Abstract
Background
The relationship between HLA-DRB1 alleles and asthma is controversial. The purpose of this study was to evaluate the relationship between HLA-DRB1 alleles and risk of asthma.
Material/Methods
We searched PubMed, Chinese National Knowledge Infrastructure (CNKI), Wan Fang (Chinese) database, and Chinese Biomedical Medical databases (CBM) to find studies on the relationship between HLA-DRB1 alleles and risk of asthma. We calculated the pooled odds ratio (OR) and 95% confidence interval (CI) using STATA 12.0. Finally, a total of 24 studies were included in this meta-analysis.
Results
The results revealed that DRB1*03 was positively associated with risk of asthma (OR=1.51, 95%CI=1.27–1.80), and DRB1*15 was negatively associated with risk of asthma (OR=0.63, 95%CI=0.42–0.93), but no association was found in other HLA-DRB1 alleles. Subgroup analysis by age revealed that DRB1*03, DRB1*04, DRB1*09, and DRB1*15 were associated with asthma in children. Subgroup analysis by ethnicity showed that DRB1*03 and DRB1*15 were associated with asthma in whites, and DRB1*07 and DRB1*14 were associated with asthma in Asians.
Conclusions
This results of this meta-analysis suggest that HLA-DRB1 alleles are associated with asthma.
MeSH Keywords: Anti-Asthmatic Agents; HLA Antigens; Meta-Analysis; Polymorphism, Single Nucleotide
Background
Asthma is characterized by recurrent episodes of airway obstruction, which reverse either spontaneously or after use of medication, and is usually associated with bronchial hyper-responsiveness and evidence of chronic airway inflammation [1]. There are more than 300 million people suffering from asthma worldwide, and it occurs in all countries regardless of the level of development [2]. Asthma prevalence increased from 7.3% in 2001 to 8.4% in 2010, when 25.7 million persons had asthma in the United States [3]. Asthma can increase mortality and morbidity, and is an important public health challenge [4]. Therefore, prevention and control of asthma have great significance.
Asthma is a multifactor disease caused by interactions between genetic and environmental factors. Genetic studies have attracted much attention with the development of genome-wide association studies and genome-wide linkage studies. For example, IREB2 gene rs2568494 polymorphism has been shown to be associated with susceptibility to chronic obstructive pulmonary disease [5]. IL13 gene polymorphisms contribute to the development of pediatric asthma [6]. The human leukocyte antigen (HLA) super-locus is a genomic region in chromosome position 6p21 that encodes the 6 classical transplantation HLA genes and at least 132 protein-coding genes that have important roles in the regulation of the immune system, as well as some other fundamental molecular and cellular processes [7]. The HLA region associated with asthma is the DR loci [8]. Cho et al. [9] found that HLA-DRB1*07 AND HLA-DRB1*04 were associated with asthma in Korean adults. Lama et al. suggested a significantly higher frequency of HLA-DRB1*03 in asthmatics than in controls among the Indian pediatric population [10]. Kauppinen et al. [11] showed that HLA-DRB1*0101, HLA-DRB1*0301 and HLA-DRB1*0404 were associated with asthma in people with allergic asthma in Finland [11]. However, some other studies have found no statistically significant associations between HLA-DRB1 alleles and asthma [12,13].
In view of several contradictory conclusions based on small sample size studies, we performed the current meta-analysis to determine if there is an association between HLA-DRB1 alleles and asthma.
Material and Methods
Literature search
PubMed, Chinese National Knowledge Infrastructure (CNKI), Wan Fang (Chinese) databases, and Chinese Biomedical Medical databases (CBM) were searched using the search terms: ‘asthma’ or ‘asthmatic’, ‘human leukocyte antigen’ or ‘HLA’, and ‘polymorphism’ or ‘mutation’ or ‘variant’ or ‘allele’. The date of the last search was December 10, 2015.
Inclusion and exclusion criteria
Inclusion criteria were: cohort study or case-control; enough data available; and English or Chinese language.
Data extraction
Two reviewers (Yingshui Yao and Jie Li) selected all potential studies separately. If there was disagreement, the reviewers would solve it by discussion or judgement by a third reviewer (Lijun Zhu). The following data were extracted: first author, year of publication, country, ethnicity, age (children or not), number of HLA-DRB1 alleles studied, detection methods, and numbers of cases and controls.
