. Clin Pharmacokinet. 2004;43(12):803–821. doi: 10.2165/00003088-200443120-00003. [DOI] [PubMed] [Google Scholar]
- 30.Medicines & Healthcare products Regulatory Agency, UK. Public assessment report: Budenofalk 9mg gastro-resistant granules (budesonide) - PL 08637/0020. UK/H/2778/001/DC. http://www.mhra.gov.uk/home/groups/par/documents/websiteresources/con111557.pdf. Updated 2011. Accessed 3/3, 2016.
- 31.Nyman-Pantelidis M, Nilsson A, Wagner ZG, Borga O. Pharmacokinetics and retrograde colonic spread of budesonide enemas in patients with distal ulcerative colitis. Aliment Pharmacol Ther. 1994;8(6):617–622. doi: 10.1111/j.1365-2036.1994.tb00339.x. [DOI] [PubMed] [Google Scholar]
- 32*.Danielsson A, Edsbacker S, Lofberg R, et al. Pharmacokinetics of budesonide enema in patients with distal ulcerative colitis or proctitis. Aliment Pharmacol Ther. 1993;7(4):401–407. doi: 10.1111/j.1365-2036.1993.tb00113.x. (Updated pharmacokinetics data on budesonide enema) [DOI] [PubMed] [Google Scholar]
- 33*.Brunner M, Vogelsang H, Greinwald R, et al. Colonic spread and serum pharmacokinetics of budesonide foam in patients with mildly to moderately active ulcerative colitis. Aliment Pharmacol Ther. 2005;22(5):463–470. doi: 10.1111/j.1365-2036.2005.02571.x. (Updated pharmacokinetics data on budesonide foam) [DOI] [PubMed] [Google Scholar]
- 34*.Rubin DT, Sandborn WJ, Bosworth B, et al. Budesonide foam has a favorable safety profile for inducing remission in mild-to-moderate ulcerative proctitis or proctosigmoiditis. Dig Dis Sci. 2015;60(11):3408–3417. doi: 10.1007/s10620-015-3868-5. (Provides safety data analysis for the use of budesonide foam in UC patients) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seidegard J. Reduction of the inhibitory effect of ketoconazole on budesonide pharmacokinetics by separation of their time of administration. Clin Pharmacol Ther. 2000;68(1):13–17. doi: 10.1067/mcp.2000.106895. [DOI] [PubMed] [Google Scholar]
- 36.Seidegard J, Randvall G, Nyberg L, Borga O. Grapefruit juice interaction with oral budesonide: Equal effect on immediate-release and delayed-release formulations. Pharmazie. 2009;64(7):461–465. [PubMed] [Google Scholar]
- 37.Sherlock ME, Seow CH, Steinhart AH, Griffiths AM. Oral budesonide for induction of remission in ulcerative colitis. Cochrane Database Syst Rev. 2010;10:CD007698. doi: 10.1002/14651858.CD007698.pub2. [DOI] [PubMed] [Google Scholar]
- 38*.Lofberg R, Danielsson A, Suhr O, et al. Oral budesonide versus prednisolone in patients with active extensive and left-sided ulcerative colitis. Gastroenterology. 1996;110(6):1713–1718. doi: 10.1053/gast.1996.v110.pm8964395. (Only study with comparison between oral budesonide and systemic steroid in UC patients) [DOI] [PubMed] [Google Scholar]
- 39**.Gross V, Bunganic I, Belousova EA, et al. 3g mesalazine granules are superior to 9mg budesonide for achieving remission in active ulcerative colitis: A double-blind, double-dummy, randomised trial. J Crohns Colitis. 2011;5(2):129–138. doi: 10.1016/j.crohns.2010.11.006. (Only study with comparison between oral budesonide and 5-ASA in UC patients) [DOI] [PubMed] [Google Scholar]
- 40*.D’Haens GR, Kovacs A, Vergauwe P, et al. Clinical trial: Preliminary efficacy and safety study of a new budesonide-MMX(R) 9 mg extended-release tablets in patients with active left-sided ulcerative colitis. J Crohns Colitis. 2010;4(2):153–160. doi: 10.1016/j.crohns.2009.09.007. (Earlier trial assessing budesonide-MMX in UC) [DOI] [PubMed] [Google Scholar]
- 41**.Sandborn WJ, Travis S, Moro L, et al. Once-daily budesonide MMX(R) extended-release tablets induce remission in patients with mild to moderate ulcerative colitis: Results from the CORE I study. Gastroenterology. 2012;143(5):1218–26.e1. doi: 10.1053/j.gastro.2012.08.003. (One of the two pivotal trials resulting in FDA approval of budesonide-MMX in UC patients) [DOI] [PubMed] [Google Scholar]
- 42**.Travis SP, Danese S, Kupcinskas L, et al. Once-daily budesonide MMX in active, mild-to-moderate ulcerative colitis: Results from the randomised CORE II study. Gut. 2014;63(3):433–441. doi: 10.1136/gutjnl-2012-304258. (One of the two pivotal trials resulting in FDA approval of budesonide-MMX in UC patients) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43*.Rubin D, Russell C, William S, et al. O-001 budesonide MMX(R) 9 mg for inducing remission in patients with mild-to-moderate ulcerative colitis not adequately controlled with oral 5-ASAs. Inflamm Bowel Dis. 2014;20(Supplement 1):S1. (Assessed the efficacy of budesonide-MMX in particular subpopulation of UC patients who failed 5-ASA- available in abstract format only) [Google Scholar]
