Abstract
Purpose: The purpose of this study was to clarify relationships between intraoperative blood loss (IBL) and long-term postsurgical survival in lung cancer patients.
Methods: We retrospectively analyzed 1336 patients undergoing surgery: lobectomy in 1016, sublobar resection in 174, pneumonectomy in 106, and combined resection with adjacent organs in 40. The lobectomy group was stratified further by pathologic stages; overall survival difference was examined according to amount of IBL.
Results: Volume of IBL differed significantly according to surgical procedure when all patients were included. Within the lobectomy group, IBL differed significantly between gender, pathologic stage, histologic type (adenocarcinoma vs. non-adenocarcinoma), and year of operation (1983 to 2002 vs. 2003 to 2012). After stratification by pathologic stage, survival differed with IBL for stages IB to IIIB. Multivariate analysis identified gender, patients age (<69 vs. ≥69), pathologic stage (IA to IIB vs. IIIA to IV), year of operation, histologic type, and IBL as significant predictors of survival.
Conclusion: Since degree of IBL is an independent predictor of overall survival after lung cancer resection, IBL should be minimized carefully during surgery.
Keywords: intraoperative blood loss, lobectomy, lung cancer, prognosis, surgery
Introduction
Since volume of intraoperative blood loss (IBL) reflects degree of surgical invasiveness, IBL could affect patient outcome. However, associations between IBL and long-term postoperative survival in patients with lung cancer have been reported only rarely. Generally, IBL is likely to increase as tumors progress, since difficulty of complete tumor removal depends on extent of tumor invasion. If a survival difference according to IBL existed in patients at the same pathologic stage who underwent resection by the same surgical method, a survival difference could have been caused by IBL or IBL-related events such as perioperative blood transfusion.
Perioperative blood transfusion frequently has been reported to worsen prognosis in patients with various malignant tumors1–4) including lung cancer.5,6) In some of these reports, immune suppression after blood transfusion was emphasized as the reason for a worse prognosis. However, conflicting results denying adverse effects of transfusion also have been reported.7–11) In a systematic review12) analyzing 5378 patients with lung cancer from a total of 19 articles, half of the papers mentioned significantly increased risk of recurrence associated with transfusion; the other half noted no adverse effects of transfusion. The authors of the review concluded that no consensus was evident from the presented evidence about whether blood transfusions were associated with poorer outcomes after resection for lung cancer. Since blood products transfused have changed considerably over the past 30 years –from whole blood to packed red blood cells (PRBC) and leukocyte-depleted RBC– one would encounter difficulty in analyzing the effects of the various transfusions given over this long period.
Perhaps more informative than blood transfusion, greater volume of IBL has been reported to be a negative prognostic factor for patients with cancers of prostate,13) pancreas,14) colon and rectum,15) and liver.16,17) We therefore retrospectively analyzed the relationships between IBL and various clinicopathologic factors in lung cancer patients who underwent surgery in order to clarify whether extent of IBL affected long-term postoperative patient survival.
Patients and Methods
From January 1983 to December 2012, a total of 1336 patients underwent resection of lung cancer in our hospital. Operative records and pathologic reports in our database of lung cancer were reviewed to obtain the necessary information. Operative methods included lobectomy or bilobectomy in 1016, sublobar resection including segmentectomy and wedge resection in 174, pneumonectomy in 106, and combined resection with adjacent organs in 40. Standard dissection of lymph nodes (ND2a) was performed in lobectomy or larger resection. Sampling of lymph nodes was done in segmentectomy, and no lymphadenectomy was done in wedge resection. A final diagnosis of lung cancer was obtained from the resected lung specimen in all cases. Postoperative pathologic stages of patients were determined according to the most recent international staging criteria for lung cancer, published by the International Association for the Study of Lung Cancer in 2009.18) The mean postoperative follow-up period was 37 ± 34 months (range, 1 to 219). This retrospective study was approved by the Institutional Review Board of our institution.
Statistical software used was StatView ver 5.0 (SAS Institute Inc., Cary, North Carolina, USA). Values were compared between 2 groups by the non-parametric Mann- Whitney U test, while values were compared among 3 groups or more by the Kruskal-Wallis test. Estimated overall survival rate after surgery was calculated by the Kaplan-Meier method, and survival differences between patient groups were tested by the log-rank test. In survival studies to confirm the effects of IBL, patients in each group were divided into 2 subgroups of equal size according to amount of IBL (median or less vs. exceeding the median). A multivariate analysis was carried out according to the Cox regression model to identify independent risk factors. Values of p below 0.05 were considered significant for all statistical tests.
