Abstract
Purpose: The aim of this study is to elucidate the impact of preoperative and postoperative pulmonary hypertension (PH) on long-term clinical outcomes after mitral valve repair for degenerative mitral regurgitation.
Methods: A total of 654 patients who underwent mitral valve repair for degenerative mitral regurgitation between 1991 and 2010 were retrospectively reviewed. Patients were divided into PH(+) group (137 patients) and PH(–) group (517 patients). Follow-up was complete in 99.0%. The median follow-up duration was 7.5 years.
Results: Patients in PH(+) group were older, more symptomatic and had higher tricuspid regurgitation grade. Thirty-day mortality was not different between 2 groups (p = 0.975). Long-term survival rate was lower in PH(+) group; 10-year survival rate after the operation was 85.2% ± 4.0% in PH(+) group and 89.7% ± 1.8% in PH(–) group (Log-rank, p = 0.019). The incidence of late cardiac events were not different between groups, however, the recurrence of PH was more frequent in PH(+) group. The recurrence of PH had an adverse impact on survival rate, late cardiac events and symptoms. Univariate analysis showed age and preoperative tricuspid regurgitation grade were the predictors of PH recurrence.
Conclusion: Early surgical indication should be advocated for degenerative mitral regurgitation before the progression of pulmonary hypertension and tricuspid regurgitation.
Keywords: pulmonary hypertension, mitral valve repair, degenerative mitral regurgitation
Introduction
Pulmonary hypertension (PH) may occur with the gradual progression of mitral regurgitation (MR) in patients with mitral valve (MV) prolapse and the presence of PH has been reported to have negative impact on operative outcomes.1–6) Current American College of Cardiology/American Heart Association guideline states that mitral valve surgery is reasonable for asymptomatic patients with chronic severe MR, preserved left ventricular function and pulmonary hypertension (pulmonary artery systolic pressure greater than 50 mmHg at rest or greater than 60 mmHg with exercise) as a class IIa indication based on level C evidence.7)
Recently, Ghoreishi, et al. reported that preoperative PH adversely affected operative mortality, morbidity and long-term death.8) They insisted that MV surgery should be referred before systolic pulmonary artery pressure exceeds 40 mmHg. However, their data did not report the impact of postoperative residual PH on long-term outcomes such as freedom from heart failure, atrial fibrillation, or symptoms.
Pulmonary hypertension is expected to improve after successful treatment for left-sided valve disease. On the other hand, Goldstone, et al. reported residual PH was common after MV repair for degenerative disease and was associated with postoperative mortality and morbidity.9) Thus the impact of preoperative and postoperative PH on surgical outcomes still remains unclear, which leads to the situation where PH cannot be a strong indicator for surgery.
The aim of this study is to elucidate the impact of preoperative PH on long-term survival and cardiac functions after MV repair for degenerative MR. We also investigated the impact of postoperative residual and recurrent PH on late cardiac events and symptoms.
Methods
The data analysis for this retrospective study was approved by the Institutional Review Board of Kobe City Medical Center General Hospital and the Board waived the need for patients’ consent.
Patient population
From January 1991 to December 2010, 1138 patients underwent mitral valve surgery (949 mitral valve repair and 169 mitral valve replacement) at Kobe City Medical Center General Hospital. Among patients who underwent mitral valve repair, 247 patients (26.0%) who had type I or III MR were excluded. Patients who had congenital MR as well as patients who underwent concomitant procedures for aortic valve were also excluded. As a result, 654 patients who had type II, degenerative mitral regurgitation, remained. We retrospectively analyzed these patients.
Definitions
Preoperative systolic pulmonary artery pressure (sPAP) was measured with Doppler echocardiography (modified Bernoulli equation: 4 × [tricuspid regurgitation jet velocity]2 + right atrial pressure [10 mmHg]). We defined the pulmonary hypertension (PH) as sPAP more than 50 mmHg according to the guideline’s cutline value.7) We divided the patients who had preoperative PH [PH(+) group: 137 patients] and who did not have PH [PH(–) group: 517 patients].
Major adverse cardiac and cerebrovascular events were defined as cardiac death, readmission for cardiac reasons, myocardial infarction, stroke and transient ischemic attack.