- 44.Sandborn WJ, Danese S, D’Haens G, et al. Induction of clinical and colonoscopic remission of mild-to-moderate ulcerative colitis with budesonide MMX 9 mg: Pooled analysis of two phase 3 studies. Aliment Pharmacol Ther. 2015;41(5):409–418. doi: 10.1111/apt.13076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45*.Sherlock ME, MacDonald JK, Griffiths AM, Steinhart AH, Seow CH. Oral budesonide for induction of remission in ulcerative colitis. Cochrane Database Syst Rev. 2015;10:CD007698. doi: 10.1002/14651858.CD007698.pub3. (Cochrane review of the trials assessing the efficacy of oral budesonide in UC patients. also provided subgroup analysis) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46*.Sandborn WJ, Danese S, Ballard ED, et al. Su2080 efficacy of budesonide MMx(r) 6 mg QD for the maintenance of remission in patients with ulcerative colitis: Results from a phase III, 12 month safety and extended use study. Gastroenterology. 2012;142(5, Supplement 1):S-564. (Only study with maintenance data for oral budesonide-MMX in UC patients- available in abstract format only) [Google Scholar]
- 47.KELLER R, STOLL R, FOERSTER EC, GUTSCHE N, DOMSCHKE W. Oral budesonide therapy for steroid-dependent ulcerative colitis: A pilot trial. Aliment Pharmacol Ther. 1997;11(6):1047–1052. doi: 10.1046/j.1365-2036.1997.00263.x. [DOI] [PubMed] [Google Scholar]
- 48*.Danielsson A, Lofberg R, Persson T, et al. A steroid enema, budesonide, lacking systemic effects for the treatment of distal ulcerative colitis or proctitis. Scand J Gastroenterol. 1992;27(1):9–12. doi: 10.3109/00365529209011158. (One of the earlier trials assessing the efficacy of rectal budesonide in patients with left-sided UC) [DOI] [PubMed] [Google Scholar]
- 49*.Hanauer SB, Robinson M, Pruitt R, et al. Budesonide enema for the treatment of active, distal ulcerative colitis and proctitis: A dose-ranging study. U.S. budesonide enema study group. Gastroenterology. 1998;115(3):525–532. doi: 10.1016/s0016-5085(98)70131-3. (Only study assessed the efficacy of different doses per application of rectal budesonide) [DOI] [PubMed] [Google Scholar]
- 50**.Sandborn WJ, Bosworth B, Zakko S, et al. Budesonide foam induces remission in patients with mild to moderate ulcerative proctitis and ulcerative proctosigmoiditis. Gastroenterology. 2015;148(4):740–750.e2. doi: 10.1053/j.gastro.2015.01.037. (Results of the two pivotal studies assessing the efficacy of rectal budesonide foam on Uc and resulted in FDA approval) [DOI] [PubMed] [Google Scholar]
- 51*.Naganuma M, Aoyama N, Suzuki Y, et al. Twice-daily budesonide 2-mg foam induces complete mucosal healing in patients with distal ulcerative colitis. J Crohns Colitis. 2015 doi: 10.1093/ecco-jcc/jjv208. doi: jjv208 [pii]. (Provide comparison between BID and daily dosing of rectal budesonide for induction of remission in patients with left-sided UC) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52**.Lindgren S, Lofberg R, Bergholm L, et al. Effect of budesonide enema on remission and relapse rate in distal ulcerative colitis and proctitis. Scand J Gastroenterol. 2002;37(6):705–710. doi: 10.1080/00365520212512. (Only study with maintenance data on the efficacy of rectal budesonide in left sided UC) [DOI] [PubMed] [Google Scholar]
- 53*.Gross V, Bar-Meir S, Lavy A, et al. Budesonide foam versus budesonide enema in active ulcerative proctitis and proctosigmoiditis. Aliment Pharmacol Ther. 2006;23(2):303–312. doi: 10.1111/j.1365-2036.2006.02743.x. (One of the trials comparing the efficacy of rectal budesonide to other forms of rectal corticosteroids) [DOI] [PubMed] [Google Scholar]
- 54*.Danielsson A, Hellers G, Lyrenas E, et al. A controlled randomized trial of budesonide versus prednisolone retention enemas in active distal ulcerative colitis. Scand J Gastroenterol. 1987;22(8):987–992. doi: 10.3109/00365528708991947. (One of the trials comparing the efficacy of rectal budesonide to other forms of rectal corticosteroids) [DOI] [PubMed] [Google Scholar]
- 55*.Matzen P. Budesonide enema in distal ulcerative colitis. A randomized dose-response trial with prednisolone enema as positive control. the danish budesonide study group. Scand J Gastroenterol. 1991;26(12):1225–1230. doi: 10.3109/00365529108998618. (One of the trials comparing the efficacy of rectal budesonide to other forms of rectal corticosteroids) [DOI] [PubMed] [Google Scholar]
