Abstract
Purpose: It is clinically difficult to differentiate between primary lung cancer (PLC) and metastasis from breast cancer (MBC) in the diagnosis of a solitary pulmonary nodule (SPN) observed in a patient with past history of breast cancer. We evaluated several clinical, radiological and pathological variables in patients with SPN in an attempt to identify reliable markers to differentiate them.
Methods: Retrospectively we reviewed the clinical, radiological and pathological characteristics of 64 patients with a history of breast cancer resection who subsequently underwent surgical resection of an indeterminate SPN in our institute.
Results: The patients with MBC were significantly younger (p = 0.01). Among CT findings, presence of a solid opacity (p <0.01), well-defined tumor (p <0.01) and absence of an air bronchogram (p <0.01) were significantly associated with MBC. Among the intraoperative frozen section pathologic findings, the absence of lepidic or papillary patterns (p <0.01) and the presence of strong fibrosis in the tumor (p <0.01) were significantly correlated with MBC.
Conclusion: Although some cases are difficult to confirm the definitive diagnoses of SPN, combining CT and intraoperative pathological findings might enable us to distinguish SPN between MBC and PLC prior to postoperative examinations.
Keywords: solitary pulmonary nodule, metastasis from breast cancer, primary lung cancer
Introduction
In patients presenting with a solitary pulmonary nodule (SPN) after breast cancer resection, the differential diagnosis between primary lung cancer (PLC) and pulmonary metastasis from breast cancer (MBC) is very crucial. An SPN developing in a patient with confirmed breast cancer either previously or currently provides a significant diagnostic challenge. However, it has been reported that an SPN in a patient with a history of breast cancer more probably indicates a primary lung cancer.1) That requires accurate evaluation for diagnosis and selection of the appropriate treatment. Many cases can be difficult to differentiate PLC, MBC and benign pulmonary tumor preoperatively. When a definitive diagnosis of developing SPN cannot be confirmed by bronchoscopic evaluation or computed tomography (CT) guided needle biopsy, surgical biopsy is indicated. If partial resection of the lung confirms that the SPN is MBC and the surgical margin is cancer-free, the incision is closed. If PLC is diagnosed, anatomical resection with systematic nodal dissection is the typical treatment of choice. However, some cases are difficult to differentiate MBC from PLC even by intraoperative frozen section examination. In an attempt to establish diagnostic criteria to differentiate MBC and PLC pre- or intraoperatively, we reviewed the clinical, radiological and pathological characteristics of patients who had been treated in our institution.
Materials and Methods
From July 1992 through December 2011, a total of 4108 patients underwent pulmonary resection at our institution. From among these patients, 64 patients who had undergone breast cancer resection followed by subsequent surgical resection of an indeterminate SPN were enrolled. In this survey, patients who had other nodules in other organs which were highly suspected as tumor metastases, who did not receive high-resolution CT (HRCT) preoperatively or who were diagnosed to have a SPN pathologically by preoperative bronchoscopic biopsy or CT guided biopsy were excluded. We also excluded benign SPN such as hamartoma and inflammatory pseudo-tumors.
The preoperative evaluation included physical examination, blood chemistry analysis, tumor marker evaluation, chest X-ray examination, chest and abdominal HRCT evaluation, bone scintigraphy, and positron emission tomography (PET) evaluation or combined PET-CT evaluation. The disease free interval (DFI) was measured in months between the date of breast cancer resection and that of SPN detection. If the SPN was diagnosed as PLC by intraoperative frozen section, we performed standard lung resection and systematic lymph node dissection. If diagnosed as MBC and the cut-end was confirmed to be cancer-free pathologically, we did not perform further resection and closed the incision.
All chest HRCT scans were reviewed independently by two of the authors (T.K. and T.H.) who were blinded to the personal data of patients, and the following radiological features on high resolution CT were recorded: opacity (pure solid/part solid), border definition (well/ill defined), edge (smooth/irregular), spiculation (+/–), vascular convergence (+/–), pleural indentation (+/–), air bronchograms (+/–), location (peripheral: outer one third of the lung field/central), and laterality (right/left). Evaluation discrepancies between observers were resolved by consensus.
