Abstract
Background
Circadian variation in renal toxicity of aminoglycosides has been demonstrated in animal and human studies. People with CF are frequently prescribed aminoglycosides. Altered pharmacokinetics of aminoglycosides are predictive of toxicity.
Aim
To investigate whether the time of day of aminoglycoside administration modulates renal excretion of tobramycin and toxicity in children with CF. To determine whether circadian rhythms are disrupted in children with CF during hospital admission.
Methods
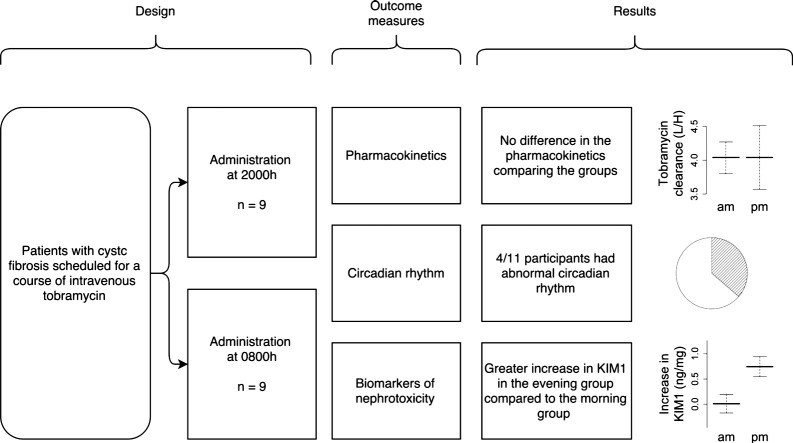
Children (age 5–18 years) with CF scheduled for tobramycin therapy were randomly allocated to receive tobramycin at 0800 or 2000 h. Serum tobramycin levels were drawn at 1 h and between 3.5 and 5 h post-infusion between days 5 and 9 of therapy. Melatonin levels were measured serially at intervals from 1800 h in the evening until 1200 h on the next day. Circadian rhythm was categorised as normal when dim light melatonin onset was demonstrated between 1800 and 2200 h and/or peak melatonin levels were observed during the night. Weight and spirometry were measured at the start and end of the therapy. Urinary biomarkers of kidney toxicity (KIM1, NAG, NGAL, IL-18 and CysC) were assayed at the start and end of the course of tobramycin.
Results
Eighteen children were recruited to the study. There were no differences in renal clearance between the morning and evening groups. The increase in urinary KIM-1 was greater in the evening dosage group compared to the morning group (mean difference, 0.73 ng/mg; 95% CI, 0.14 to 1.32; p = 0.018). There were no differences in the other urinary biomarkers. There was normal circadian rhythm in 7/11 participants (64%).
Conclusions
Renal elimination of tobramycin was not affected by the time of day of administration. Urinary KIM-1 raises the possibility of greater nephrotoxicity with evening administration. Four children showed disturbed circadian rhythm and high melatonin levels (ClinicalTrials.gov NCT01207245).
Keywords: Cystic fibrosis, Aminoglycosides, Toxicity, Circadian rhythm
Graphical abstract
1. Introduction
Despite recent advances in cystic fibrosis (CF) therapeutics, the mainstay of treatment of pulmonary exacerbations remains aggressive antibiotic management. In the UK, registry reports indicate that patients receive a median of 21 days of intravenous antibiotics per year [1]. Aminoglycosides are commonly administered in CF, but have been implicated in acute [2], [3] and chronic [4] kidney disease. Patients with CF may have other risk factors for renal impairment including CF-related diabetes, concomitant nephrotoxic drugs and immunosuppressive treatment post-transplant.
Aminoglycoside toxicity can be minimised by prescribing tobramycin in preference to gentamicin [3] and careful therapeutic drug monitoring [5]. Extended interval once daily dosing is less nephrotoxic in cystic fibrosis [6], and has been adopted as a standard of care in many centres [7]. However, time of day of administration varies according to unit and patient preference.
Time of day of administration of once daily aminoglycosides could influence toxicity in patients with cystic fibrosis. In animal models, circadian variation in aminoglycoside pharmacokinetics and toxicity has been demonstrated [8], [9], although in human subjects, the data are conflicting [10], [11], [12], [13], [14]. In a post hoc analysis of a randomised trial of once versus thrice daily administration of tobramycin in CF (TOPIC) [15], thrice daily administration was associated with a higher elimination rate constant (i.e. faster elimination) than the once daily administration. Once daily dosing was mainly administered in the evenings. The PK of thrice daily dosing represented a daily average, indicating that morning elimination was more rapid. Therefore, we hypothesized that a once daily dose administered in the morning may be more rapidly eliminated and hence safer than an evening dose. A predictable intrinsic circadian variability in elimination of tobramycin would provide an opportunity for a cost neutral and easily implementable strategy to reduce the toxicity of intravenous tobramycin.
We report a randomised pharmacokinetic comparison of once daily tobramycin in cystic fibrosis, comparing morning with evening dosing, using a primary pharmacokinetic endpoint. As one observational study found increased toxicity with evening administration without a measurable difference in pharmacokinetics [11], we evaluated subclinical kidney toxicity with urinary biomarkers. As an intact circadian rhythm is essential for a predictable diurnal variability in tobramycin elimination, we additionally assessed circadian rhythm with melatonin, a robust marker of circadian rhythm [16].
2. Methods
2.1. Study design and participants
This open label, randomised controlled clinical trial was conducted in four centres across the Midlands region of the UK (Nottingham University Hospital NHS Trust, University Hospitals Leicester NHS Trust, Lincoln County Hospital and Pilgrim Hospital). The study was approved by the regulatory authority (MHRA) and the National Research Ethics Service (NRES) and was registered on ClinicalTrials.gov (NCT01207245).