Surgical techniques
We approached through median sternotomy in all patients. Standard cardiopulmonary bypass techniques were used, including bicaval cannulation. Myocardial protection was achieved with antegrade and retrograde intermittent cold blood cardioplegia. A left atrial incision was applied in most patients. The techniques of mitral valve repair were described by Carpentier10) and David and colleagues.11) Prolapse of the posterior leaflet was mostly corrected by resection and suture of mitral leaflets. Prolapse of the anterior leaflet was mostly corrected by chordal replacement with polytetrafluoroethylene (Gore-Tex; W.L. Gore and Associates Inc., Flagstaff, Arizona, USA) sutures. The concomitant procedures were performed in 243 patients (37.2%). The details of surgical procedures are shown in Table 1.
Table 1.
Details of surgical procedure
| Operative procedure for mitral valve | N = 654 |
| Leaflet resection and suture, n (%) | 548 (83.8%) |
| Artificial chordal reconstruction, n (%) | 296 (45.3%) |
| Folding plasty, n (%) | 59 (9.0%) |
| Auto-pericardial patch, n (%) | 30 (4.6%) |
| Ring annuloplasty, n (%) | 576 (88.1%) |
| Concomitant procedures | |
| Tricuspid annuloplasty | 162 (19.3%) |
| Maze procedure, n (%) | 87 (13.3%) |
| Coronary artery bypass grafting, n (%) | 35 (5.4%) |
| Closure of ASD or PFO, n (%) | 34 (5.2%) |
| Cardiopulmonary bypass time, min | 147 ± 48 |
| Aortic cross-clamp time, min | 104 ± 36 |
ASD: atrial septal defect; PFO: patent foramen ovale
Follow-up examinations and management
We followed up the patients at the outpatient clinic or with a telephone survey, and the follow-up was completed in 648 patients (99.0%). The median follow-up duration was 7.5 years. Postoperative echocardiographic follow-up was generally performed before discharge and at the outpatient clinic at 1, 5, 10 years after the operation. The median length of the echocardiographic follow- up was 5.5 years. Follow-up echocardiographic data was obtained in 129 patients at 1 year, in 88 patients at 5 years and in 57 patients at 10 years after the operation.
Statistical analysis
The continuous data in this study are expressed as mean ± standard deviation and range. Categorical variables were compared with the χ2 or Fisher’s exact tests, and continuous variables were compared with unpaired t or Wilcoxon tests. Survival and freedom from events were calculated with the Kaplan-Meier method. An univariate Cox hazard regression analysis were used to identify predictors for late moderate TR. Statistical analysis was performed with StatView (SAS Institute, Cary, North Carolina, USA).
Results
Preoperative patient characteristics
The patients’ characteristics and preoperative echocardiographic data are shown in Table 2. Of note, patients in PH(+) group were older, more symptomatic, and had higher grade of functional tricuspid regurgitation.
Table 2.
Preoperative clinical characteristics
| Variables | PH+ (n = 137) | PH– (n = 517) | p value |
|---|---|---|---|
| Mean age, year | 61.9 ± 14.5 | 55.0 ± 14.4 | <0.001 |
| Male, n (%) | 74 (54.0%) | 311 (60.2%) | 0.194 |
| BSA, m2 | 1.53 ± 0.21 | 1.62 ± 0.20 | <0.001 |
| Hypertension, n (%) | 65 (47.4%) | 186 (36.0%) | 0.014 |
| Ischemic heart disease, n (%) | 11 (8.0%) | 28 (5.4%) | 0.251 |
| History of cardiac operation, n (%) | 2 (1.5%) | 6 (1.2%) | 0.878 |