Results
Including all 1336 patients, IBL varied with operative choices closely linked to extent of lung cancer progression; greatest blood loss was observed in patients undergoing combined resection, followed in turn by patients undergoing pnemonectomy; lobectomy; and lastly, sublobar resection (p <0.0001, Table 1). After confirming that operative methods were associated significantly with IBL, further analyses were limited to the lobectomy group.
Table 1.
Intraoperative blood loss in surgery for lung cancer by procedure
| Procedure | Patient number | IBL (mL, mean ± SD) | p valuea |
|---|---|---|---|
| Lobectomy | 1016 | 445 ± 449 | |
| Sublobar resection | 174 | 88 ± 166 | |
| Pneumonectomy | 106 | 908 ± 790 | |
| Combined resection | 40 | 1171 ± 810 | <0.0001 |
| Total | 1336 | 457 ± 524 |
a: Kruskal-Wallis test. IBL: intraoperative blood loss; SD: standard deviation
Profiles of 1016 patients undergoing lobectomy are shown in Table 2. In this group, a significant survival difference was obtained between subgroups defined by volume of IBL (p <0.0001). Additionally, IBL differed significantly by gender (p <0.0001), pathologic stage (p <0.0001), histologic type (adenocarcinoma vs. non-adenocarcinoma; p <0.0001), and year of operation (1983 to 2002 vs. 2003 to 2012; p <0.0001).
Table 2.
Intraoperative blood loss and survival in 1016 patients who underwent lobectomy for lung cancer
| Patient number | IBL (mL, mean ± SD) | p value | 5-year survival (%) | p value | |
|---|---|---|---|---|---|
| IBL | |||||
| ≤318 mL | 509 | 176 ± 81 | 73.6 | ||
| >318 mL | 507 | 713 ± 504 | <0.0001a | 45.0 | <0.0001b |
| Gender | |||||
| Male | 640 | 525 ± 510 | 49.8 | ||
| Female | 376 | 307 ± 269 | <0.0001a | 73.9 | <0.0001b |
| Age | |||||
| <69 | 553 | 431 ± 403 | 62.9 | ||
| ≥69 | 463 | 460 ± 499 | 0.7622a | 53.1 | 0.0082b |
| Pathologic stage | |||||
| IA | 402 | 330 ± 322 | 81.7 | ||
| IB | 220 | 415 ± 434 | 62.6 | ||
| IIA | 92 | 499 ± 403 | 56.9 | ||
| IIB | 71 | 644 ± 609 | 24.5 | ||
| IIIA | 192 | 568 ± 517 | 31.8 | ||
| IIIB | 10 | 722 ± 613 | 20.0 | ||
| IV | 29 | 674 ± 680 | <0.0001c | 10.2 | <0.0001b |
| IA–IIB | 785 | 402 ± 408 | 67.7 | ||
| IIIA–IV | 231 | 588 ± 543 | <0.0001a | 28.8 | <0.0001b |
| Histologic type | |||||
| Ad | 660 | 373 ± 354 | 63.6 | ||
| Non-Ad | 356 | 577 ± 564 | <0.0001a | 48.9 | <0.0001b |
| Year of operation | |||||
| 1983–2002 | 555 | 588 ± 485 | 47.6 | ||
| 2003–2012 | 461 | 272 ± 328 | <0.0001a | 72.4 | <0.0001b |
| Total | 1016 | 445 ± 449 | 58.3 | ||
a: Mann-Whitney U test, b: Log-rank test, c: Kruskal-Wallis test. Ad: adenocarcinoma; IBL: intraoperative blood loss; Non-Ad: non-adenocarcinoma; SD: standard deviation
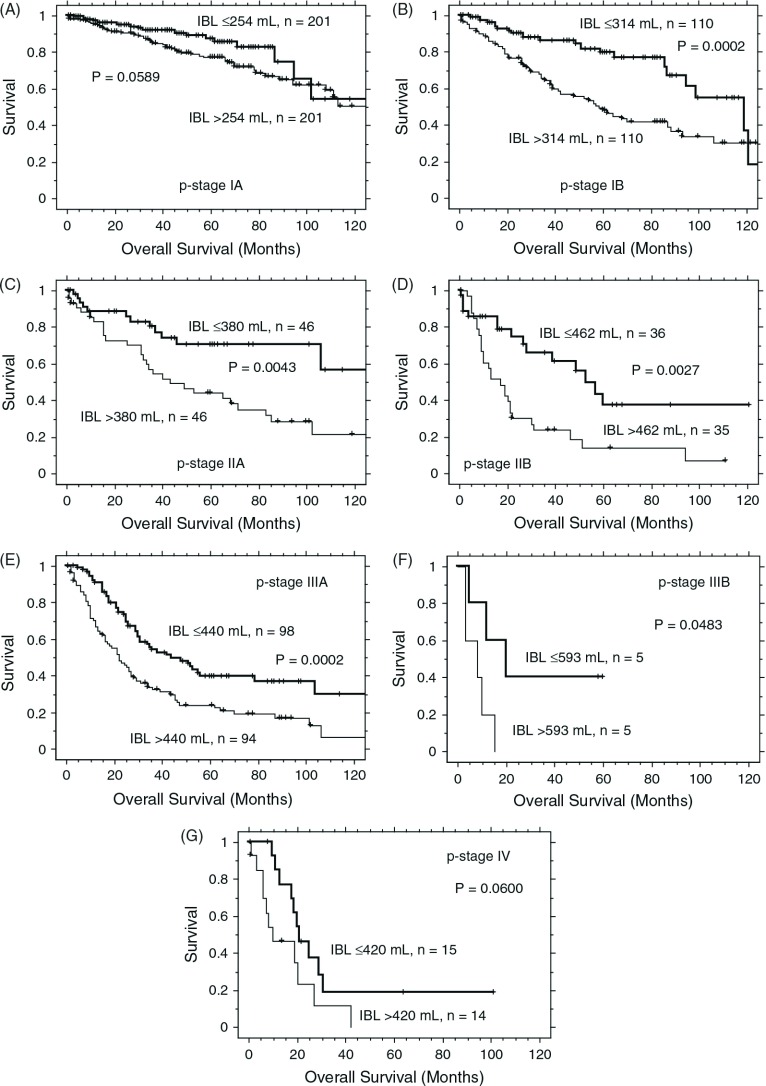
In each stratified disease stage within the lobectomy group, a significant survival difference related to IBL was found in pathologic (p-) stages IB (p = 0.0002), IIA (p = 0.0043), IIB (p = 0.0027), IIIA (p = 0.0002), and IIIB (p = 0.0483); differences in p values showed a lesser trend in p-stages IA (p = 0.0589) and IV (p = 0.0600, Fig. 1A–1G).