Patients with CF between 5 and 18 years of age were eligible for inclusion when their treating physician decided to administer a course of tobramycin. CF was defined as clinical features of CF in the presence of positive sweat test and/or presence of two mutations known to be associated with CF. Study exclusion criteria included history of acute kidney injury, recipients of solid organ transplant or hypersensitivity to tobramycin. Patients were recruited from May 2011 until December 2012. Informed consent and assent were obtained from the parents/legal guardians and children, respectively.
2.2. Drug treatment and pharmacokinetics measurement
All patients received tobramycin and at least a second intravenous antibiotic which was not pre-specified in the protocol. The dose of tobramycin was determined as per the local unit's protocol, which was in line with the CF Trust Antibiotic Guideline (10 mg/kg/day) [17]. Participants were randomly assigned to tobramycin administered at either 0800 or 2000 h (infused over 30 min). Randomisation used a remote, web-based system (Nottingham Clinical Trials Unit), and randomised participants to each arm in a 1:1 ratio without blocks. Pharmacokinetic (PK) samples were drawn once in the middle of the course (day 7 ± 2). Samples were taken at 1 h and between 3.5 and 5 h from the end of the infusion. Samples were analysed using a homogenous enzyme immunoassay technique (Beckman Coulter Inc., California, USA). Tobramycin PK parameters were measured based on a one-compartment model using MwPharm Version 3.7 (Mediware a.s., Prague, Czech Republic). The PK parameters determined were elimination rate constant (Kel h− 1), clearance (CL, L/h), volume of distribution (V, L/kg) and area-under-the-concentration-time curve (AUC, mg × h/L). Concomitant medications were recorded (see Results).
2.3. Urinary biomarkers of nephrotoxicity
Urine samples were collected on day one (prior to treatment) and at the end of the 14-day treatment course. Samples were placed on ice immediately, and centrifuged at 4 °C for 15 min at 1000g, and 1 tablet of protease inhibitor (cOmplete, Mini; Roche Applied Science) was added to 10 ml of supernatant. Samples were aliquotted and stored at − 80 °C for processing in batch. The biomarker panel consisted of kidney injury molecule-1 (KIM1), cystatin C (CysC), neutrophil gelatinase associated lipocalin (NGAL), interleukin-18 (IL-18) and N-acetyl-β-d-glucosaminidase (NAG). KIM1, NAG, CysC and IL-18 were assayed with a sandwich ELISA according to the manufacturer's instructions (RnD Systems catalogue numbers DKM100, DLCN20 and DSCTC0, and MBL International catalogue number 7620). NAG was assayed with a modification of a kit supplied by Bioquant (catalogue number BQ062A-EAKP). The data were analysed using the CalibFit package in R (version 2.15.1). Urinary biomarkers were normalised to urinary creatinine levels to control for urinary dilution effects.
2.4. Melatonin measurements and analysis
Saliva samples were collected for melatonin analysis by passive drooling into collection containers (UltraSal-2™, Oasis Diagnostics® Corporation, Vancouver, USA). Samples were collected on day 7 ± 2 at 1800, 1900, 2030, 2130, and 2400 h and on the next day at 0600 and 1200 h. If a participant was asleep for an overnight sample, the sample was omitted. The room lights were dimmed to less than 30 Lx from approximately one hour before the saliva collection was commenced until waking in the morning, and participants were advised to take precautions to prevent contamination with food. Samples visibly contaminated by blood or food were discarded. The analysis was undertaken by radioimmunoassay method (BÜHLMANN direct saliva melatonin RIA, Schönenbuch, Switzerland). Dim light melatonin onset (DLMO) was defined as the time when saliva melatonin levels increased to ≥ 4 pg/ml rising from a lower baseline value [18]. For the study purposes, a normal circadian pattern was defined as either (i) DLMO observed between 1800 and 2200 h, or (ii) a rise in the melatonin level during the night with a fall in the daytime [19], [20]. Midnight, early morning and midday samples were collected to identify any abnormal rhythms such as phase reversal or low melatonin levels. Two investigators independently categorised the participants as having normal or abnormal circadian melatonin (APP and KJ) with a third investigator adjudicating (BK). A sleep quality questionnaire was completed, including a visual analogue score (from 0 to 10) to describe sleep quality during one night in the hospital. Visual analogue scores of 7–10, 4–6 and 0–3 were defined as good quality sleep, moderate quality sleep and poor quality sleep, respectively.
2.5. Other measurements
Serum creatinine levels, weight and height were measured at the beginning of the course. Glomerular filtration rate was estimated (eGFR) with the Schwartz formula [21]. Weight was measured at the end of the treatment course. Spirometry was performed by appropriately trained staff on the ward measuring forced expiratory volume in 1 s (FEV1), forced vital capacity (FVC) and FEV1/FVC at the beginning and end of course of IV treatment according to ATS/ERS Task Force recommendations [22].
2.6. Outcome measures and statistical analysis
The primary outcome measure of the study was the renal elimination rate constant for intravenous tobramycin when given in the morning (0800 h) compared to evening administration (2000 h). The secondary outcome measures were (i) change in biomarker levels over the course of treatment, (ii) the presence or absence of circadian rhythm in children with CF and (iii) clinical parameters including the change in weight and lung function during the course of therapy. We defined the minimum clinically important difference in Kelr of 30% between the two groups. We used distribution data from the TOPIC study (sd of Kelr 0.00035 h− 1/ml/min/1.73 m2) [6] to inform our power calculation. To achieve a power of 95% to detect a 30% difference in Kelr, a sample size of 8 patients in each arm was required (Minitab version 15, Minitab Inc., PA, USA). We planned for 10 in each arm to allow for a dropout rate of 20%. Participants who had results available for weight, height, serum creatinine, tobramycin dose and tobramycin serum levels including the timing for any one day of the therapy were included in the primary analysis.