Statistical analysis
The pooled OR with 95% CI was analyzed using the Z test to assess the strength of the associations between HLA-DRB1 alleles and asthma. Heterogeneity assumption was evaluated by the χ2-based Q-test and I2 test [14]. If P>0.10 (Q-test) or I2 <50%, the fixed-effects model (the Mantel-Haenszel method) was used [15]. Otherwise, the random-effects model (the DerSimonian and Laird method) was used [16]. The Begg’s rank correlation method and the Egger’s linear regression method were used to assess potential publication bias [17,18]. The meta-analysis was carried out using STATA version 12.0 (Stata Corp, College Station, TX) software. P<0.05 meant there was statistically significant. All P values are 2-tailed.
Results
Characteristics of studies
We finally identified 24 studies, including 2346 cases and 5506 controls [9–12,19–38]. The retrieval process is presented in Figure 1. The characteristics of each study are shown in Table 1. Thirteen studies were conducted in whites and 12 studies were conducted in Asians. Nine studies were conducted in children. We also extracted the number of DRB1 alleles for each study.
Figure 1.
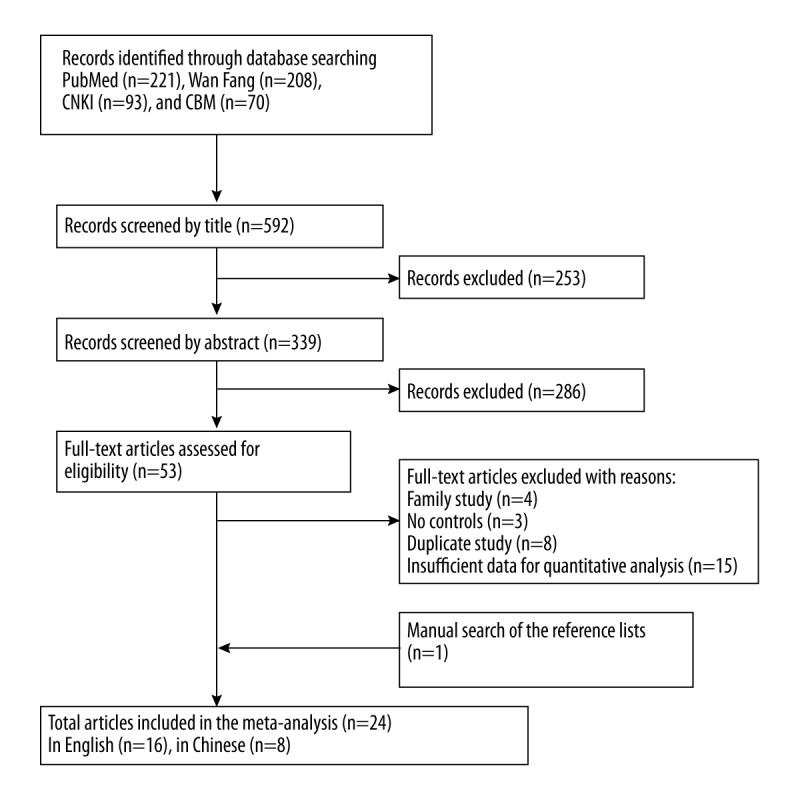
Flow chart of this meta-analysis.
Table 1.
Characteristics of studies included in the meta-analysis.
| First author | Year | Country (ethnicity) | Children or not | No. of DRB1 alleles studied | Detection methods | No. of cases | No. of controls |
|---|---|---|---|---|---|---|---|
| Bignon JS | 1994 | Italy (whites) | Not | 2 | PCR-SSP | 56 | 32 |
| Soriano JB | 1997 | Spain (whites) | Not | 11 | PCR-SSP | 145 | 168 |
| Cho SH | 2000 | Korea (Asians) | Not | 11 | PCR-SSP | 91 | 98 |
| Horne C | 2000 | Canada (whites) | Not | 6 | PCR-SSP | 56 | 63 |
| Mapp CE | 2000 | Italy (whites) | Not | 2 | PCR-SSP | 67 | 128 |
| Wang CG | 2000 | China (Asians) | Not | 3 | PCR-SSP | 64 | 104 |
| Li L | 2003 | China (Asians) | Children | 9 | PCR-SSOP | 40 | 92 |
| Choi JH | 2004 | Korea (Asians) | Not | 3 | PCR-SSP | 149 | 91 |
| Li CP | 2005 | China (Asians) | Not | 3 | PCR-SSP | 60 | 30 |
| Lu JR | 2006 | China (Asians) | Children | 11 | PCR-SSP | 78 | 82 |
| Juhn YJ | 2007 | USA (whites) | Children | 2 | PCR-SSP | 81 | 231 |
| Munthe-Kaas MC | 2007 | Norway (whites) | Children | 7 | PCR-SSOP | 330 | 1260 |
| Wang JW | 2007 | China (Asians) | Children | 12 | PCR-SSP | 45 | 45 |
| Movahedi M | 2008 | Iran (whites) | Children | 12 | PCR-SSP | 112 | 80 |