The available pathology slides from surgical specimens, including intraoperative frozen sections, were blinded for personal information and reviewed by two of the authors (T.K. and G.I.). We recorded the following histological characteristics, which were obtained by consensus: histological subtypes (lepidic or papillary/others), and fibrosis (high/medium or low). If a tumor showed a fibrotic focus larger than half the diameter of the entire tumor, we classified the tumor as having high fibrosis. If the tumor showed little central fibrosis, we classified it as having low fibrosis, and the remainders were classified as having medium level of fibrosis. We made the final pathologic diagnosis based on hematoxylin and eosin staining, comparing with previously specimen slide of resected breast cancer, with additional immunohistochemical staining of the following markers: thyroid transcription factor 1 (TTF-1), estrogen receptor (ER), progesterone receptor (PgR) and human epidermal growth factor receptor type 2 (HER2).
The Fisher’s exact test was used to compare the categorical variables. Continuous variables were compared using the Mann-Whitney U test. A p-value of less than 0.05 was considered to represent a statistically significant difference. All statistical analyses were performed using StatView software (version 5.0 for Windows; SAS Institute Inc., Cary, North Carolina, USA).
Data collection and analyses were approved, and, as the research was a retrospective review of chart data and specimens and no personally identifiable information was included, the need to obtain written informed consent was waived by our institutional review board in March 2012.
Results
At first we examined the clinical data among these 64 patients. All the MBC patients except 1 were women, with a median age of 64 years (range: 36–85). The median age of the patients with PLC was 67 (range: 46–85), and that of the patients with MBC was 61 (range: 36–79). MBC patients were significantly younger than PLC patients (p = 0.01). We did not observe any significant differences in sex, smoking status, preoperative serum tumor marker levels (including carcinoembryonic antigen, cancer antigen 15-3 and 125), pathological stage in breast cancer or DFI between MBC and PLC patients as shown in Table 1.
Table 1.
Clinical characteristics of MBC and PLC
| Factors | MBC (n = 27) | PLC (n = 37) | p-value | |
|---|---|---|---|---|
| Sex | woman | 26 | 37 | 0.42 |
| man | 1 | 0 | ||
| Age (yr) (median, range) | 61 (36–79) | 67 (46–85) | 0.01 | |
| Smoking | ever | 4 | 7 | 0.75 |
| never | 23 | 30 | ||
| Tumor markers | normal | 24 | 29 | 0.33 |
| abnormal | 3 | 8 | ||
| P-stage in breast cancer | I | 17 | 22 | 0.77 |
| II | 10 | 15 | ||
| DFI (months) (median, range) | 48 (13–180) | 72 (0–312) | 0.98 |
Two-category comparison was performed by Fisher’s exact test. Continuous data were compared using Mann-Whitney U test. Tumor markers including carcinoembryonic antigen, cancer antigen 15-3 and 125. P-stage: pathological stage; DFI: disease free interval; MBC: metastasis from breast cancer; PLC: primary lung cancer
Secondly, we evaluate the radiological variables based on the preoperative chest CT scans. The findings are summarized in Table 2. The median PLC diameter was 2.2 cm (range: 0.7–2.9), which was significantly larger than that of MBC (1.6 cm; range: 0.6–2.8, p <0.01). In MBC compared with PLC, pure solid opacity (p <0.01), well border definition (p <0.01) and absence of air bronchograms (p <0.01) were statistically characteristic feature on CT scans. There were no significant differences in edge regularity, spiculation, vascular convergence, pleural indentation, location and laterality between these groups.
Table 2.