At trial initiation, we planned to employ a one-compartment Bayesian PK model using estimated glomerular filtration rate (calculated with the Schwartz formula) within the model priors. However, data from the CEFIT study (manuscript submitted, ISRCTN44660887) became available during the conduct of this study. These results indicate that in the CF population, the Schwartz formula poorly assesses eGFR. In the final analysis presented herein, we employed a one-compartment model calculating total body clearance without separating metabolic (which accounts for around 10%–15% of clearance in individuals with normal renal function) and renal clearance. This avoided the need to use an inaccurate calculated eGFR in the model.
We compared the clearance, elimination rate constant and half-life between the groups. For biomarker data, we compared the absolute change (increase) in biomarker levels from the start to the end of the course of antibiotic for the morning versus the evening group. Data were tested for normality using QQ plots, and the Shapiro–Wilk test for normality. Where data were not normally distributed, deterministic transformations (log, reciprocal, square root and powers) were explored. If the data or suitable transforms of the data were found to be normally distributed, a Student's T-test was used to compare means. If the data were not normally distributed, a Wilcoxon test was used to compare groups. The study results were analysed using IBM SPSS, Statistics for Windows, version 20 (IBM Corp., Armonk, NY, USA) and R version 2.15.2 [23].
3. Results
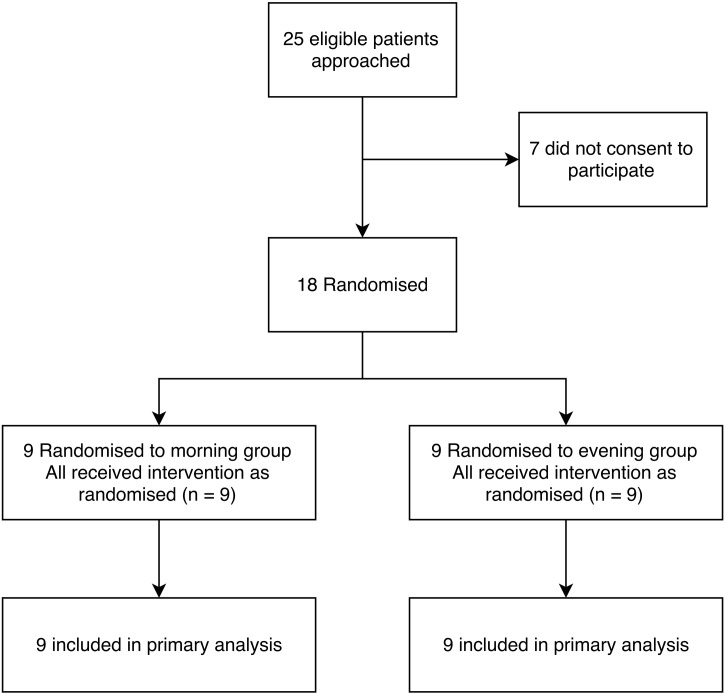
Twenty-five eligible subjects were identified and approached (Fig. 1). Consent was given by 18 patients who were randomised to morning (N = 9) or evening groups (N = 9). Both groups were similar in characteristics (Table 1) including age, sex, initial weight, height, serum creatinine and estimated GFR. The second antibiotic was not pre-specified in the protocol. In most cases, this was ceftazidime or meropenem (see Table 4). No patient was on a concomitant nephrotoxic drug (e.g. high dose ibuprofen). Concomitant medications in each group are given in Table 4.
Fig. 1.
Consort diagram of included participants.
Table 1.
Demographics of enrolled patients.
| Characteristics | Morning Median (IQR) | Evening Median (IQR) |
|---|---|---|
| N | 9 | 9 |
| Age (years) | 12.5 (12.2–15.5) | 14.5 (12.8–14.9) |
| Weight (Kg) | 39.2 (30.4–52.0) | 42.0 (38.5–50.7) |
| Height (cm) | 146 (137–165) | 164 (162–167) |
| Serum Creatinine (mmol/L) | 57 (40–60) | 56 (50–79) |
| eGFR (ml/min/1.73 m2) | 135 (123–165) | 121 (100–159) |
Table 4.
Concomitant medications.
| Medication | Morning group frequency | Evening group frequency |
|---|---|---|
| Ceftazidime | 8 | 9 |
| Meropenem | 2 | 2 |
| Co-trimxazole | 1 | 0 |
| Azithromycin | 5 | 8 |
| Beclomethasone | 0 | 1 |
| Calcichew D3 forte | 1 | 1 |
| Cholecalciferol | 1 | 1 |
| Clenil modulite | 0 | 1 |
| Creon | 7 | 8 |
| Dornase alfa | 6 | 9 |
| Doxycycline | 0 | 1 |
| Flixotide | 0 | 1 |
| Flucloxacillin | 1 | 0 |
| Furosemide | 0 | 1 |
| Hypertonic saline | 3 | 2 |
| Insulin | 0 | 1 |
| Itraconazole | 1 | 1 |
| Monteleukast | 1 | 0 |
| Movicol | 2 | 1 |
| Multivitamin | 8 | 5 |
| Nutritional supplement | 0 | 2 |
| Nasonex | 1 | 0 |
| Nystatin | 6 | 5 |
| Omeprazole | 5 | 6 |
| Prednisolone | 1 | 0 |
| Propanolol | 0 | 1 |
| Salbutamol | 8 | 5 |
| Seretide 50 | 2 | 1 |
| Sodium picosulphate | 0 | 1 |
| Spironolactone | 0 | 1 |
| Symbicort | 0 | 1 |
| Terbutaline | 0 | 1 |
| Ursodeocycholic acid | 3 | 4 |
| Vitamin A & D | 1 | 2 |
| Vitamin E | 6 | 8 |
| Vitamin K | 1 | 3 |
3.1. Pharmacokinetic results
There was no evidence for a difference in the primary outcome of pharmacokinetics of the two groups (Table 2). This finding did not change when a Schwarz formula calculated eGFR was included in the model (data not shown). Lung function and weight at the initiation and end of the intravenous therapy were similar between groups (Table 2).