| Choi JH | 2009 | Korea (Asians) | Not | 3 | PCR-SSP | 84 | 174 |
| Ivković-Jureković I | 2011 | Croatia (whites) | Children | 8 | PCR-SSOP | 143 | 163 |
| Zhu SF | 2011 | China (Asians) | Not | 2 | PCR-SSP | 45 | 46 |
| Chen LP | 2012 | China (Asians) | Not | 1 | PCR-SSP | 100 | 100 |
| Kauppinen A | 2012 | Finland (whites) | Not | 6 | PCR-SSP | 40 | 151 |
| Dzurilla M | 2013 | Slovakia (whites) | Not | 13 | PCR-SSP | 109 | 130 |
| Xie QL | 2013 | China (Asians) | No | 1 | PCR-SSP | 84 | 168 |
| Mishra MN | 2014 | India (whites) | Children | 13 | PCR-SSP | 103 | 152 |
| Bottero P | 2014 | Italy (whites) | Not | 8 | PCR-SSP | 159 | 1808 |
| Lama M | 2014 | India (whites) | Children | 4 | PCR-SSP | 105 | 110 |
Meta-analysis of HLA-DRB1 alleles and risk of asthma
We took 19 HLA-DRB1 alleles into consideration, including 13 HLA-DRB1 allele families and 6 HLA-DRB1-specific alleles. The detailed results of the association between HLA-DRB1 alleles and asthma are presented in Table 2.
Table 2.
Meta-analysis of associations between HLA-DRB1 alleles and asthma.
| Alleles | No. of study | Case | Control | Heterogeneity | Model | Test of association | P value for Egger’s (Begg’s) test | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| χ2 | P value | I2(%) | OR (95%CI) | Z | P | ||||||
| 01 | 10 | 180/1216 | 306/2336 | 15.31 | 0.083 | 41.2 | F | 1.04 (0.84–1.28) | 0.36 | 0.721 | 0.366 (0.210) |
| 03 | 11 | 276/1389 | 607/4247 | 12.32 | 0.264 | 18.9 | F | 1.51 (1.27–1.80) | 4.61 | 0.000 | 0.728 (1.000) |
| 04 | 14 | 359/1601 | 839/4449 | 40.61 | 0.000 | 68.0 | R | 1.09 (0.82–1.46) | 0.61 | 0.540 | 0.904 (0.913) |
| 07 | 12 | 300/1415 | 774/4108 | 27.32 | 0.004 | 59.7 | R | 1.23 (0.91–1.67) | 1.35 | 0.177 | 0.040 (0.115) |
| 08 | 8 | 86/723 | 98/847 | 7.68 | 0.361 | 8.9 | F | 1.02 (0.74–1.41) | 0.13 | 0.896 | 0.103 (1.000) |
| 09 | 7 | 57/725 | 86/2530 | 3.38 | 0.760 | 0.0 | F | 1.39 (0.93–2.09) | 1.59 | 0.111 | 0.575 (1.000) |
| 10 | 7 | 44/611 | 47/767 | 6.47 | 0.373 | 7.2 | F | 1.23 (0.79–1.91) | 0.92 | 0.358 | 0.569 (0.764) |
| 11 | 11 | 314/1355 | 1238/4078 | 18.85 | 0.042 | 47.0 | R | 1.18 (0.89–1.56) | 1.16 | 0.245 | 0.732 (0.876) |
| 12 | 10 | 92/987 | 138/2765 | 9.12 | 0.426 | 1.3 | F | 1.18 (0.86–1.61) | 1.04 | 0.298 | 0.599 (0.858) |
| 13 | 10 | 242/1277 | 766/3996 | 12.63 | 0.180 | 28.8 | F | 0.93 (0.77–1.11) | 0.84 | 0.403 | 0.979 (0.474) |
| 14 | 9 | 97/819 | 318/2651 | 21.48 | 0.006 | 62.8 | R | 0.93 (0.57–1.54) | 0.28 | 0.783 | 0.410 (0.754) |