| Urgent/Emergent operation, n (%) | 3 (2.2%) | 2 (0.4%) | 0.109 |
| Chronic atrial fibrillation, n (%) | 39 (28.5%) | 119 (23.0%) | 0.185 |
| Active bacterial endocarditis, n (%) | 3 (2.2%) | 15 (2.9%) | 0.874 |
| History of cardiac failure, n (%) | 68 (49.6%) | 154 (29.8%) | <0.001 |
| NYHA functional class | |||
| I, n (%) | 2 (1.5%) | 10 (1.9%) | 0.992 |
| II, n (%) | 84 (61.3%) | 387 (74.9%) | 0.002 |
| III, n (%) | 41 (29.9%) | 104 (20.1%) | 0.014 |
| IV, n (%) | 10 (7.3%) | 16 (3.1%) | 0.025 |
| Prolapse region | |||
| anterior, n (%) | 9 (6.6%) | 67 (13.0%) | 0.038 |
| posterior, n (%) | 85 (62.0%) | 261 (50.5%) | 0.016 |
| both, n (%) | 43 (31.4%) | 189 (36.5%) | 0.261 |
| LVDd, mm | 55.5 ± 6.5 | 55.4 ± 6.9 | 0.835 |
| LVDs, mm | 33.7 ± 6.5 | 34.0 ± 6.8 | 0.709 |
| LVEF, % | 68.4 ± 7.3 | 66.1 ± 8.0 | 0.004 |
| LAD, mm | 49.6 ± 9.5 | 45.8 ± 9.1 | <0.001 |
| Regurgitant volume, ml | 80.8 ± 53.3 | 73.5 ± 69.3 | 0.308 |
| Regurgitant fraction, % | 50.6 ± 29.8 | 45.9 ± 28.9 | 0.133 |
| Effective regurgitant orifice, cm2 | 0.47 ± 0.68 | 0.36 ± 0.43 | 0.030 |
| TR grade | |||
| mild or less, n (%) | 49 (35.8%) | 430 (83.2%) | <0.001 |
| moderate, n (%) | 60 (43.8%) | 65 (12.6%) | <0.001 |
| severe, n (%) | 28 (20.4%) | 22 (4.2%) | <0.001 |
PH: pulmonary hypertension; BSA: body surface area; NYHA: New York Heart Association; LVDd: left ventricular diastolic diameter; LVDs: left ventricular systolic diameter; LVEF: left ventricular ejection fraction; LAD: left atrial diameter; TR: tricuspid regurgitation
Survival
Thirty-day mortality occurred in 2 patients of PH(+) group (1.5%) and 5 patients of PH(–) group (1.0%), which was not different between 2 groups (p = 0.975). The overall long-term survival rate was better in PH(–) group; 5-year survival rate after the operation was 92.8% ± 2.3% in PH(+) group and 96.2% ± 0.9% in PH(–) group, 10-year survival rate after the operation was 85.2 %± 4.0% in PH(+) group and 89.7% ± 1.8% in PH(–) group (Log-rank, p = 0.019).
There were 20 late deaths in PH(+) group, of which 9 were cardiac-related. The leading cause of cardiac death was stroke. There were 42 late deaths in PH(–) group, about half of which were cardiac-related. The leading cause of late death was also stroke (Table 3).
Table 3.
Details of late death
| PH(+) group (n = 20) | n |
| Cardiac | 9 (50%) |
| Stroke | 6 |
| Arrhythmia | 2 |
| Congestive heart failure | 1 |
| Non-cardiac | 9 (50%) |
| PH(–) group (n = 42) | |
| Cardiac | 20 (48%) |
| Stroke | 12 |
| Operative death at subsequent | 3 |
| Re-operation | |
| Congestive heart failure | 2 |
| Other | 3 |
| Non-cardiac | 19 (45%) |
| Unknown | 3 (7%) |
Late major adverse cardiac and cerebrovascular events
The freedom rate from major adverse cardiac and cerebrovascular events was lower in PH(+) group, however, it was not different significantly; 5-year freedom rate after the operation was 90.9% ± 2.7% in PH(+) group and 92.6% ± 1.2% in PH(–) group, 10-year freedom rate after the operation was 83.0% ± 4.2% in PH(+) group and 84.7% ± 2.1% in PH(–) group (Log-rank, p = 0.262).
Postoperative residual and recurrent PH
In PH(+) group, 2 patients (1.5%) showed residual PH (>50 mmHg) at discharge. The preoperative sPAP of these patients were 80 and 98 mmHg. In PH(–) group, there was no residual PH at discharge.