Table 2.
Pharmacokinetic and clinical results.
| Morning | Evening | |
|---|---|---|
| n | 9 | 9 |
| Kel (h− 1) | 0.41 [0.36 to 0.44] | 0.36 [0.34 to 0.46] |
| Clearance (L/h) | 4.1 [3.77 to 4.47] | 3.54 [3.39 to 4.51] |
| T1/2 (h) | 1.66 [1.49 to 1.88] | 1.89 [1.49 to 2.04] |
| Vd (l/kg) | 0.26 [0.22 to 0.31] | 0.24 [0.21 to 0.32] |
| AUC (h.mg/L) | 4.15 [3.09 to 4.85] | 3.72 [3.53 to 5.35] |
| Weight gain (kg) | 1.2 [0.7 to 1.5] | 0.6 [0.2 to 0.7] |
| ∆FEV1 (End − Initial) (L) | 0.19 [0.07 to 2.20] | 0.23 [0.19 to 0.28] |
| ∆FVC (End − Initial) (L) | 0.32 [− 0.01 to 0.38] | 0.18 [− 0.01 to 0.29] |
Results are expressed as median [inter-quartile range]. No differences were found between groups when compared with the Wilcoxon signed rank test.
3.2. Biomarkers of kidney toxicity
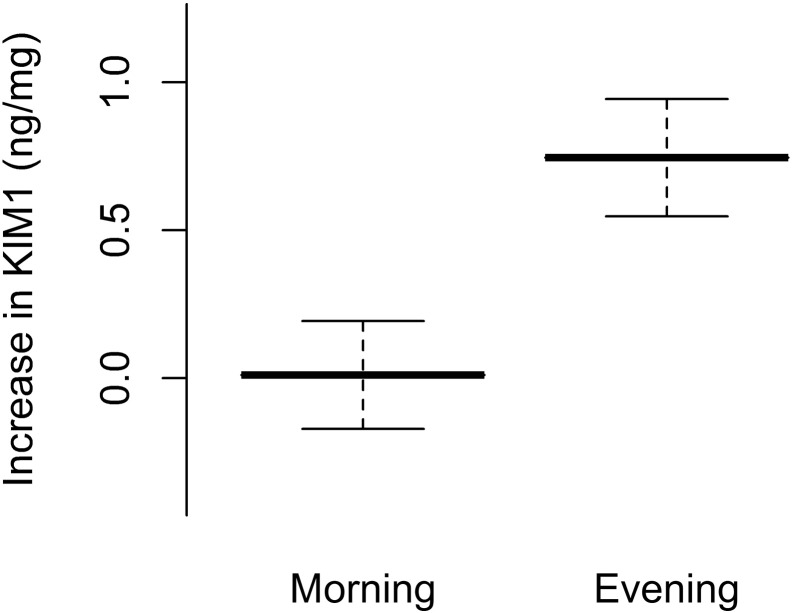
Biomarkers (kidney injury molecule-1 (KIM1), cystatin C (CysC), neutrophil gelatinase associated lipocalin (NGAL), interleukin-18 (IL-18) and N-acetyl-β-d-glucosaminidase (NAG)) were assayed. Levels were available for 14 participants. Four participants dropped out prior to the final day of the study (three were changed from tobramycin to an alternative antibiotic prior to the end of the course, and one withdrew consent). For each biomarker assayed, the increase for each participant was calculated and the mean difference in the increase for the morning and evening groups was calculated (with a log transformation of the data where appropriate) (Table 3). KIM1 showed a greater rise in the evening compared to the morning (p < 0.05), suggesting greater renal toxicity with evening administration (Fig. 2).
Table 3.
Difference in biomarker increase over the treatment course.
| Biomarker | Mean change in morning group (95% CI)a | Mean change in evening group (95% CI)a | Difference in biomarker increase (95% CI)b | p-value |
|---|---|---|---|---|
| KIM1/Cr (ng/mg) | 0.01 (− 0.43 to 0.46) | 0.74 (0.26 to 1.22) | 0.73 (0.14 to 1.32) | 0.018 |
| CysC/Cr (ng/mg) | − 16.6 (− 51.0 to 17.8) | 29.6 (− 45.2 to 104.3) | 46.2 (− 30.7 to 123.0) | 0.20c |
| NGAL/Cr (ng/mg) | 10.1 (− 144.7 to 164.9) | 12.9 (− 76.9 – 102.8) | − 0.01 (− 2.47 to 2.43)d | 0.99 |
| IL-18/Cr (pg/mg) | − 5.72 (− 34.3 to 22.9) | 139.3 (− 221.1 to 499.7) | 0.2 (− 0.6 to 1.0)d | 0.59 |
| NAG/Cr (IU/mg) | 0.037 (0.002 to 0.07) | 0.052 (− 0.001 to 1.034) | 0.79 (− 0.94 to 2.52)d | 0.33 |
A positive value indicates that the biomarker level increased over the course of antibiotics.
A positive difference indicates that the increase in the evening group was greater than the rise in the morning group.
Indicates p value calculated with Wilcoxon signed rank test (other p values calculated with Student’s T-test).
Indicates a log transform has been applied to the data (log transformed data shown in this column) before performing the T-test. Urinary biomarker levels were normalised to urinary creatinine to control for urinary concentration.