| 15 | 6 | 136/808 | 389/1832 | 11.91 | 0.036 | 58.0 | R | 0.63 (0.42–0.93) | 2.31 | 0.021 | 0.762 (0.707) |
| 16 | 4 | 42/433 | 63/527 | 0.67 | 0.881 | 0.0 | F | 0.74 (0.49–1.13) | 1.41 | 0.160 | 0.866 (1.000) |
| 0101 | 5 | 60/441 | 100/559 | 16.57 | 0.002 | 75.9 | R | 0.85 (0.39–1.83) | 0.42 | 0.673 | 0.039 (0.027) |
| 0301 | 6 | 55/477 | 85/521 | 8.31 | 0.140 | 39.8 | F | 0.75 (0.51–1.12) | 1.42 | 0.156 | 0.404 (0.452) |
| 0701 | 4 | 51/280 | 92/488 | 2.51 | 0.473 | 0.0 | F | 0.75 (0.50–1.12) | 1.43 | 0.153 | 0.076 (0.308) |
| 0901 | 7 | 112/483 | 129/674 | 35.76 | 0.000 | 83.2 | R | 1.23 (0.50–3.05) | 0.45 | 0.649 | 0.786 (0.764) |
| 1001 | 6 | 20/401 | 36/612 | 5.71 | 0.336 | 12.4 | F | 0.81 (0.46–1.43) | 0.73 | 0.466 | 0.895 (1.000) |
| 1401 | 4 | 20/264 | 18/326 | 1.97 | 0.578 | 0.0 | F | 1.20 (0.60–2.41) | 0.51 | 0.608 | 0.868 (0.734) |
Among the allele families, significant associations were found in HLA-DRB1*03 and HLA-DRB1*15 alleles (DRB1*03: OR=1.51, 95%CI=1.27–1.80; DRB1*15: OR=0.63, 95%CI=0.42–0.93). HLA-DRB1*03 was significantly increased the risk of asthma and HLA-DRB1*15 was associated with significantly decreased risk of asthma. No evidence of correlations was found in other allele families. Heterogeneity was observed in DRB1*04, DRB1*07, DRB1*11, DRB1*14, and DRB1*15 (P>0.10 or I2<50%). Thus, the random-effect model was used to analyze these alleles. Begg’s and Egger’s tests were carried out to estimate the publication bias, and the results showed that there was no publication bias among any of the allele families (P>0.05). There was no significant difference between asthma and the specific alleles (DRB1*0101, DRB1*0301, DRB1*0701, DRB1*0901, DRB1*1001, DRB1*1401). Among these specific alleles, heterogeneity was observed in DRB1*0101 and DRB1*0301 (P>0.10 or I2<50%). Publication bias was found in DRB1*0101 (P<0.05).
Subgroup analysis by ethnicity and age were carried out among allele families. For ethnicity (categorized as whites and Asians), DRB1*03 significantly increased the risk of asthma and DRB1*15 significantly decreased the risk of asthma in whites. DRB1*07 significantly increased the risk of asthma and DRB1*14 significantly decreased the risk of asthma in Asians. For age (categorized as children or not), DRB1*03, DRB1*04, and DRB1*09 suggested the risk-enhancing role and DRB1*15 suggested a protective role. The detailed information of the subgroup analysis is presented in Table 3.
Table 3.
Meta-analysis stratified by ethnicity and age.