Radiological characteristics of MBC and PLC
| Factors | MBC (n = 27) | PLC (n = 37) | p-value | |
|---|---|---|---|---|
| Diameter (cm) (median, range) | 1.6 (0.6–2.8) | 2.2 (0.7–2.9) | <0.01 | |
| Opacity | pure solid | 27 | 10 | <0.01 |
| part solid | 0 | 27 | ||
| Border | well-defined | 26 | 15 | <0.01 |
| ill-defined | 1 | 22 | ||
| Edge | smooth | 1 | 1 | >0.99 |
| irregular | 26 | 36 | ||
| Spiculation | present | 5 | 8 | 0.76 |
| absent | 22 | 29 | ||
| Vascular convergence | present | 10 | 19 | 0.26 |
| absent | 17 | 18 | ||
| Pleural indentation | present | 9 | 20 | 0.10 |
| absent | 18 | 17 | ||
| Air bronchograms | present | 1 | 22 | <0.01 |
| absent | 26 | 15 | ||
| Location | peripheral | 16 | 21 | 0.22 |
| central | 11 | 16 | ||
| Laterality | right | 9 | 20 | 0.10 |
| left | 18 | 17 |
Two-category comparison was performed by Fisher’s exact test. Continuous data were compared using Mann-Whitney U test. MBC: metastasis from breast cancer; PLC: primary lung cancer
Finally, we reviewed available intraoperative frozen section slides retrospectively. Unfortunately they were suitable for evaluation in 18 of 27 MBC patients and in 17 of 37 PLC patients. There were no cases in which the diagnosis by intraoperative frozen section differed from that by permanent section; the findings are summarized in Table 3. In MBC, the predominant subtypes were acinar and solid patterns, and lepidic and papillary patterns were not observed. Even when the primary breast cancer lesion showed a papillotubular pattern, MBC lesion comprised both solid and acinar patterns. However, lepidic and papillary patterns were observed in all PLC cases. Although the MBC tumors were significantly smaller, a high level of fibrosis was observed in the tumor center in more than 60% of cases (p <0.01).
Table 3.
Pathological characteristics of MBC and PLC
| Factors | MBC (n = 18) | PLC (n = 17) | p-value | |
|---|---|---|---|---|
| Lepidic or Papillary | present | 0 | 17 | <0.01 |