Fig. 2.
Increase in urinary KIM1 evening versus morning administration group.
Urinary KIM1 was collected at the start and end of therapy. Lines represent the mean and standard error of the mean. Levels were normalised to urinary creatinine to control for urinary concentration (ng KIM1 per mg creatinine).
3.3. Sleep–wake cycle
A salivary melatonin profile was obtained for a subgroup of 11 patients. Seven had an intact circadian rhythm, and four had altered rhythm. Two patients with abnormal rhythm were in the evening group, and two were in the morning group. The small number of patients prevented comparisons of pharmacokinetics and biomarkers in individuals with and without normal circadian rhythm.
4. Discussion
In a randomised trial comparing dosage time of once daily tobramycin in CF, we did not find a difference in pharmacokinetics between groups administered tobramycin in the morning compared to the evening. However, in the pre-specified secondary endpoint of change in urinary biomarker levels, we found that urinary KIM1 increased over the treatment course in participants who received tobramycin in the evening, whereas it remained approximately static in the morning group. Thus, we have found some evidence of increased toxicity in evening administration of tobramycin in the absence of altered pharmacokinetics. A significant proportion (4/11) of participants did not have an intact circadian rhythm.
Ours is the first published randomised study to investigate circadian variability of pharmacokinetics of tobramycin in children with cystic fibrosis. Once daily aminoglycoside regimens have been adopted widely since the publication of the TOPIC study. In the UK, 90% of paediatric centres and 79% of adult centres currently use once daily aminoglycosides [7]. Tobramycin is eliminated principally by the kidneys [24], and there is a circadian rhythm of glomerular filtration [25]. As toxicity is increased with greater serum exposure to the drug, more rapid elimination results in reduced toxicity. The present study was powered to detect a 30% difference in elimination rate constant between the two groups. We took samples for pharmacokinetic analysis between one and three half-lives of tobramycin—the optimum time to detect differences in elimination rate [28]. We found no evidence for a difference in pharmacokinetics in tobramycin administered in the morning compared to the evening.
We report tobramycin clearance as the primary endpoint. At the trial initiation, we powered for a difference in Kelr (the renal elimination rate constant). However, we found in the CEFIT study (ISRCTN44660887, manuscript submitted for publication) that the Schwartz formula is an unreliable estimator of glomerular filtration rate. As MwPharm takes into account estimated GFR when calculating Kelr, we carefully considered whether Kelr is the correct parameter to report, and concluded that Kelr has a high chance of bias due to the inaccuracies in eGFR. We therefore herein report total body tobramycin clearance, Kel (total body elimination rate constant) and the half-life, as these do not depend upon eGFR. We also undertook our original planned analysis of Kelr, and also found no difference between groups (see online supplement).
The increase in KIM1 (one of our biomarker secondary endpoints) suggests increased toxicity associated with evening administration. This result must be viewed with caution, as the other four biomarkers did not show evidence of a difference in toxicity. As KIM1 was the only biomarker showing a difference between groups, and given the multiple testing and the small sample size, there is a risk of type I error. However, KIM1 may be the most sensitive marker of subclinical AKI in the setting of aminoglycoside toxicity. McWilliam found that KIM1 outperformed NGAL and NAG in neonates exposed to aminoglycosides [26]. Additionally, KIM1 is elevated after exposing children with CF to aminoglycoside [27]. From our results, we do not believe that we can yet recommend that morning administration is safer than evening administration, but rather we believe that it is worthwhile to investigate this in a future study with a larger study size powered specifically for the KIM1 endpoint.
KIM-1 is an emerging biomarker with evidence of potential clinical utility accumulating in the literature at a rapid rate. Urinary KIM1 is a transmembrane protein with extracellular immunoglobulin and mucin domains [28]. After an acute nephrotoxic insult to the proximal tubule, urinary levels of KIM1 increase [29], and KIM1 has been investigated as a marker of severe acute kidney injury in a variety of settings [30]. Recently, Shao et al.'s report on the meta-analysis of its role in acute kidney injury has been published [31]. Within this meta-analysis, studies found a range of cutoffs for dichotomising patients into high and low risk of AKI extending from 0.4 to 2.8 ng/mg. This heterogeneity likely reflects differences in assays used in the primary studies, different definitions of AKI, and different populations of patients. Our results (difference between the increase from baseline when comparing the morning versus evening group of 0.73 mg) falls toward the lower end of this range. It is noteworthy that Shao's meta-analysis found some evidence of publication bias.
We avoided pre-specifying a cutoff for KIM1 at the protocol phase of our study. Our study was to pilot the biomarker endpoints to address the relative toxicity of a morning versus evening group. Data from Al-Aloul et al. [4] indicate that each course is associated with a small reduction in kidney function which builds up cumulatively over time. Much of this toxicity in individual courses is likely to be subclinical and would not meet the criteria for AKI. Therefore, in our study, we have not aimed to determine if patients developed AKI, but take the view that an elevated biomarker level implies greater toxicity (either subclinical or clinical). Our intention in this present study was not to determine if a difference between the rates of AKI exists between the two groups (for which we could measure the proportion of patients meeting the clinical criteria for AKI), but to establish if biomarkers support the notion that a morning dose is less nephrotoxic than the evening group. In this regard, our study is more akin to that of McWilliam et al. [26], who studied the profile of KIM1 and other biomarkers in neonates to determine if they could be used to assess subclinical nephrotoxicity in patients after exposure to aminoglycosides in the absence of AKI.