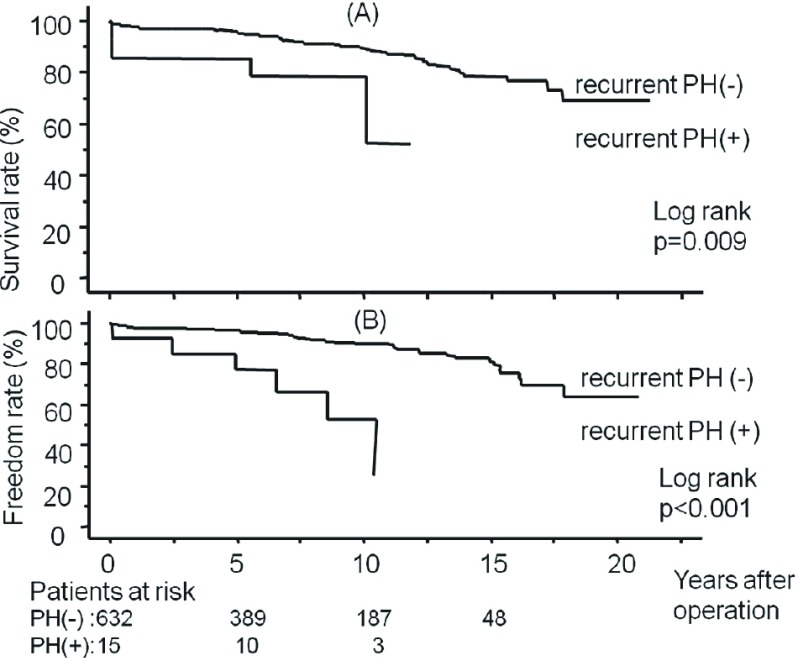
During the follow-up period, 8 of PH(+) patients and 7 of PH (–) patients showed recurrent PH (>50 mmHg). The freedom rate from recurrent PH was significantly lower in PH(+) group; 5-year freedom rate after the operation was 94.4% ± 2.5% in PH(+) group and 98.4% ± 0.8% in PH(–) group, 10-year freedom rate after the operation was 90.7% ± 3.5% in PH(+) group and 98.0% ± 0.9% in PH(–) group (Log-rank, p = 0.003). The survival rate and freedom rate from congestive heart failure were significantly lower in patients with recurrent PH than in patients without recurrent PH (Fig. 1). A total of 10 out of 15 patients who revealed recurrent PH fell into atrial fibrillation at follow-up. The latest New York Heart Association (NYHA) functional class was higher in the patients who revealed recurrent PH than the patients who did not (1.7 ± 0.7 vs. 1.2 ± 0.4, p <0.001).
Fig. 1.
The overall survival rate after mitral valve repair in patients with recurrent pulmonary hypertension (PH) and without recurrent PH (A) and the freedom rate from congestive heart failure after mitral valve repair in patients with recurrent PH and without recurrent PH (B).
Predictors of PH recurrence
An univariate analysis was performed to determine the predictors of postoperative PH recurrence. Age and preoperative TR grade became the predictors for postoperative PH recurrence. Preoperative sPAP, left ventricular dilatation, left ventricular function, atrial fibrillation, left atrial diameter and postoperative recurrence of severe mitral regurgitation did not become the predictors (Table 4).
Table 4.
Predictors of postoperative PH recurrence
| Variables | Odds ratio | 95% CI | p-value |
|---|---|---|---|
| Age | 1.081 | 1.007–1.160 | 0.031 |
| Pre TR grade | 3.690 | 1.637–8.322 | 0.002 |
| Pre sPAP | 1.022 | 0.985–1.048 | 0.167 |
| Pre LVDd | 1.029 | 0.403–2.627 | 0.953 |
| Pre LVEF | 0.953 | 0.981–1.023 | 0.186 |
| Pre atrial fibrillation | 0.953 | 0.258–3.521 | 0.943 |
| Pre LA diameter | 0.863 | 0.689–3.161 | 0.705 |
| Post MR recurrence | 1.569 | 0.221–23.33 | 0.996 |
PH: pulmonary hypertension; TR: tricuspid regurgitation; sPAP: systolic pulmonary artery pressure; LVDd: left ventricular diastolic diameter; LVEF: left ventricular ejection fraction; LA: left atrium; MR: mitral regurgitation
Discussion
Preoperative PH is reported to be a major risk factor for right ventricular failure and perioperative mortality undergoing mitral valve surgery.12–14) Ghoreishi, et al. also reported that operative mortality after mitral valve operation for mitral regurgitation was correlated with the degree of preoperative PH.8) In this study, we focused on the impact of preoperative PH after mitral valve repair for degenerative mitral regurgitation. We defined PH as sPAP more than 50 mmHg according to the guideline’s cutline value. Patients in PH(+) group were older, more symptomatic, had higher TR grade, and higher effective regurgitant orifice than PH(–) group. Left ventricular function and incidence of atrial fibrillation were similar between 2 groups, which was different from the previous study.8) In this study, the 30-day mortality was 1.5% in PH(+) group and 1.0% in PH(–) group (1.0%), which was not different between 2 groups (p = 0.975). Ghoreishi, et al. made a subgroup analysis of 284 patients who had isolated degenerative mitral regurgitation.8) They reported that operative mortality was 0.8% in that population, which was similar with our result. Our results and their study showed preoperative PH did not make a significant impact on operative mortality among the patients with degenerative mitral regurgitation.