| absent | 18 | 0 | ||
| Fibrosis | high | 11 | 1 | <0.01 |
| middle/low | 7 | 16 |
Two-category comparison was performed by Fisher’s exact test. MBC: metastasis from breast cancer; PLC: primary lung cancer
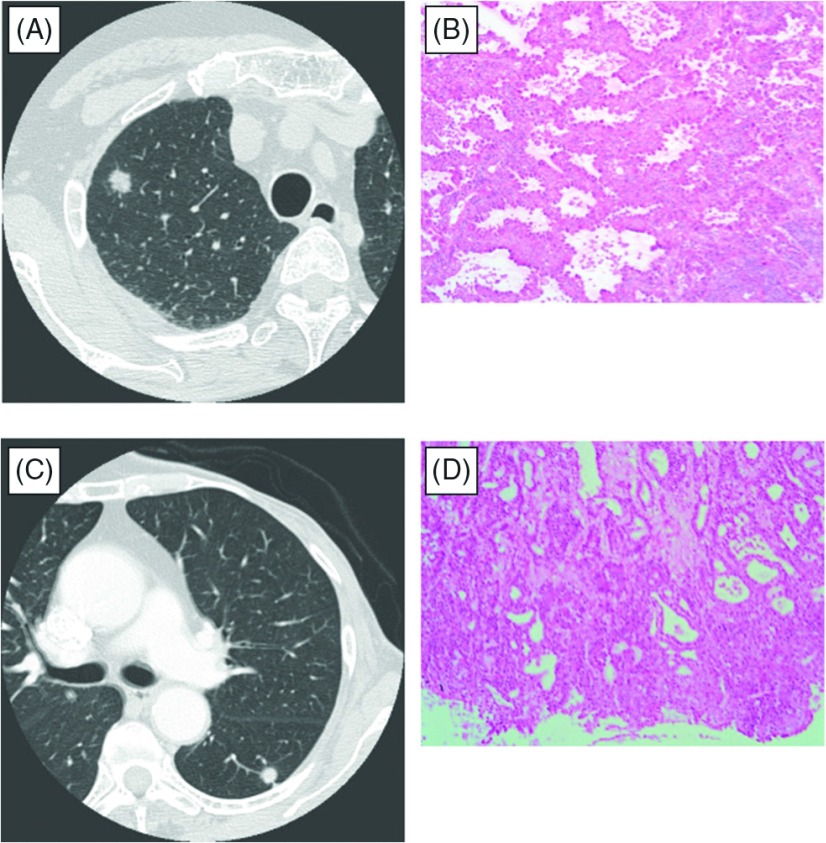
Two representative cases were described in Fig. 1. Both patients had previously undergone breast cancer resection, and their subsequent SPNs were detected on follow-up chest X-ray examinations. These tumors had solid opacity, well defined border and both did not have air bronchograms. The differential diagnosis between PLC and MBC was not possible from radiological findings (Fig. 1A and 1C). Wedge resections were performed and the intraoperative frozen section specimens are shown in Fig. 1B and 1D. In Fig. 1A and 1B, a lepidic pattern was observed in the tumor periphery, which yielded the diagnosis of PLC. We performed lobectomy and lymph node dissection. The final pathologic diagnosis was PLC. In Fig. 1C and 1D, no lepidic or papillary patterns were observed in the tumor periphery, however acinar patterns were observed. Additionally, the tumor in this case showed a high level of fibrosis, and the tumor was highly suspected to be MBC. We closed the incision, and postoperative immunohistochemical staining results confirmed the diagnosis of MBC.
Fig. 1.
Two representative cases with very similar computed tomography findings (A, C). In the primary lung cancer tumor, a lepidic pattern was observed in the tumor periphery (B). In the metastatic tumor, only an acinar pattern was observed in the tumor periphery (D).
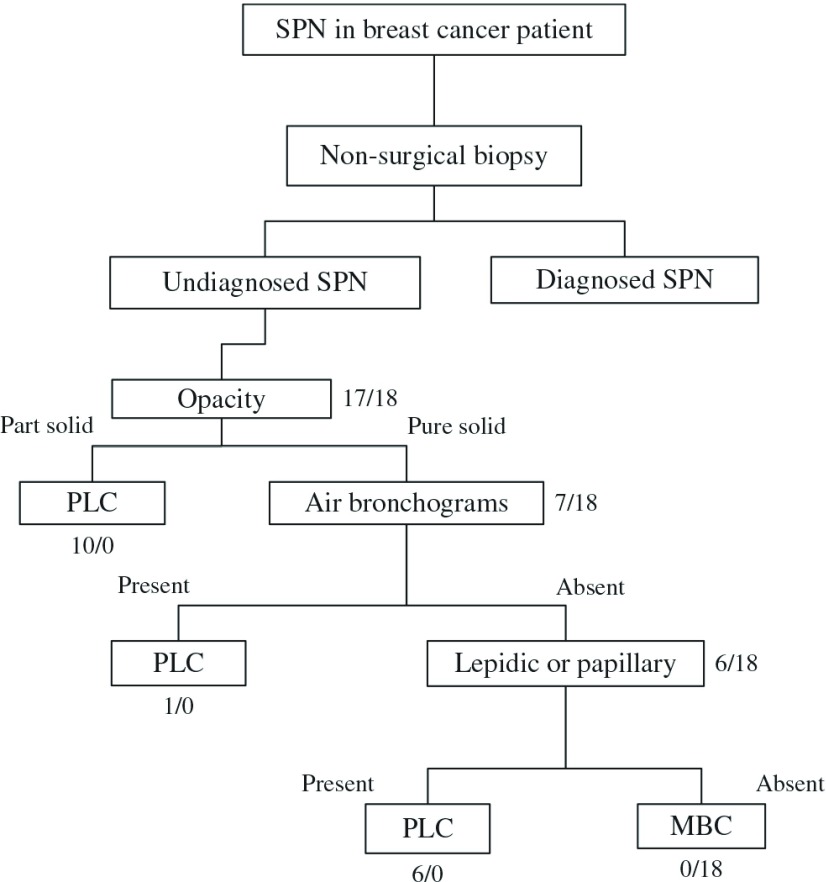
Based on the current findings, we propose a diagnostic flow chart (Fig. 2). By combining the level of solid opacity on CT with the lack of lepidic or papillary patterns on frozen sections, MBC and PLC tumors were clearly distinguishable in the current study. This chart might enable us to distinguish MBC from PLC intraoperatively.
