Abstract
Purpose: Preoperative radiological predictions of pathological invasiveness must be objective and reproducible in addition to being accurate when considering limited surgery for early lung cancer.
Methods: Two cohorts were used for the analysis. Two independent observers traced lesion edges and measured areas and proportions of solid component on tumor images with the largest diameter by high resolution computed tomography images and “Image J” software.
Results: The value of the intraclass correlation was 0.997 (95% confidence interval [CI], 0.996–0.998) for the area of solid component and 0.979 (95%CI, 0.958–0.986) for the proportion of solid component, suggesting such parameters were reliable in terms of reproducibility. Az value was 0.898 (95%CI, 0.842–0.953) for the area of solid component and 0.882 (95%CI, 0.816–0.949) for the proportion of solid component, demonstrating 2 parameters were both highly predictive of non-invasive adenocarcinoma. The optimal prediction of non-invasive adenocarcinoma with a cut-off value of 7.5 mm2 for the area of solid component resulted in a sensitivity of 85.3% and specificity of 86.2% in Cohort 1 and a sensitivity of 66.7% and specificity of 88.5% in Cohort 2.
Conclusion: Image analysis using “Image J” software was promising for predicting non-invasive adenocarcinoma with its limited inter-observer variability and high predictive performance.
Keywords: pathology, tomography, X-ray computed, carcinoma, non-small-cell lung, radiology
Introduction
As a result of recent advances in computed tomography (CT) imaging and the prevalence of lung cancer screening currently being performed using helical CT, detection of small and early lung cancers that are invisible on chest X-rays is increasing in both Japan and the United States.1,2) Lung cancers detected by helical CT are mostly adenocarcinomas in the periphery of the lung.3) Such adenocarcinomas frequently display a ground-glass nodule (GGN) appearance that indicates a hazy increase of lung attenuation on high resolution CT (HRCT). Lung adenocarcinomas with GGN on HRCT images rarely involve lymph-node metastasis and lung adenocarcinomas ≤2 cm in size with GGN are considered to be good candidates for limited resection although lobectomy remains the mainstay surgical treatment for lung cancers.4,5)
Although several methods for radiological prediction of pathological invasiveness have been proposed, their objectivity and reproducibility among observers have not been examined as yet.6–8) Definitions of early lung adenocarcinoma using computer software have recently been reported that seem quite promising.9,10) In this study, we examined predictive performance for radiological evaluation of non-invasive adenocarcinoma of the lung, which would be an appropriate candidate for limited resection, using Image J software provided by the National Institutes of Health.11)
Materials and Methods
We retrospectively reviewed the medical charts for patients clinically staged as IA with primary lung adenocarcinomas ≤2 cm who underwent surgery at The University of Tokyo Hospital and whose HRCT images were available in the Digital Imaging and Communications in Medicine (DICOM) format. The study consisted of 2 patient cohorts with Cohort 1 including 157 such patients who underwent surgery from January 2001 to December 2008 and Cohort 2 including 41 patients who fulfilled the above-mentioned criteria and received surgery from January 2009 to December 2009. Cohort 1 was used as a “test set” for the purpose of calculating the appropriate cut-off values for the prediction of pathological findings. Such values were then applied to Cohort 2 which was used as a “validation set” to verify the predictive performance.
Preoperative staging included conducting a chest CT scan with liver and adrenal glands in the scanned area and brain CT if there were any signs or symptoms of brain metastasis. Contrast materials were generally used although they were omitted in certain cases because of contraindications or the preferences of the attending doctors. The slice thickness of HRCT images were 1–3 mm.