Fig. 1.
Kaplan-Meier survival curves according to the amount of intraoperative blood loss (IBL) for patients undergoing lobectomy at various pathologic (p-) stages. (A) p-IA, (B) p-IB, (C) p-IIA, (D) p-IIB, (E) p-IIIA, (F) p-IIIB, and (G) p-IV. In each instance, the median amount of IBL was used as the cut off value between equal size groups. Significance was found in p-stage IB to IIIB but not in p-stages IA and IV.
Univariate analysis by the log-rank test identified IBL, gender, patient age (<69 vs. ≥69), p-stage (IA to IIB vs. IIIA to IV), histologic type, and year of operation as significant predictors of survival (Table 2). Multivariate analysis identified all of these factors including IBL as significant predictors of survival (Table 3).
Table 3.
Multivariate analysis by the Cox regression model in patients undergoing lobectomy
| Characteristics | p valuea | Hazard ratio (95% CI) |
|---|---|---|
| IBL, ≤median | 0.0004 | 0.647 (0.510–0.822) |
| Gender, female | <0.0001 | 0.551 (0.428–0.710) |
| Age <69 | 0.0035 | 0.735 (0.598–0.904) |
| p-stage, IA-IIB | <0.0001 | 0.360 (0.291–0.445) |
| Histologic type, Ad | 0.0201 | 0.774 (0.623–0.961) |
| Year of operation, 2003–2012 | <0.0001 | 0.528 (0.408–0.684) |
a: Cox regression model. Ad: adenocarcinoma; CI: confidence interval; IBL: intraoperative blood loss
Discussion
This study found IBL in surgery for lung cancer to be associated closely with operative procedure, gender, disease stage, histologic type, and year of operation. Difference of IBL between both gender may be due to difference in average body size and difference in proportion of adenocarcinoma. Since non-adenocarcinomas including squamous cell carcinomas are more centrally located and locally invasive compared to adenocarcinomas, IBL is speculated to increase in non-adenocarcinomas. The large number of factors could confound attempts to evaluate effects of IBL on patient outcome. Since large IBL is likely in surgery for advanced lung cancer with a worse initial prognosis, high IBL might be indirectly associated with shorter survival. Therefore, in order to compare similar patients, we grouped patients undergoing lobectomy according to p-stage. Significant survival differences by IBL were obtained in p-stages IB, IIA, IIB, IIIA, and IIIB. In a multivariate analysis including known prognostic factors, IBL remained an independent predictor of survival in patients undergoing lobectomy. In survival analysis, postoperative death within 30 days after operation was found in 10 cases (0.98%) in the lobectomy group. Nine cases were postoperative infection or pneumonia including acute exacerbation of interstitial pneumonia. Death due to intraoperative massive bleeding was only one case in p-stage IV. Removal of these cases from the analysis did not change the survival results concerning IBL.