| Alleles | Ethnicity | Age | ||||||
|---|---|---|---|---|---|---|---|---|
| Whites | Asians | Children | Not | |||||
| I2 (%) | OR (95%CI) | I2 (%) | OR (95%CI) | I2 (%) | OR (95%CI) | I2 (%) | OR (95%CI) | |
| 01 | 42.7 | 1.10 (0.88–1.38) | 40.5 | 0.73 (0.42–1.26) | 52.5 | 1.11 (0.84–1.45) | 31.3 | 0.95 (0.69–1.32) |
| 03 | 24.2 | 1.54 (1.28–1.84) | 20.9 | 1.13 (0.56–2.30) | 27.2 | 1.71 (1.37–2.14) | 0.0 | 1.25 (0.94–1.65) |
| 04 | 50.6 | 1.22 (0.95–1.57) | 80.2 | 0.82 (0.34–1.95) | 53.5 | 1.38 (1.01–1.87) | 77.3 | 0.70 (0.39–1.24) |
| 07 | 12.9 | 1.01 (0.82–1.23) | 63.6 | 2.82 (1.13–7.09) | 0.0 | 1.18 (0.92–1.50) | 82.2 | 1.54 (0.77–3.05) |
| 08 | 0.0 | 1.42 (0.88–2.31) | 40.5 | 0.77 (0.50–1.20) | 30.9 | 0.82 (0.52–1.29) | 0.0 | 1.29 (0.81–2.05) |
| 09 | 0.0 | 0.98 (0.37–2.57) | 17.6 | 1.50 (0.96–2.36) | 0.0 | 1.88 (1.08–3.27) | 0.0 | 0.97 (0.52–1.79) |
| 10 | 0.0 | 0.93 (0.52–1.67) | 0.0 | 1.82 (0.91–3.62) | 17.8 | 1.30 (0.78–2.15) | 25.8 | 1.04 (0.42–2.60) |
| 11 | 63.8 | 1.21 (0.87–1.67) | 0.0 | 1.01 (0.49–2.05) | 38.6 | 1.16 (0.80–1.68) | 66.8 | 1.20 (0.71–2.00) |
| 12 | 22.4 | 1.30 (0.83–2.02) | 0.0 | 1.08 (0.70–1.66) | 37.0 | 1.34 (0.93–1.95) | 0.0 | 0.87 (0.49–1.54) |
| 13 | 23.0 | 0.91 (0.75–1.10) | 54.1 | 1.09 (0.62–1.90) | 0.0 | 0.97 (0.76–1.23) | 60.6 | 0.88 (0.68–1.15) |
| 14 | 0.0 | 1.35 (0.98–1.86) | 52.5 | 0.38 (0.15–0.96) | 48.5 | 0.70 (0.20–2.50) | 71.6 | 0.98 (0.54–1.79) |
| 15 | 34.6 | 0.68 (0.50–0.93) | 83.8 | 0.41 (0.06–2.68) | 56.4 | 0.57 (0.37–0.87) | – | 1.01 (0.56–1.82) |
| 16 | 0.0 | 0.70 (0.44–1.12) | – | 0.94 (0.36–2.45) | 0.0 | 0.71 (0.44–1.15) | – | 0.84 (0.36–1.97) |
Discussion
Asthma is a complex heterogeneous respiratory disease. The pathogenesis of asthma involves many different cells. IgE and an imbalance between T helper cell 1 (Th1) and T helper cell 2 (Th2) are thought to play a key role in the pathogenesis of asthma [39–41]. The human major histocompatibility complex (MHC) is localized to chromosome 6p21 and is thought to play a role in regulating inflammation on T helper cells [7,42,43]. For the past 2 decades, many studies have focused on the relationship between HLA-DRB1 alleles and asthma, which may help to disclose the pathogenesis of asthma [44–48]. Use of meta-analysis could resolve the inconsistent results, which have harmful effect on false-positive and false-negative associations [49]. Thus, we performed this meta-analysis to clarify the association between HLA-DRB1 alleles and asthma.
Two alleles (DRB1*03 and DRB1*15) were found have statistically significant associations with risk of asthma among 13 HLA-DRB1 allele families. The results showed that individuals who carry HLA DRB1*03 have a 51% higher risk of asthma compared with those who do not carry this allele. Similar conclusions were reported in other studies. A study by Juhn et al. [28] found that the 12-year cumulative incidence of asthma by age among children who carry HLA DRB1*03 was 33%, compared to 24.2% among those who did not carry this allele. Lama et al. [10] demonstrated a significantly higher frequency of HLA-DRB1*03 in asthmatics than in controls. HLA DRB1 may have an effect on asthma through regulating Th1 vs. Th2 immune response. Murray et al. [43] showed that the interaction between T cell receptor, peptide, and major histocompatibility complex (MHC) can determine Th1/Th2 dominance through a differential T cell receptor affinity and ligand density between different MHC genes. In this study, we also found that DRB1*15 was a protective factor for asthma. A similar protective role of DRB1*15 was reported in other studies [30,37]. Although the precise mechanism of this effect is still unknown, we hypothesis that HLA-DRB1 alleles have different effects on asthma. In addition, we also examined the association between HLA-DRB1 specific alleles and asthma, but no significant difference was found between them. Because of the limited number of studies, we only chose 6 HLA-DRB1-specific alleles in this meta-analysis. These results of specific alleles still require evaluation by further studies.
Considering ethnicity and age, we performed subgroup analysis in HLA-DRB1 allele families. DRB1*03 significantly increased the risk of asthma and DRB1*15 significantly decreased the risk of asthma in whites. DRB1*07 significantly increased the risk of asthma and DRB1*14 significantly decreased the risk of asthma in Asians. For age, DRB1*03, DRB1*04, and DRB1*09 were suggested to affect risk and DRB1*15 was suggested to have a protective role. Publication bias is another factor to consider in meta-analysis. In this meta-analysis, the Begg’s rank correlation method and the Egger’s linear regression method showed that there was no publication bias in any alleles except DRB1*0101.