The observation that KIM1 increases with evening but not morning administration supports the hypothesis that an evening dose is more toxic than a morning dose. Prins and colleagues also found increased renal toxicity (as evaluated with a change in serum creatinine level) with evening administration of aminoglycosides in the absence of pharmacokinetic differences [11]. This observational study was performed in adults with sepsis, and hence other factors could explain the increased kidney damage in the evening administration group. Within this context, it remains possible to postulate that increased toxicity in the evening may occur via a mechanism independent of a circadian rhythm in pharmacokinetics. An alternate explanation, which incorporates pharmacokinetics, would be that the evening dose is inherently more nephrotoxic, and has the effect of gradually prolonging elimination over the two-week course of antibiotics. This might exacerbate the effect of the evening dose late in the course of treatment. As we measured pharmacokinetics midway through the course of tobramycin, we are unable to exclude this possibility.
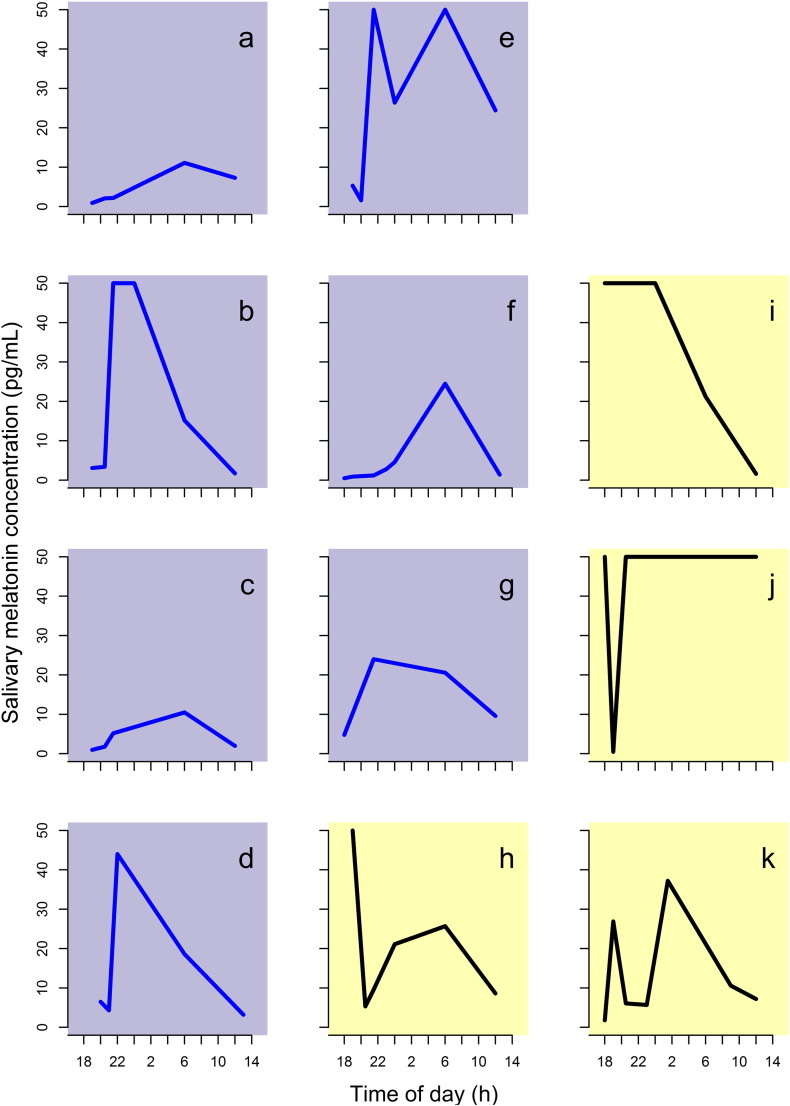
We measured salivary melatonin profiles to investigate circadian rhythm (Fig. 3). This is the first time circadian rhythm has been investigated in an inpatient CF population with melatonin. The results were available in 11 (60%) participants. A normal rhythm was demonstrated in 7/11 children; altered or no rhythm was observed in 4/11 patients. We have ruled out participants self-administering melatonin as a potential confounder for these results. A limitation of our analysis is that we assessed sleep–wake cycles once during the admission. To allow for adjustment to hospital environment, the melatonin profile was collected in the middle of the course of antibiotics. We cannot exclude that the sleep–wake cycle changed during the admission. Indeed, we waited until the middle of the course to give the rhythm time to entrain to the hospital routine. However, these results suggest that circadian rhythm is disrupted in a large proportion of hospitalised patients with CF. Alternately, other factors may lead to increased melatonin levels, as it has several roles including antioxidant properties.
Fig. 3.
Melatonin profiles.
Each plot represents the profile for an individual participant. Purple background (a–g): normal; Yellow Background (h–k): abnormal. For definitions of normal and abnormal, please see methods.
There are several limitations to our study. Whilst participants were randomly allocated to groups, and allocation concealment was maintained with a web-based randomisation schedule provided by the Nottingham Clinical Trials Unit, the two arms of the group were not blinded. There was concern that blinding would negatively impact upon recruitment by doubling the number of interventions administered to each child. We therefore elected to run a non-blinded study. The small number of participants precluded the assessment of potential interactions between circadian rhythm and pharmacokinetics or biomarkers.
Although the literature describing the role of KIM1 is steadily increasing, with multiple studies demonstrating its usefulness as a biomarker of kidney injury in a variety of settings, the clinical implications of a rise in KIM1 for the individual patient is not yet known, and requires future work. The ideal study to address the question of whether morning versus evening administration is safer would demonstrate a reduction in a clinically defined AKI endpoint using the pRIFLE or AKIN criteria. This would require a much larger sample size. We therefore view the study presented herein as providing evidence to support a programme of work towards larger confirmatory studies.