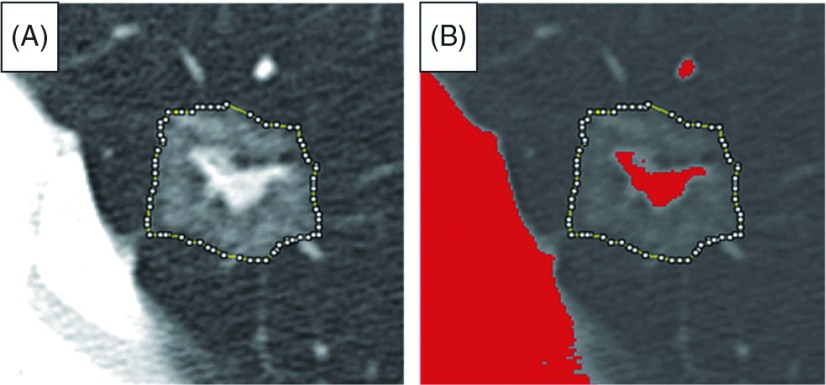
The DICOM file that included the largest lesion diameter was used for analysis and we performed the following procedure using Image J software:11) window level and window width were set at –600 and 1400 Hounsfield units (HUs), respectively. We traced the edge of the lesion using segmented line selections with the magnifying glass and scrolling tool (Fig. 1). Using the histogram feature, we were able to calculate the total number of pixels within the lesion and their distribution. We defined solid component as pixels whose CT numbers were greater than –160 HUs according to a previously reported study.10) Image J software automatically set the bin width in a histogram so we simply added together the number of pixels for intervals with CT numbers greater than –160 HUs and then calculated the area of solid component and proportion of solid component within the lesion. Information on pixel size was obtained from the DICOM header data using the “Show Info” function. Based on another earlier study, GGN was defined as CT appearance in which the internal density of a nodule was low and visualization of the bronchovascular structures in the area was still possible.4)
Fig. 1.
Tracing lesion edge with Image J software. (A) Tracing lesion edge using segmented line selections. (B) Solid component in lesion shown in red with solid component defined as pixels with computed tomography number greater than –160 Hounsfield units.
Two independent observers (Y.Y. and M.S.), who were unaware of the pathological findings, traced lesion edges using Image J software and the radiological information was compared with pathological findings to investigate radiological predictions of non-invasive adenocarcinoma for Cohort 1 cases. Cohort 2 cases were analyzed by 1 observer (YY).
Distribution of the 2 groups was assessed using the t-test for continuous variables and inter-observer variability was determined by calculating intraclass correlation and the corresponding 95% confidence interval (CI).12) Predictive performances were evaluated by receiver operating characteristic (ROC) analysis and areas under the respective curves were represented by Az values. The cut-off value of the parameter for optimal prediction was chosen so as to maximize the Youden index13) and all statistical analyses were performed with SAS 9.2 software (SAS Institute Inc., Cary, North Carolina, USA) and the SPSS statistical package, version 11.0 for Windows (SPSS Inc., Chicago, Illinois, USA).
Staging and pathological findings were based on the 7th TNM staging and 2004 WHO classification.14,15) Bronchioloalveolar carcinoma (BAC) was defined as a non-invasive adenocarcinoma that showed growth of neoplastic cells along pre-existing alveolar structures without evidence of stromal, vascular or pleural invasion. Pathological diagnosis was determined as a consensus of at least 2 board-certified pathologists.
This retrospective study was approved by the institutional review board and written informed consent from each patient was waived for the study.
Results
Patient characteristics are listed in Table 1. Cohort 1 included 34 BACs and 123 adenocarcinomas with mixed subtypes while Cohort 2 included 15 BACs and 26 adenocarcinomas with mixed subtypes. Classification of BAC was all non-mucinous subtype except for 1 mucinous BAC in Cohort 1.
Table 1.