There are some limitations to the present study that should be addressed. First, we only chose some of the HLA-DRB1-specific alleles in this study due to the scarcity of data in the literature. Second, the relationship between the HLA-DRB1 alleles and asthma did not consider confounding factors such as sex, lifestyle factors, and other risk factors. It is best to adjust these factors when conducting the studies. Third, asthma is the result of the interaction of genetic and environmental factors, but we could not extract sufficient data on these factors from the primary publications. Thus, this meta-analysis could not elucidate gene-gene and gene-environment interactions. More studies should be designed to analyze these associations in the future. Results of the present study must be interpreted cautiously in light of its limitations.
Conclusions
Despite these limitations, this meta-analysis suggests that DRB1*03 is positively associated with asthma risk and DRB1*15 is negatively associated with asthma risk. Additional studies on the association between DRB1-specific alleles and asthma are required.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Source of support: Departmental sources
References
- 1.Martinez FD, Vercelli D. Asthma Lancet. 2013;(382):1360–72. doi: 10.1016/S0140-6736(13)61536-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olin JT, Wechsler ME. Asthma: Pathogenesis and novel drugs for treatment. BMJ. 2014:349 g5517. doi: 10.1136/bmj.g5517. [DOI] [PubMed] [Google Scholar]
- 3.Akinbami LJ, Moorman JE, Bailey C, et al. NCHS Data Brief, 20121–8. Trends in asthma prevalence, health care use, and mortality in the United States, 2001–2010. [PubMed] [Google Scholar]
- 4.Ali Z, Dirks CG, Ulrik CS. Long-term mortality among adults with asthma: A 25-year follow-up of 1,075 outpatients with asthma. Chest. 2013;143:1649–55. doi: 10.1378/chest.12-2289. [DOI] [PubMed] [Google Scholar]
- 5.Du Y, Xue Y, Xiao W. Association of IREB2 gene rs2568494 polymorphism with risk of chronic obstructive pulmonary disease: A meta-analysis. Med Sci Monit. 2016;22:177–82. doi: 10.12659/MSM.894524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu Z, Li P, Wang J, et al. A meta-analysis of IL-13 polymorphisms and pediatric asthma risk. Med Sci Monit. 2014;20:2617–23. doi: 10.12659/MSM.891017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shiina T, Hosomichi K, Inoko H, et al. The HLA genomic loci map: Expression, interaction, diversity and disease. J Hum Genet. 2009;54:15–39. doi: 10.1038/jhg.2008.5. [DOI] [PubMed] [Google Scholar]
- 8.Kontakioti E, Domvri K, Papakosta D, et al. HLA and asthma phenotypes/endotypes: A review. Hum Immunol. 2014;75:930–39. doi: 10.1016/j.humimm.2014.06.022. [DOI] [PubMed] [Google Scholar]
- 9.Cho SH, Kim YK, Oh OH, et al. Association of HLA-DRB1(*)07 and DRB1(*)04 to citrus red mite (Panonychus citri) and house dust mite sensitive asthma. Clin Exp Allergy. 2000;30:1568–75. doi: 10.1046/j.1365-2222.2000.00915.x. [DOI] [PubMed] [Google Scholar]
- 10.Lama M, Chatterjee M, Chaudhuri TK. A study of the association of childhood asthma with HLA alleles in the population of Siliguri, West Bengal, India. Tissue Antigens. 2014;84:316–20. doi: 10.1111/tan.12403. [DOI] [PubMed] [Google Scholar]
- 11.Kauppinen A, Perasaari J, Taivainen A, et al. Association of HLA class II alleles with sensitization to cow dander Bos d 2, an important occupational allergen. Immunobiology. 2012;217:8–12. doi: 10.1016/j.imbio.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 12.Dzurilla M, Vrlik M, Homolova M, et al. No association between bronchial asthma and HLA-DRB1, -DQB1 alleles in the Slovak population. Bratisl Lek Listy. 2013;114:93–95. doi: 10.4149/bll_2013_021. [DOI] [PubMed] [Google Scholar]