5. Conclusions
This randomised controlled parallel group pharmacokinetic study found no difference in the renal elimination rate of intravenous tobramycin in those administered it in the morning versus the evening. In a pre-specified secondary endpoint biomarker, we found some evidence of reduced toxicity in the morning group, but this result clearly requires confirmation in a future larger study. The mechanism of this diurnal nephrotoxicity independent of tobramycin elimination requires further exploration. Altered circadian rhythm, found by aberrant melatonin levels, was observed in a large proportion of children with CF.
Acknowledgements
We would like to thank all the participants and their families who took part in this research. We thank the cystic fibrosis teams at Nottingham University Hospitals NHS Trust (in particular Mrs. Amanda Ward, and Drs. Bhatt and Bertenshaw), United Lincolnshire Hospitals NHS Trust (Drs. Crawford and Chingale), and University Hospitals Leicester NHS Trust (Dr. Gaillard). We thank the NIHR-CRN (formally MCRN-EAST) for adopting and supporting the study. APP was funded by a NIHR Doctoral Research Fellowship (DRF-2009-02-112). This paper summarises independent research funded by the National Institute for Health Research (NIHR) under its Research for Patient Benefit (RfPB) Programme (Grant Reference Number PB-PG-1207-15025). The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.jcf.2015.07.012.
Appendix A. Supplementary data
Supplementary materials.
References
- 1.Trust U.K.C.F. CF Trust; 2014. UK Cystic Fibrosis Registry. [Google Scholar]
- 2.Bertenshaw C., Watson A.R., Lewis S., Smyth A., Bertenshaw C., Watson A.R. Survey of acute renal failure in patients with cystic fibrosis in the UK. Thorax. 2007;62(6):541–545. doi: 10.1136/thx.2006.067595. [2007 June 1] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smyth A., Lewis S., Bertenshaw C., Choonara I., McGaw J., Watson A. Case–control study of acute renal failure in patients with cystic fibrosis in the UK. Thorax. 2008;63(6):532–535. doi: 10.1136/thx.2007.088757. [2008 June 1] [DOI] [PubMed] [Google Scholar]
- 4.Al-Aloul M., Miller H., Alapati S., Stockton P., L M., Walshaw M. Renal impairment in cystic fibrosis patients due to repeated intravenous aminoglycoside use. Pediatr Pulmonol. 2005;39(1):15–20. doi: 10.1002/ppul.20138. [DOI] [PubMed] [Google Scholar]
- 5.Touw D.J., Vinks A.A., Heijerman H.G., Bakker W. Validation of tobramycin monitoring in adolescent and adult patients with cystic fibrosis. Ther Drug Monit. Feb 1993;15(1):52–59. doi: 10.1097/00007691-199302000-00010. [PubMed PMID: 8451782. Epub 1993/02/01. eng.] [DOI] [PubMed] [Google Scholar]
- 6.Smyth A., Tan K.H.V., Hyman-Taylor P., Mulheran M., Lewis S., Stableforth D. Once versus three-times daily regimens of tobramycin treatment for pulmonary exacerbations of cystic fibrosis—the TOPIC study: a randomised controlled trial. Lancet. 2005;365(9459):573–578. doi: 10.1016/S0140-6736(05)17906-9. [DOI] [PubMed] [Google Scholar]
- 7.Smyth A.R., Campbell E.L. Prescribing practices for intravenous aminoglycosides in UK Cystic Fibrosis clinics: A questionnaire survey. J Cyst Fibros. Jul 2014;13(4):424–427. doi: 10.1016/j.jcf.2013.11.007. [PubMed PMID: ISI:000338814400013. English.] [DOI] [PubMed] [Google Scholar]
- 8.Pariat C., Courtois P., Cambar J., Piriou A., Bouquet S. Circadian variations in the renal toxicity of gentamicin in rats. Toxicol Lett. Feb 1988;40(2):175–182. doi: 10.1016/0378-4274(88)90159-2. [PubMed PMID: ISI:A1988L918900010. English.] [DOI] [PubMed] [Google Scholar]
- 9.Lin L.S., Grenier L., Theriault G., Gourde P., Yoshiyama Y., Bergeron M.G. Nephrotoxicity of low-doses of tobramycin in rats—Effect of the time of administration. Life Sci. 1994;55(3):169–177. doi: 10.1016/0024-3205(94)00877-9. [PubMed PMID: ISI:A1994NQ58000002. English.] [DOI] [PubMed] [Google Scholar]
- 10.Bleyzac N., Allard-Latour B., Laffont A., Mouret J., Jelliffe R., Maire P. Diurnal changes in the pharmacokinetic behavior of amikacin. Ther Drug Monit. Jun 2000;22(3):307–312. doi: 10.1097/00007691-200006000-00012. [PubMed PMID: 10850398. eng.] [DOI] [PubMed] [Google Scholar]
- 11.Prins J.M., Weverling G.J., van Ketel R.J., Speelman P. Circadian variations in serum levels and the renal toxicity of aminoglycosides in patients. Clin Pharmacol Ther. 1997;62(1):106–111. doi: 10.1016/S0009-9236(97)90156-9. [DOI] [PubMed] [Google Scholar]
- 12.Lucht F., Tigaud S., Esposito G., Cougnard J., Fargier M.P., Peyramond D. Chronokinetic study of netilmicin in man. Eur J Clin Pharmacol. 1990;39(2):199–201. doi: 10.1007/BF00280062. [PubMed PMID: ISI:A1990DV45200023. English.] [DOI] [PubMed] [Google Scholar]