Patient characteristics
| Cohort 1 (n = 157) | Cohort 2 (n = 41) | ||||
|---|---|---|---|---|---|
| BAC | AwMS | BAC | AwMS | ||
| Gender | Male | 19 | 68 | 6 | 13 |
| Female | 15 | 55 | 9 | 13 | |
| Mean age, Years | 67.1 | 66.0 | 61.9 | 68.3 | |
| Smokers, % | 58.8 | 54.5 | 53.3 | 57.7 | |
| Mean size, cm | 1.3 | 1.6 | 1.4 | 1.4 | |
| Surgery | Lobectomy | 11 | 98 | 4 | 18 |
| Segmentectomy | 2 | 5 | 4 | 3 | |
| Wedge Resection | 21 | 20 | 7 | 5 | |
BAC: bronchioloalveolar carcinoma; AwMS: adenocarcinoma with mixed subtypes
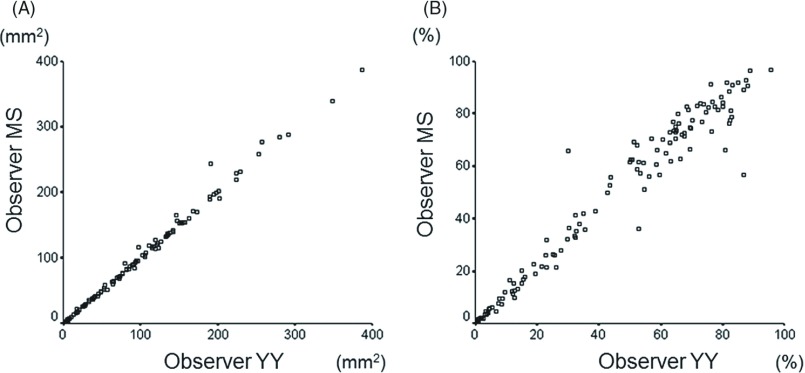
The value of intraclass correlation indicating inter-observer variability was 0.997 (95%CI, 0.996–0.998) for the area of solid component and 0.979 (95%CI, 0.958–0.986) for the proportion of solid component (Fig. 2).
Fig. 2.
Scatter plots of values measured by 2 independent observers. Each small circle indicates value measured with values measured by Observer YY shown in X-axis and Observer MS in Y-axis. (A) Scatter plot for area of solid component. (B) Scatter plot for proportion of solid component.
The mean area of solid component for 34 BACs was 9.1 mm2 ranging from 0 to 89.6 mm2 and 90.9 mm2 ranging from 0 to 386.2 mm2 for 123 adenocarcinomas with mixed subtypes in Cohort 1 with the area of solid component significantly smaller for BACs (p <0.001; t-test). The mean proportion of solid component for the 34 BACs was 7.8% ranging from 0% to 71% and 46.1% ranging from 0% to 96.1% for the 123 adenocarcinomas with mixed subtypes with the proportion of solid component once again significantly smaller for BACs (p <0.001; t-test).
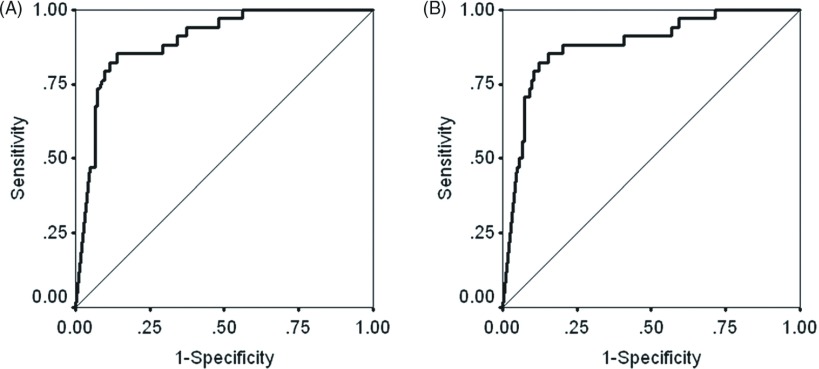
The Az value indicating predictive performance for BACs was 0.898 (95%CI, 0.842–0.953) for the area of solid component and 0.882 (95%CI, 0.816–0.949) for the proportion of solid component although the difference was not statistically significant (p = 0.121) (Fig. 3).
Fig. 3.
Receiver operating characteristic curves of area of solid component (A) and proportion of solid component (B) for the prediction of non-invasive adenocarcinoma.
Cut-off values maximizing the Youden index for optimal prediction of BAC were 7.5 mm2 for the area of solid component and 4.2% for the proportion of solid component. When the cut-off value for the area of solid component was set at 7.5 mm2, the sensitivity, specificity, positive predictive value and negative predictive value were 85.3%, 86.2%, 63.0% and 95.5%, respectively (Table 2). When the cut-off value for the proportion of solid component was set at 4.2%, the sensitivity, specificity, positive predictive value and negative predictive value were 82.4%, 87.8%, 65.1% and 94.7%, respectively (Table 2).