- 13.Rihs HP, Barbalho-Krolls T, Huber H, et al. No evidence for the influence of HLA class II in alleles in isocyanate-induced asthma. Am J Ind Med. 1997;32:522–27. doi: 10.1002/(sici)1097-0274(199711)32:5<522::aid-ajim13>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 14.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 15.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–48. [PubMed] [Google Scholar]
- 16.Rogers WJ. What is the optimal tool to define appropriate therapy: The randomized clinical trial, meta-analysis, or outcomes research? Commentary. Curr Opin Cardiol. 1994;9:401–3. [PubMed] [Google Scholar]
- 17.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101. [PubMed] [Google Scholar]
- 18.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bignon JS, Aron Y, Ju LY, et al. HLA class II alleles in isocyanate-induced asthma. Am J Respir Crit Care Med. 1994;149:71–75. doi: 10.1164/ajrccm.149.1.8111601. [DOI] [PubMed] [Google Scholar]
- 20.Soriano JB, Ercilla G, Sunyer J, et al. HLA class II genes in soybean epidemic asthma patients. Am J Respir Crit Care Med. 1997;156:1394–98. doi: 10.1164/ajrccm.156.5.9701064. [DOI] [PubMed] [Google Scholar]
- 21.Horne C, Quintana PJ, Keown PA, et al. Distribution of DRB1 and DQB1 HLA class II alleles in occupational asthma due to western red cedar. Eur Respir J. 2000;15:911–14. doi: 10.1034/j.1399-3003.2000.15e17.x. [DOI] [PubMed] [Google Scholar]
- 22.Mapp CE, Beghe B, Balboni A, et al. Association between HLA genes and susceptibility to toluene diisocyanate-induced asthma. Clin Exp Allergy. 2000;30:651–56. doi: 10.1046/j.1365-2222.2000.00807.x. [DOI] [PubMed] [Google Scholar]
- 23.Chunfang M, Runfang G. Study of HLA-DRB1 allele in bronchial asthma. Shanxi Clin Med. 2000;9:417–19. [Google Scholar]
- 24.Lin L, Lanfang C, Ticheng G, et al. Study on gene frquency of HLA-DRB1 expression in children with mycoplasma pneumonia and bronchial. Journal of Clinical Pediatrics. 2003;21:29–31. [Google Scholar]
- 25.Choi JH, Lee KW, Oh HB, et al. HLA association in aspirin-intolerant asthma: DPB1*0301 as a strong marker in a Korean population. J Allergy Clin Immunol. 2004;113:562–64. doi: 10.1016/j.jaci.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 26.Chaopin L, Qinggui Y, Li T. Relationship between HLA-DRB1 gene and acarian asthma. Acta Universitatis Medicinalis Anhui. 2003;40:244–46. [Google Scholar]
- 27.Jirong L, Lixin J, Chengxun W. A study of correlation between childhood asthma and HLA. Journal of Clinical Pediatrics. 2006;24:16–24. [Google Scholar]
- 28.Juhn YJ, Kita H, Lee LA, et al. Childhood asthma and human leukocyte antigen type. Tissue Antigens. 2007;69:38–46. doi: 10.1111/j.1399-0039.2006.00719.x. [DOI] [PubMed] [Google Scholar]
- 29.Munthe-Kaas MC, Carlsen KL, Carlsen KH, et al. HLA Dr-Dq haplotypes and the TNFA-308 polymorphism: Associations with asthma and allergy. Allergy. 2007;62:991–98. doi: 10.1111/j.1398-9995.2007.01377.x. [DOI] [PubMed] [Google Scholar]
- 30.Jiawei W, Gesheng W, Licheng D, et al. [Immunogenetic analysis of leukocyte antigen DRB1 and DQB1 in childhood asthma]. Zhejiang Practical Medicine. 2007;12:240–41. [in Chinese] [Google Scholar]
- 31.Movahedi M, Moin M, Gharagozlou M, et al. Association of HLA class II alleles with childhood asthma and Total IgE levels. Iran J Allergy Asthma Immunol. 2008;7:215–20. [PubMed] [Google Scholar]
- 32.Choi JH, Lee KW, Kim CW, et al. The HLA DRB1*1501-DQB1*0602-DPB1*0501 haplotype is a risk factor for toluene diisocyanate-induced occupational asthma. Int Arch Allergy Immunol. 2009;150:156–63. doi: 10.1159/000218118. [DOI] [PubMed] [Google Scholar]
- 33.Ivkovic-Jurekovic I, Zunec R, Balog V, et al. The distribution of HLA alleles among children with atopic asthma in Croatia. Coll Antropol. 2011;35:1243–49. [PubMed] [Google Scholar]