On the other hand, Goldstone, et al. reported residual PH, not a preoperative PH, was a significant risk factor for postoperative mortality and morbidity.9) They reported that 46% of the patients had residual PH after mitral valve repair. In our study, residual PH at discharge was only seen in 2 patients (1.5%) in PH(+) group. The difference may be due to the definition of residual PH and timing of measurement of PAP. Goldstone, et al. defined residual PH as mean PAP of ≥25 mmHg and they measured PAP on postoperative day 1. However, our study showed that PAP decreased greatly by discharge after successful repair for degenerative mitral regurgitation and incidence of residual PH defined as sPAP ≥50 mmHg was rare.
Ghoreishi, et al. also sPAP was also a predictor for late death.8) They demonstrated that long-term survival progressively decreased with increasing preoperative sPAP grade. The subgroup analysis for isolated degenerative mitral regurgitation also revealed that preoperative sPAP became a predictor of decreased long-term survival. Our study showed the long-term survival rate was worse in PH(+) group, which was compatible with previous studies. The previous study, however, did not demonstrate the causes of late death. We could detect most of the causes of late death, about half of which were cardiac. The leading cause of late death was stroke. The late death was prominent in the patients who had recurrence of PH. In this setting, most of these patients were suffered from atrial fibrillation and some of them revealed stroke despite warfarin therapy.
During the follow-up period, 8 of PH(+) patients and 7 of PH(–) patients showed postoperative PH recurrence. PH recurrence was more seen in the patients with preoperative PH. Goldstone, et al. reported that residual PH was associated with postoperative complications, however, not associated with late survival. In contrast, in our study, the recurrence of PH had an adverse impact on survival rate and late cardiac events. It also adversely affected the prevalence of atrial fibrillation and patients’ symptoms. Most of the patients were suffered from atrial fibrillation and the NYHA functional class was higher in the patients with PH recurrence than without. The prediction and prevention of late recurrence of PH should be important. An univariate analysis showed that age and preoperative TR grade were the predictors of PH recurrence. Interestingly, preoperative pulmonary artery pressure and postoperative MR recurrence were not associated with PH recurrence.
Three principal mechanisms are implicated in the development of PH in the setting of mitral valve disease. (1) passive retrograde transmission of elevated left atrial pressure resulting from mitral disease; (2) reactive pulmonary vasoconstriction; and (3) pulmonary vascular remodeling. The first point should be eliminated in the patients who underwent successful mitral surgery. So the compliance of pulmonary vascular component should be related to postoperative PH recurrence. Patients with high age and high preoperative TR grade are estimated to have poor compliance of pulmonary vascular component, which would lead to pulmonary vascular remodeling. In these patients, pulmonary vasodilators, including bosentan, nitric oxide, sildenafil and epoprostenol might be useful adjuncts. We have not experienced these products yet, though. Targeted postoperative optimization therapy should be studied further, because it could reduce the incidence of PH recurrence and its adverse effect of postoperative course.
The limitations of our study included its retrospective method and the difference of preoperative patients’ characteristics. The small sample of patients and incomplete clinical and echocardiographic are also limitations. We calculated PAP by Bernoulli equation, which may be different from the real PAP measured by right heart catheterization.