Table 2.
Optimal predictive performance of area and proportion of consolidation for non-invasive adenocarcinoma
| Cohort 1 (n = 157): Test set |
Cohort 2 (n = 41): Validation set |
|||
|---|---|---|---|---|
| BAC | AwMS | BAC | AwMS | |
| Area of consolidation | ||||
| ≤7.5 mm2 | 29 | 17 | 10 | 3 |
| >7.5 mm2 | 5 | 106 | 5 | 23 |
| Proportion of consolidation | ||||
| ≤4.2% | 28 | 15 | 10 | 3 |
| >4.2% | 6 | 108 | 5 | 23 |
BAC: bronchioloalveolar carcinoma; AwMS: adenocarcinoma with mixed subtypes
Predictive performance was validated in Cohort 2 with 41 patients. When the cut-off value of 7.5 mm2 for the area of solid component was applied, the sensitivity, specificity, positive predictive value and negative predictive value were 66.7%, 88.5%, 76.9% and 82.1%, respectively (Table 2). When the cut-off value of 4.2% for the proportion of solid component was applied, the sensitivity, specificity, positive predictive value and negative predictive value were 66.7%, 88.5%, 76.9% and 82.1%, respectively (Table 2).
Discussion
The proportion of solid component and the tumor shadow disappearance rate have both been reported to enhance the pathological malignancy of lung adenocarcinomas ≤2 cm in size.7,8) Those 2 studies were performed in a single institution, however, and inter-observer variability, which is critical in a multi-institutional setting, was not considered by the authors. In addition, differences in CT scanning equipment as well as window level and window width settings can influence results, but some of those problems will be resolved by using computer software.9,10) We also examined the accuracy of the predictive performance of Image J software by applying the cut-off values obtained from the test set to a second cohort of patients used as the validation set.
In this study, we examined the area of solid component and proportion of solid component in the lesion. Tracing the edge of a lesion with GGN in the periphery is difficult in some cases because of faint and ambiguous margins and could be a cause of inter-observer variability for the proportion of solid component although the area of solid component in the lesion is considered to be less influenced by tracing.
Our study demonstrated the intraclass correlation of both the area of solid component and the proportion of solid component was high suggesting such parameters were reliable in terms of reproducibility among more than 1 observer. The intraclass correlation value for the area of solid component tended to be higher than such value for the proportion of solid component. The intraclass correlation of the area of the entire lesion calculated by the 2 observers independently tracing the edge of the lesion with segmented line selections using Image J software was 0.928 (95%CI, 0.891–0.948) and this lower value indicated tracing of faint GGN in the periphery varied between the observers. We assume the proportion of solid component in GGN lesions will vary among observers because the proportion of solid component equals the pixel number of solid component divided by the total pixel number for the entire lesion. We believe radiological prediction using the area of solid component, therefore, is superior to the proportion of solid component for the selection of candidates for limited resection given such inter-observer variability.
Predictive performance of HRCT images and Image J software for non-invasive adenocarcinoma was analyzed by calculating Az values for both the area of solid component and the proportion of solid component. The results demonstrated the 2 parameters were both highly predictive of non-invasive adenocarcinoma, but we have to be cautious because cut-off values were determined in a mathematically applied method separate and apart from their clinical validity. Although Az values were high, the respective ranges for the area of solid component and proportion of solid component overlapped between BACs and adenocarcinomas with mixed subtypes. When we predict early lung adenocarcinoma and select suitable candidates for sublobar resection in a clinical setting, we need to set cut-off values so as to make specificity sufficiently high.