In our present study, we focused on IBL without considering perioperative blood transfusion, which had been identified as a factor worsening survival after resection of lung cancer in several studies.5,6) An initially postulated basis for worse prognosis involves immune modulation effects from passenger leukocytes present in allogeneic whole blood transfusions.19) Recently however, even leukocyte-depleted blood transfusion was reported to be associated with decreased survival in lung cancer resection patients.20) Another study fully disagreed, concluding that neither allogeneic nor autologous blood transfusion had an independent, adverse survival impact in lung cancer patients treated with radical resection.11) Numbers of patients requiring perioperative blood transfusion in lung cancer surgery have decreased dramatically since IBL has been decreased by new, less invasive surgical procedures such as video-assisted thoracoscopic surgery. In this study we analyzed IBL rather than blood transfusion to avoid difficulties in interpreting data influenced by changing blood product preferences.
Though we cannot deny possible adverse effects of blood transfusion in this study, we hypothesized that massive IBL can directly promote spread of cancer cells into circulating blood and induce immune dysfunction by decreasing numbers of leukocytes available to engage in anti-tumor immunity. An experimental study using a rat model showed that blood loss, 1 day prior to tumor challenge had a profound stimulating effect on tumor growth, while natural killer (NK) cell activity of cells from the spleen was significantly depressed 24 h after blood loss.21) Since NK cells are considered to participate in clearance of tumor cells from the circulation, enhanced tumor growth observed after blood loss might be caused by depressed NK function. Similarly, in a mouse model, NK cell cytotoxicity was significantly decreased 4 days after operative blood loss; the decrease correlated with the total amount of blood loss.22) Clinical studies have suggested that blood loss and surgical injury suppress cell-mediated immune responses through depression of macrophage antigen presentation capacity, depressed mitogenic response of T lymphocytes, and decreased T-helper 1 (Th1) lymphokines.23) Recent clinical trials in patients with non-small cell lung cancer using antibody-mediated immune checkpoint molecule blockade24,25) demonstrated importance of tumor immunity in controlling tumor growth. Therefore, a large IBL with or without blood transfusion likely would worsen the prognosis of patients undergoing resection of lung cancer.
Though statistically significant survival differences related to IBL were found in p-stages IB to IIIB, significance was not found in p-stages IA and IV. In p-stage IA, complete removal of cancer cells usually can be accomplished by surgery alone, so postoperative immune suppression from operative bleeding might not significantly affect relapse of lung cancer. In contrast, in p-stage IV, cancer cells already have spread through the circulation at the time of surgery, so immune suppression from bleeding may have limited effect on survival.
Only retrospective studies can provide data concerning association between IBL and postoperative survival, since prospective studies controlling for IBL are unethical. Since retrospective studies involve many confounding factors, multivariate analysis is essential for interpretation of study results. As IBL was selected as an independent predictor of survival among known prognostic factors, we believe that IBL can be an important determinant of patient outcome.
Conclusion
Our study identified degree of IBL as an independent predictor of overall survival after lung cancer resection. Since implications extend considerably beyond the perioperative period, we should take pains to minimize IBL in surgery for lung cancer in order to improve long-term outcome.
Disclosure Statement
No conflict of interest is declared.