Fig. 2.
Proposed diagnostic flow chart of SPN in breast cancer patients (numbers are PLC/MBC cases). SPN: solitary pulmonary nodule; PLC: primary lung cancer; MBC: metastasis from breast cancer.
Discussion
The surgical indication and procedure for pulmonary metastases from breast cancer have been controversial among breast surgeons, medical oncologists, and thoracic surgeons. However it is very important that definitive diagnosis of SPN after breast cancer resection is essential in selecting the optimum treatment. Percutaneous fine-needle CT-guided biopsy and transbronchial biopsy are useful and should be performed in order to confirm the definitive diagnosis of developing SPN pathologically. However, the diagnostic yield of these procedures is highly dependent on the experience of the performing physician, tumor location, and SPN size, and invalid specimens are frequently obtained. The resection of SPN is typically vital in order to establish a definitive diagnosis and subsequent treatment options. Although intraoperative frozen section examination is often performed in order to select the appropriate surgical extent, intraoperative differentiation between PLC and MBC can also be difficult. Postoperative evaluation for definitive diagnosis is sometimes necessary using immunohistochemical staining, such as TTF-1, hormone receptors.2,3) Rena, et al. reported that, among 65 patients who underwent surgery for a malignant SPN after a curative resection for breast cancer, the SPN was diagnosed as PLC in 61% and as MBC in 39%.1) The corresponding incidence rates at our institution were 58% and 42%, respectively. Even after breast cancer resection, SPN may more likely be PLC. As the standard surgical extent for resection in MBC and PLC differs, intraoperative definitive diagnosis is imperative.
In this study, we reviewed the clinical, radiological and pathological characteristics of indeterminate SPN patients, in an attempt to establish a method to differentiate them pre- or intraoperatively. Of the clinical characteristics studied, only age was statistically significantly different between PLC and MBC patients. This may be considered natural, as the incidence of breast cancer peaks at a younger age than lung cancer. Although a clear cut-off age was not determined to distinguish MBC from PLC in the present study, the younger age may be available and helpful information to distinguish MBC and PLC. We did not detect a significant difference in DFI. This fact may be compatible with the characteristic feature that breast cancer could recur even 10 years after the initial treatment.
Radiologically, tumor diameter on CT was significantly smaller in MBC than in PLC tumors, however a clear cut-off size to differentiate MBC and PLC could not be defined. Pure solid opacity, well-defined border and an absence of air bronchograms were significantly associated with MBC. These features might reflect the histologic differences in tumor progression in the lung between MBC and PLC.
Interestingly, all the MBCs consisted of acinar and/or solid pattern. There were no MBCs with lepidic or papillary patterns in this study. This fact may be because MBC cells hardly grow in alveolar replacement pattern. To distinguish MBC from pure acinar or solid PLC might be difficult. However such types of PLC are rare. More than 90% of pulmonary adenocarcinomas are of mixed subtypes. Pure acinar and pure solid adenocarcinomas are reported to occur in 1% and 2% of PLCs, respectively.4) Since almost all the patients are women, who are unlikely to have smoking habit, lung adenocarcinoma related with smoking such as solid type occurs rarely in them. Among the pathological characteristics, we do not emphasize the occurrence of a high level of fibrosis in the tumor center. This is because a medium or low level of fibrosis, which is characteristic of PLC, was observed in a considerable number of the MBC tumors.