In conclusion, both preoperative PH and postoperative PH recurrence adversely affected clinical outcomes after mitral valve repair for degenerative mitral regurgitaiton. In the current guideline, PH is classified as class II indication. If patients were referred for early surgery, they would be less likely to experience irreversible pulmonary vascular remodeling. We believe early surgical indication should be advocated for degenerative mitral regurgitation before the progression of pulmonary hypertension and tricuspid regurgitation.
Disclosure Statement
The authors have no conflicts of interest to disclose.
References
- 1).Montant P, Chenot F, Robert A, et al. Long-term survival in asymptomatic patients with severe degenerative mitral regurgitation: a propensity score-based comparison between an early surgical strategy and a conservative treatment approach. J Thorac Cardiovasc Surg 2009; 138: 1339-48. [DOI] [PubMed] [Google Scholar]
- 2).Le Tourneau T, Richardson M, Juthier F, et al. Echocardiography predictors and prognostic value of pulmonary artery systolic pressure in chronic organic mitral regurgitation. Heart 2010; 96: 1311-7. [DOI] [PubMed] [Google Scholar]
- 3).Barbieri A, Bursi F, Grigioni F, et al. Prognostic and therapeutic implications of pulmonary hypertension complicating degenerative mitral regurgitation due to flail leaflet: a multicenter long-term international study. Eur Heart J 2011; 32: 751-9. [DOI] [PubMed] [Google Scholar]
- 4).Kang DH, Kim JH, Rim JH, et al. Comparison of early surgery versus conventional treatment in asymptomatic severe mitral regurgitation. Circulation 2009; 119: 797-804. [DOI] [PubMed] [Google Scholar]
- 5).Fukumoto Y, Shimokawa H. Recent progress in the management of pulmonary hypertension. Circ J 2011; 75: 1801-10. [DOI] [PubMed] [Google Scholar]
- 6).Tatebe S, Fukumoto Y, Sugimura K, et al. Clinical significance of reactive post-capillary pulmonary hypertension in patients with left heart disease. Circ J 2012; 76: 1235-44. [DOI] [PubMed] [Google Scholar]
- 7).Bonow RO, Carabello BA, Chatterjee K, et al. 2008 Focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation 2008; 118: e523-661. [DOI] [PubMed] [Google Scholar]
- 8).Ghoreishi M, Evans CF, DeFilippi CR, et al. Pulmonary hypertension adversely affects short- and long-term survival after mitral valve operation for mitral regurgitation: implications for timing of surgery. J Thorac Cardiovasc Surg 2011; 142: 1439-52. [DOI] [PubMed] [Google Scholar]
- 9).Goldstone AB, Chikwe J, Pinney SP, et al. Incidence, epidemiology, and prognosis of residual pulmonary hypertension after mitral valve repair for degenerative mitral regurgitation. Am J Cardiol 2011; 107: 755-60. [DOI] [PubMed] [Google Scholar]
- 10).Carpentier A. Cardiac valve surgery—the “French correction”. J Thorac Cardiovasc Surg 1983; 86: 323-37. [PubMed] [Google Scholar]
- 11).David TE, Bos J, Rakowski H. Mitral valve repair by replacement of chordae tendineae with polytetrafluoroethylene sutures. J Thorac Cardiovasc Surg 1991; 101: 495-501. [PubMed] [Google Scholar]
- 12).Vincens JJ, Temizer D, Post JR, et al. Long-term outcome of cardiac surgery in patients with mitral stenosis and severe pulmonary hypertension. Circulation 1995; 92: II137-42. [DOI] [PubMed] [Google Scholar]
- 13).Crawford MH, Souchek J, Oprian CA, et al. Determinants of survival and left ventricular performance after mitral valve replacement. Department of Veterans Affairs Cooperative Study on Valvular Heart Disease. Circulation 1990; 81: 1173-81. [DOI] [PubMed] [Google Scholar]
- 14).Roques F, Nashef SA, Michel P, et al. Risk factors and outcome in European cardiac surgery: analysis of the EuroSCORE multinational database of 19030 patients. Eur J Cardiothorac Surg 1999; 15: 816-22. [DOI] [PubMed] [Google Scholar]