References
- 1).Bongioannini G, Vercellino M, Rugiu MG, et al. Influence of perioperative transfusion therapy on the recurrence potential of locally advanced laryngeal carcinoma. ORL J Otorhinolaryngol Relat Spec 1990; 52: 260-4. [DOI] [PubMed] [Google Scholar]
- 2).Tartter PI. The association of perioperative blood transfusion with colorectal cancer recurrence. Ann Surg 1992; 216: 633-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).Tachibana M, Tabara H, Kotoh T, et al. Prognostic significance of perioperative blood transfusions in resectable thoracic esophageal cancer. Am J Gastroenterol 1999; 94: 757-65. [DOI] [PubMed] [Google Scholar]
- 4).Chau JK, Harris JR, Seikaly HR. Transfusion as a predictor of recurrence and survival in head and neck cancer surgery patients. J Otolaryngol Head Neck Surg 2010; 39: 516-22. [PubMed] [Google Scholar]
- 5).Tartter PI, Burrows L, Kirschner P. Perioperative blood transfusion adversely affects prognosis after resection of Stage I (subset N0) non-oat cell lung cancer. J Thorac Cardiovasc Surg 1984; 88: 659-62. [PubMed] [Google Scholar]
- 6).Little AG, Wu HS, Ferguson MK, et al. Perioperative blood transfusion adversely affects prognosis of patients with stage I non-small-cell lung cancer. Am J Surg 1990; 160: 630-2; discussion 633. [DOI] [PubMed] [Google Scholar]
- 7).Keller SM, Groshen S, Martini N, et al. Blood transfusion and lung cancer recurrence. Cancer 1988; 62: 606-10. [DOI] [PubMed] [Google Scholar]
- 8).Garau I, Benito E, Bosch FX, et al. Blood transfusion has no effect on colorectal cancer survival. A population-based study. Eur J Cancer 1994; 30A: 759-64. [DOI] [PubMed] [Google Scholar]
- 9).Heslin MJ, Gaynor JJ, Newman E, et al. Effect of perioperative blood transfusion on recurrence and survival in 232 primary high-grade extremity sarcoma patients. Ann Surg Oncol 1994; 1: 189-97. [DOI] [PubMed] [Google Scholar]
- 10).Monk BJ, Tewari K, Gamboa-Vujicic G, et al. Does perioperative blood transfusion affect survival in patients with cervical cancer treated with radical hysterectomy. Obstet Gynecol 1995; 85: 343-8. [DOI] [PubMed] [Google Scholar]
- 11).Rzyman W, Dziadziuszko R, Skokowski J, et al. The influence of blood transfusion on survival in operated non-small cell lung cancer patients. J Thorac Cardiovasc Surg 2003; 126: 755-60. [DOI] [PubMed] [Google Scholar]
- 12).Churchhouse AM, Mathews TJ, McBride OM, et al. Does blood transfusion increase the chance of recurrence in patients undergoing surgery for lung cancer. Interact Cardiovasc Thorac Surg 2012; 14: 85-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).Oefelein MG, Colangelo LA, Rademaker AW, et al. Intraoperative blood loss and prognosis in prostate cancer patients undergoing radical retropubic prostatectomy. J Urol 1995; 154: 442-7. [DOI] [PubMed] [Google Scholar]
- 14).Nagai S, Fujii T, Kodera Y, et al. Impact of operative blood loss on survival in invasive ductal adenocarcinoma of the pancreas. Pancreas 2011; 40: 3-9. [DOI] [PubMed] [Google Scholar]
- 15).Lehnert T, Methner M, Pollok A, et al. Multivisceral resection for locally advanced primary colon and rectal cancer: an analysis of prognostic factors in 201 patients. Ann Surg 2002; 235: 217-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16).Katz SC, Shia J, Liau KH, et al. Operative blood loss independently predicts recurrence and survival after resection of hepatocellular carcinoma. Ann Surg 2009; 249: 617-23. [DOI] [PubMed] [Google Scholar]
- 17).Chikamoto A, Beppu T, Masuda T, et al. Amount of operative blood loss affects the long-term outcome after liver resection for hepatocellular carcinoma. Hepatogastroenterology 2012; 59: 1213-6. [DOI] [PubMed] [Google Scholar]
- 18).Goldstraw P. International Association for the Study of Lung Cancer, Staging Manual in Thoracic Oncology. Orange Park: Editorial Rx Press, 2009. [Google Scholar]
- 19).Bordin JO, Blajchman MA. Immunosuppressive effects of allogeneic blood transfusions: implications for the patient with a malignancy. Hematol Oncol Clin North Am 1995; 9: 205-18. [PubMed] [Google Scholar]
- 20).Leukocyte-depleted blood transfusion is associated with decreased survival in resected early-stage lung cancer J Thorac Cardiovasc Surg 2012; 143: 815-9. [DOI] [PubMed] [Google Scholar]
- 21).Hoynck van Papendrecht MA, Busch OR, Jeekel J, et al. The influence of blood loss on tumour growth: effect and mechanism in an experimental model. Neth J Surg 1991; 43: 85-8. [PubMed] [Google Scholar]
- 22).Yago H, Yoshii H, Naiki M, et al. Stress and murine NK cell function: the role of blood loss. J Clin Lab Immunol 1992; 37: 123-32. [PubMed] [Google Scholar]
- 23).Angele MK, Faist E. Clinical review: immunodepression in the surgical patient and increased susceptibility to infection. Crit Care 2002; 6: 298-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24).Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012; 366: 2455-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25).Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012; 366: 2443-54. [DOI] [PMC free article] [PubMed] [Google Scholar]