- 34.Shufen Z. [Association between Human Leukocyte antigen DRB1(HLA-DRB1)gene polymorphisms and Cough variant]. Journal of Clinical Pulmonary Medicine. 2011;16:371–73. [in Chinese] [Google Scholar]
- 35.Liping C, Hong X, Jingmei L, et al. [Association between Human Leukocyte antigen-DRB1 allele and asthma]. Chinese Journal of Practical Internal Medicine. 2012;32:956–57. [in Chinese] [Google Scholar]
- 36.Qinglin X, Hong Z, Ling Q, et al. Study on the association between Human Leukocyte antigen gene-DR and -G polymorphisms and children. Chinese Journal of New Clinical Medicine. 2013;6:109–11. [Google Scholar]
- 37.Mishra MN, Dudeja P, Gupta RK. Association of HLA-Class II and IgE serum levels in pediatric asthma. Iran J Immunol. 2014;11:21–28. [PubMed] [Google Scholar]
- 38.Bottero P, Motta F, Bonini M, et al. Can HLA-DRB4 Help to Identify Asthmatic Patients at Risk of Churg-Strauss Syndrome? ISRN Rheumatol. 2014;2014:843804. doi: 10.1155/2014/843804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim Y, Lee S, Kim YS, et al. Regulation of Th1/Th2 cells in asthma development: a mathematical model. Math Biosci Eng. 2013;10:1095–133. doi: 10.3934/mbe.2013.10.1095. [DOI] [PubMed] [Google Scholar]
- 40.Brand S, Kesper DA, Teich R, et al. DNA methylation of TH1/TH2 cytokine genes affects sensitization and progress of experimental asthma. J Allergy Clin Immunol. 2012;129:1602–10e6. doi: 10.1016/j.jaci.2011.12.963. [DOI] [PubMed] [Google Scholar]
- 41.Arroyave WD, Rabito FA, Carlson JC. The relationship between a specific IgE level and asthma outcomes: results from the 2005–2006 National Health and Nutrition Examination Survey. J Allergy Clin Immunol Pract. 2013;1:501–8. doi: 10.1016/j.jaip.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 42.Lin KL, Hsieh KH, Huang JH, et al. Major histocompatibility complex (MHC) class II restriction, lymphokine production, and IgE regulation of house dust mite-specific T-cell clones. J Clin Immunol. 1992;12:271–80. doi: 10.1007/BF00918151. [DOI] [PubMed] [Google Scholar]
- 43.Murray JS. How the MHC selects Th1/Th2 immunity. Immunol Today. 1998;19:157–63. doi: 10.1016/s0167-5699(97)01237-1. [DOI] [PubMed] [Google Scholar]
- 44.Knutsen AP, Vijay HM, Kariuki B, et al. Association of IL-4RA single nucleotide polymorphisms, HLA-DR and HLA-DQ in children with Alternaria-sensitive moderate-severe asthma. Clin Mol Allergy. 2010;8:5. doi: 10.1186/1476-7961-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martyn MB, Molis W, Jacobson RM, et al. Human leukocyte antigen type and progression from onset of symptoms to development of asthma. Allergy Asthma Proc. 2010;31:120–25. doi: 10.2500/aap.2010.31.3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hanchard NA, Jacobson RM, Poland GA, et al. An assessment of the association between childhood asthma and HLA DRB1*03 using extended haplotype analysis. Tissue Antigens. 2010;76:491–94. doi: 10.1111/j.1399-0039.2010.01548.x. [DOI] [PubMed] [Google Scholar]
- 47.Smit LA, Strachan DP, Vermeulen R, et al. Human leukocyte antigen class II variants and adult-onset asthma: Does occupational allergen exposure play a role? Eur Respir J. 2014;44:1234–42. doi: 10.1183/09031936.00068014. [DOI] [PubMed] [Google Scholar]
- 48.Muro M, Mondejar-Lopez P, Moya-Quiles MR, et al. HLA-DRB1 and HLA-DQB1 genes on susceptibility to and protection from allergic bronchopulmonary aspergillosis in patients with cystic fibrosis. Microbiol Immunol. 2013;57:193–97. doi: 10.1111/1348-0421.12020. [DOI] [PubMed] [Google Scholar]
- 49.Wang J, Pan HF, Hu YT, et al. Polymorphism of IL-8 in 251 allele and gastric cancer susceptibility: A meta-analysis. Dig Dis Sci. 2010;55:1818–23. doi: 10.1007/s10620-009-0978-y. [DOI] [PubMed] [Google Scholar]