- 56*.Lofberg R, Ostergaard Thomsen O, Langholz E, et al. Budesonide versus prednisolone retention enemas in active distal ulcerative colitis. Aliment Pharmacol Ther. 1994;8(6):623–629. doi: 10.1111/j.1365-2036.1994.tb00340.x. (One of the trials comparing the efficacy of rectal budesonide to other forms of rectal corticosteroids) [DOI] [PubMed] [Google Scholar]
- 57*.Tarpila S, Turunen U, Seppala K, et al. Budesonide enema in active haemorrhagic proctitis–a controlled trial against hydrocortisone foam enema. Aliment Pharmacol Ther. 1994;8(6):591–595. doi: 10.1111/j.1365-2036.1994.tb00335.x. (One of the trials comparing the efficacy of rectal budesonide to other forms of rectal corticosteroids) [DOI] [PubMed] [Google Scholar]
- 58*.Porro GB, Prantera C, Campierit M, et al. Comparative trial of methylprednisolone and budesonide enemas in active distal ulcerative colitis. Eur J Gastroenterol Hepatol. 1994;6(2):125–130. (One of the trials comparing the efficacy of rectal budesonide to other forms of rectal corticosteroids) [Google Scholar]
- 59*.Bar-Meir S, Fidder HH, Faszczyk M, et al. Budesonide foam vs. hydrocortisone acetate foam in the treatment of active ulcerative proctosigmoiditis. Dis Colon Rectum. 2003;46(7):929–936. doi: 10.1007/s10350-004-6687-x. (One of the trials comparing the efficacy of rectal budesonide to other forms of rectal corticosteroids) [DOI] [PubMed] [Google Scholar]
- 60*.Lemann M, Galian A, Rutgeerts P, et al. Comparison of budesonide and 5-aminosalicylic acid enemas in active distal ulcerative colitis. Aliment Pharmacol Ther. 1995;9(5):557–562. doi: 10.1111/j.1365-2036.1995.tb00421.x. (One of the trials comparing the efficacy of rectal budesonide to rectal 5-ASA) [DOI] [PubMed] [Google Scholar]
- 61*.Hartmann F, Stein J, BudMesa-Study Group Clinical trial: Controlled, open, randomized multicentre study comparing the effects of treatment on quality of life, safety and efficacy of budesonide or mesalazine enemas in active left-sided ulcerative colitis. Aliment Pharmacol Ther. 2010;32(3):368–376. doi: 10.1111/j.1365-2036.2010.04354.x. (One of the trials comparing the efficacy of rectal budesonide to rectal 5-ASA) [DOI] [PubMed] [Google Scholar]
- 62*.Lichtenstein GR, Travis S, Danese S, et al. Budesonide MMX for the induction of remission of mild to moderate ulcerative colitis: A pooled safety analysis. J Crohns Colitis. 2015;9(9):738–746. doi: 10.1093/ecco-jcc/jjv101. (Provide safety data of the new budesonide-MMX formulation) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lichtenstein GR. Budesonide multi-matrix for the treatment of patients with ulcerative colitis. Dig Dis Sci. 2016;61(2):358–370. doi: 10.1007/s10620-015-3897-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Danese S, Siegel CA, Peyrin-Biroulet L. Review article: Integrating budesonide-MMX into treatment algorithms for mild-to-moderate ulcerative colitis. Aliment Pharmacol Ther. 2014;39(10):1095–1103. doi: 10.1111/apt.12712. [DOI] [PubMed] [Google Scholar]
- 65.Gionchetti P, Pratico C, Rizzello F, et al. The role of budesonide-MMX in active ulcerative colitis. Expert Rev Gastroenterol Hepatol. 2014;8(3):215–222. doi: 10.1586/17474124.2014.887437. [DOI] [PubMed] [Google Scholar]
- 66.Safdi M, DeMicco M, Sninsky C, et al. A double-blind comparison of oral versus rectal mesalamine versus combination therapy in the treatment of distal ulcerative colitis. Am J Gastroenterol. 1997;92(10):1867–1871. [PubMed] [Google Scholar]
- 67.Kuenzig ME, Rezaie A, Seow CH, et al. Budesonide for maintenance of remission in crohn’s disease. Cochrane Database Syst Rev. 2014;8:CD002913. doi: 10.1002/14651858.CD002913.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Herfarth H, Gross V, Andus T, et al. Analysis of the therapeutic efficacy of different doses of budesonide in patients with active crohn’s ileocolitis depending on disease activity and localization. Int J Colorectal Dis. 2004;19(2):147–152. doi: 10.1007/s00384-003-0529-5. [DOI] [PubMed] [Google Scholar]
- 69.Bebb JR, Scott BB. How effective are the usual treatments for ulcerative colitis? Aliment Pharmacol Ther. 2004;20(2):143–149. doi: 10.1111/j.1365-2036.2004.02018.x. [DOI] [PubMed] [Google Scholar]
- 70.GoodRx. http://www.goodrx.com/. Updated 2016. Accessed 3/3, 2016.