Non-invasive adenocarcinoma would be an ideal target for limited resection. A prospective trial involving 50 cases reported excellent results16) and another study demonstrated minimally invasive adenocarcinomas were also candidates for limited resection.17) The JCOG 0201 study defined a lung adenocarcinoma without nodal involvement, vascular invasion or lymphatic invasion as provisionally pathological non-invasive lung cancer.18) Our study adopted the 2004 WHO classification for pathological diagnosis of lung adenocarcinoma so detailed pathological analysis as to the degree of invasion based on the newly proposed IASLC/ATS/ERS classification of lung adenocarcinoma, which refers to “invasive adenocarcinoma and minimally invasive adenocarcinoma” instead, was not performed because of the retrospective nature of the study.19) Consequently, validity of the appropriate targets for limited resection and predictive performance for radiological evaluation of minimally invasive adenocarcinoma using Image J software should be examined in a future prospective trial. Even with non-invasive adenocarcinomas, however, 3 cases of possible delayed cut-end recurrences following limited resections have been reported after initially positive results.16,20)
Yoshioka, et al. reported 18F-fluorodeoxyglucose positron emission tomography (18F-FDG PET) in combination with HRCT findings could predict non-invasive non-small cell carcinoma.21) Predictive performance of 18F-FDG PET for selection of early lung adenocarcinoma should also be examined.
Limitations of this study include the fact that inter-observer variability among more than 2 observers was not considered; it was impossible to eliminate vessels with the same CT numbers as solid component because Image J software could not distinguish such vessels from solid component within lesions; and distribution of solid component within lesions was not taken into account. In addition, the cut-off value for solid component was set at –160 HUs based on a previous study conducted by other researchers10) so it can be argued such a cut-off value was not defined from the standpoint of clinical importance. There would still be variability given different methods and computer softwares being used among studies.9,10) Other cut-off values have also been proposed22,23) and the optimal cut-off value should be evaluated for solid component. Sensitivity was 85.3% for the area of sold component and 82.4% for the proportion of solid component in Cohort 1, while they were 66.7% in Cohort 2. Cohort 1 included 157 patients, while there were 41 patients in Cohort 2. BAC consisted of 21.7% (34/157) in Cohort 1, while the proportion of BAC was 36.6% in Cohort 2. These results mean that Cohort 2 had fewer patients in total and higher proportion of BAC compared to Cohort 1. Heterogeneous distributions of patients between 2 groups will explain relatively low sensitivity in Cohort 2. We will need more cases to validate cut-off values and small number of patients is also a limitation in our study.
The methods in our study will be beneficial for clinical settings because they enable more detailed analysis of HRCT images compared to current standard of measurement performed by the radiologist. Another advantage of using computer software is cases can be analyzed on a multi-institutional basis because DICOM is a standard format in the field of medical imaging.
Conclusion
In the future, therefore, a prospective study on limited resections in a multi-institutional setting should be conducted based on a definition of early lung adenocarcinoma that is both objective and reproducible. Computer software such as Image J used in this study will be a useful means to facilitate the analysis of such cases.
Disclosure Statement
The authors hereby declare that there is no conflict of interest associated with this study.
References
- 1).Kaneko M, Eguchi K, Ohmatsu H, et al. Peripheral lung cancer: screening and detection with low-dose spiral CT versus radiography. Radiology 1996; 201: 798-802. [DOI] [PubMed] [Google Scholar]
- 2).International Early Lung Cancer Action Program Investigators. Henschke CI, Yankelevitz DF, et al. Survival of patients with stage I lung cancer detected on CT screening. N Engl J Med 2006; 355: 1763-71. [DOI] [PubMed] [Google Scholar]
- 3).Flieder DB, Vazquez M, Carter D, et al. Pathologic findings of lung tumors diagnosed on baseline CT screening. Am J Surg Pathol 2006; 30: 606-13. [DOI] [PubMed] [Google Scholar]