It is known that some metastatic carcinomas in the lung may grow along the alveolar walls in a lepidic pattern simulating the appearance of primary pulmonary adenocarcinoma in situ (AIS, formerly called bronchioloalveolar carcinoma). Some metastatic pulmonary carcinomas, such as those originating from well-differentiated pancreatic carcinoma, may show the lepidic growth pattern.5) Several adenocarcinomas metastasizing from the breast to the lung were reported to show this pattern.6,7) It can be difficult to differentiate such adenocarcinomas from pulmonary AIS without the help of immunohistochemical staining. As far as we examined, we have not experienced such difficult cases to differentiate them using our proposal shown in Fig. 2. There were not any discrepancies between intraoperative and postoperative diagnosis up to now. Lepidic growth pattern in pulmonary metastases is quite rare. Whichever a pulmonary tumor showing replacement growth of alveolar structures is PLC or MBC, wedge resection of it might be appropriate as a surgical procedure.
There are some limitations in the current study; its retrospective nature and the small number of patients studied. The number of valid specimens available for pathological evaluation was even smaller. Nevertheless, we might be able to distinguish MBC from PLC intraoperatively, together with other clinic-radiological information using our proposal. We feel that these findings in the current study might indicate a potentially helpful method of differentiating MBC and PLC in SPN patients.
Conclusion
In conclusion, we identified a panel of clinical, radiological, and frozen section information to help distinguish MBC and PLC. We believe that this panel will be helpful in obtaining more reliable intraoperative diagnosis. Future prospective, large-scale studies are required to confirm these preliminary results.
Acknowledgements
The authors thank Mr. Roderick J. Turner of Toho University Medical School and Prof. J. Patrick Barron, Chairman of the Department of International Medical Communications of Tokyo Medical University for their editorial review of this manuscript.
Disclosure Statement
The authors declare no potential conflicts of interest regarding this study.
References
- 1).Rena O, Papalia E, Ruffini E, et al. The role of surgery in the management of solitary pulmonary nodule in breast cancer patients. Eur J Surg Oncol 2007; 33: 546-50. [DOI] [PubMed] [Google Scholar]
- 2).Moldvay J, Jackel M, Bogos K, et al. The role of TTF-1 in differentiating primary and metastatic lung adenocarcinomas. Pathol Oncol Res 2004; 10: 85-8. [DOI] [PubMed] [Google Scholar]
- 3).Longatto Filho A, Bisi H, Alves VA, et al. Adenocarcinoma in females detected in serous effusions. Cytomorphologic aspects and immunocytochemical reactivity to cytokeratins 7 and 20. Acta Cytol 1997; 41: 961-71. [DOI] [PubMed] [Google Scholar]
- 4).Motoi N, Szoke J, Riely GJ, et al. Lung adenocarcinoma: modification of the 2004 WHO mixed subtype to include the major histologic subtype suggests correlations between papillary and micropapillary adenocarcinoma subtypes, EGFR mutations and gene expression analysis. Am J Surg Pathol 2008; 32: 810-27. [DOI] [PubMed] [Google Scholar]
- 5).Gaeta M, Volta S, Scribano E, et al. Air-space pattern in lung metastasis from adenocarcinoma of the GI tract. J Comput Assist Tomogr 1996; 20: 300-4. [DOI] [PubMed] [Google Scholar]
- 6).Rosenblatt MB, Lisa JR, Collier F. Primary and metastatic bronciolo-alveolar carcinoma. Dis Chest 1967; 52: 147-52. [DOI] [PubMed] [Google Scholar]
- 7).Foster CS. Mucus-secreting ‘alveolar-cell’ tumour of the lung: a histochemical comparison of tumours arising within and outside the lung. Histopathology 1980; 4: 567-77. [DOI] [PubMed] [Google Scholar]