- 4).Asamura H, Suzuki K, Watanabe S, et al. A clinicopathological study of resected subcentimeter lung cancers: a favorable prognosis for ground glass opacity lesions. Ann Thorac Surg 2003; 76: 1016-22. [DOI] [PubMed] [Google Scholar]
- 5).Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995; 60: 615-22. [DOI] [PubMed] [Google Scholar]
- 6).Suzuki K, Kusumoto M, Watanabe S, et al. Radiologic classification of small adenocarcinoma of the lung: radiologic-pathologic correlation and its prognostic impact. Ann Thorac Surg 2006; 81: 413-9. [DOI] [PubMed] [Google Scholar]
- 7).Suzuki K, Asamura H, Kusumoto M, et al. “Early” peripheral lung cancer: prognostic significance of ground glass opacity on thin-section computed tomographic scan. Ann Thorac Surg 2002; 74: 1635-9. [DOI] [PubMed] [Google Scholar]
- 8).Takamochi K, Nagai K, Yoshida J, et al. Pathologic N0 status in pulmonary adenocarcinoma is predictable by combining serum carcinoembryonic antigen level and computed tomographic findings. J Thorac Cardiovasc Surg 2001; 122: 325-30. [DOI] [PubMed] [Google Scholar]
- 9).Nakata M, Sawada S, Yamashita M, et al. Objective radiologic analysis of ground-glass opacity aimed at curative limited resection for small peripheral non-small cell lung cancer. J Thorac Cardiovasc Surg 2005; 129: 1226-31. [DOI] [PubMed] [Google Scholar]
- 10).Matsuguma H, Nakahara R, Anraku M, et al. Objective definition and measurement method of ground-glass opacity for planning limited resection in patients with clinical stage IA adenocarcinoma of the lung. Eur J Cardiothorac Surg 2004; 25: 1102-6. [DOI] [PubMed] [Google Scholar]
- 11).Abramoff MD, Magelhaes PJ, Ram SJ. Image Processing with Image J. Biophotonics International 2004; 11: 36-42. [Google Scholar]
- 12).Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull 1979; 86: 420-8. [DOI] [PubMed] [Google Scholar]
- 13).Perkins NJ, Schisterman EF. The inconsistency of “optimal” cutpoints obtained using two criteria based on the receiver operating characteristic curve. Am J Epidemiol 2006; 163: 670-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).International Union Against Cancer (UICC) Sobin LH, Gospodarowicz MK, Wittekind C, eds.; TNM Classification of Malignant Tumours. 7th ed. Oxford: Wiley-Blackwell, 2009. [Google Scholar]
- 15).Travis WD, Brambilla E, Müller-Hermelink HK, et al., eds.; Pathology & Genetics of Tumours of the Lung, Thymus and Heart (World Health Organization Classification of Tumours). Lyon: IARC Press, 2004. [Google Scholar]
- 16).Yoshida J, Nagai K, Yokose T, et al. Limited resection trial for pulmonary ground-glass opacity nodules: fifty-case experience. J Thorac Cardiovasc Surg 2005; 129: 991-6. [DOI] [PubMed] [Google Scholar]
- 17).Sakurai H, Maeshima A, Watanabe S, et al. Grade of stromal invasion in small adenocarcinoma of the lung: histopathological minimal invasion and prognosis. Am J Surg Pathol 2004; 28: 198-206. [DOI] [PubMed] [Google Scholar]
- 18).Suzuki K, Koike T, Asakawa T, et al. A prospective radiological study of thin-section computed tomography to predict pathological noninvasiveness in peripheral clinical IA lung cancer (Japan Clinical Oncology Group 0201). J Thorac Oncol 2011; 6: 751-6. [DOI] [PubMed] [Google Scholar]
- 19).Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011; 6: 244-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20).Yoshida J, Ishii G, Yokose T, et al. Possible delayed cut-end recurrence after limited resection for ground-glass opacity adenocarcinoma, intraoperatively diagnosed as Noguchi type B, in three patients. J Thorac Oncol 2010; 5: 546-50. [DOI] [PubMed] [Google Scholar]
- 21).Yoshioka M, Ichiguchi O. Selection of sublobar resection for c-stage IA non-small cell lung cancer based on a combination of structural imaging by CT and functional imaging by FDG PET. Ann Thorac Cardiovasc Surg 2009; 15: 82-8. [PubMed] [Google Scholar]
- 22).Okada T, Iwano S, Ishigaki T, et al. Computer-aided diagnosis of lung cancer: definition and detection of ground-glass opacity type of nodules by high-resolution computed tomography. Jpn J Radiol 2009; 27: 91-9. [DOI] [PubMed] [Google Scholar]
- 23).Yanagawa M, Tanaka Y, Kusumoto M, et al. Automated assessment of malignant degree of small peripheral adenocarcinomas using volumetric CT data: correlation with pathologic prognostic factors. Lung Cancer 2010; 70: 286-94. [DOI] [PubMed] [Google Scholar]