- 13.Elting L., Bodey G.P., Rosenbaum B., Fainstein V. Circadian variation in serum amikacin levels. J Clin Pharmacol. Sep 1990;30(9):798–801. doi: 10.1002/j.1552-4604.1990.tb01876.x. [PubMed PMID: ISI:A1990EA46900005. English.] [DOI] [PubMed] [Google Scholar]
- 14.Fauvelle F., Perrin P., Belfayol L., Boukari M., Cherrier P., Bosio A.M. Fever and associated changes in glomerular filtration rate erase anticipated diurnal variations in aminoglycoside pharmacokinetics. Antimicrob Agents Chemother. 1994;38(3):620–623. doi: 10.1128/aac.38.3.620. [1994 March 1] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Touw D.J., Knox A.J., Smyth A. Population pharmacokinetics of tobramycin administered thrice daily and once daily in children and adults with cystic fibrosis. J Cyst Fibros. Sep 2007;6(5):327–333. doi: 10.1016/j.jcf.2006.12.007. [PubMed PMID: 17276147. Epub 2007/02/06. eng.] [DOI] [PubMed] [Google Scholar]
- 16.Klerman E.B., Gershengorn H.B., Duffy J.F., Kronauer R.E. Comparisons of the variability of three markers of the human circadian pacemaker. J Biol Rhythms. Apr 2002;17(2):181–193. doi: 10.1177/074873002129002474. [PubMed PMID: ISI:000174719700009. English.] [DOI] [PubMed] [Google Scholar]
- 17.UK CF Trust Antibiotic Working Group . Cystic Fibrosis Trust; 2009. Antibiotic treatment for cystic fibrosis. [2009. Report No.] [Google Scholar]
- 18.der Heijden KB Van, Smits M.G., Van Someren E.J.W., Gunning W.B. Prediction of melatonin efficacy by pretreatment dim light melatonin onset in children with idiopathic chronic sleep onset insomnia. J Sleep Res. Jun 2005;14(2):187–194. doi: 10.1111/j.1365-2869.2005.00451.x. [PubMed PMID: WOS:000229278600009. English.] [DOI] [PubMed] [Google Scholar]
- 19.Touitou Y., Auzeby A., Camus F., Djeridane Y. Daily profiles of salivary and urinary melatonin and steroids in healthy prepubertal boys. J Pediatr Endocrinol Metab. Nov 2009;22(11):1009–1015. doi: 10.1515/jpem.2009.22.11.1009. [PubMed PMID: WOS:000273080400004. English.] [DOI] [PubMed] [Google Scholar]
- 20.Praninskiene R., Dumalakiene I., Kemezys R., Mauricas M., Jucaite A. Diurnal melatonin patterns in children: ready to apply in clinical practice? Pediatr Neurol. Feb 2012;46(2):70–76. doi: 10.1016/j.pediatrneurol.2011.11.018. [PubMed PMID: WOS:000299863700002. English] [DOI] [PubMed] [Google Scholar]
- 21.Schwartz G.J., Haycock G.B., Edelmann C.M., Jr., Spitzer A. A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics. Aug 1976;58(2):259–263. [PubMed PMID: 951142. Epub 1976/08/01. eng.] [PubMed] [Google Scholar]
- 22.Miller M.R., Hankinson J., Brusasco V., Burgos F., Casaburi R., Coates A. Standardisation of spirometry. Eur Respir J. Aug 2005;26(2):319–338. doi: 10.1183/09031936.05.00034805. [PubMed PMID: ISI:000230874000021. English] [DOI] [PubMed] [Google Scholar]
- 23.R Development Core Team . R Foundation for Statistical Computing; Vienna: 2012. R: A language and environment for statistical computing. 2.15.0 ed. [Google Scholar]
- 24.de Groot R., Smith A.L. Antibiotic pharmacokinetics in cystic-fibrosis—Differences and clinical-significance. Clin Pharmacokinet. Oct 1987;13(4):228–253. doi: 10.2165/00003088-198713040-00002. [PubMed PMID: ISI:A1987K457500002. English] [DOI] [PubMed] [Google Scholar]
- 25.Koopman M.G., Koomen G.C.M., Krediet R.T., Demoor E.A.M., Hoek F.J., Arisz L. Circadian-rhythm of glomerular-filtration rate in normal individuals. Clin Sci. Jul 1989;77(1):105–111. doi: 10.1042/cs0770105. [PubMed PMID: ISI:A1989AB99600017. English] [DOI] [PubMed] [Google Scholar]
- 26.McWilliam S.J., Antoine D.J., Sabbisetti V., Turner M.A., Farragher T., Bonventre J.V. Mechanism-based urinary biomarkers to identify the potential for aminoglycoside-induced nephrotoxicity in premature neonates: a proof-of-concept study. PLoS One. 2012;7(8):e43809. doi: 10.1371/journal.pone.0043809. [PubMed PMID: 22937100. Pubmed Central PMCID: 3427159. Epub 2012/09/01. eng.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McWilliam S.J., Antoine D.J., Smyth R.L., Pirmohamed M. Association of urinary kidney injury molecule-1 with aminoglycoside exposure in children with cystic fibrosis. J Cyst Fibros. 2014;6(13, Supplement 2(0)):S63. [abstract] [Google Scholar]
- 28.Ichimura T., Bonventre J.V., Bailly V., Wei H., Hession C.A., Cate R.L. Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J Biol Chem. 1998;273(7):4135–4142. doi: 10.1074/jbc.273.7.4135. [1998 February 13] [DOI] [PubMed] [Google Scholar]
- 29.Ichimura T., Hung C.C., Yang S.A., Stevens J.L., Bonventre J.V. Kidney injury molecule-1: a tissue and urinary biomarker for nephrotoxicant-induced renal injury. Am J Physiol Renal Physiol. 2004;286(3):F552–F563. doi: 10.1152/ajprenal.00285.2002. [2004 March 1] [DOI] [PubMed] [Google Scholar]
- 30.Vanmassenhove J., Vanholder R., Nagler E., Van Biesen W. Urinary and serum biomarkers for the diagnosis of acute kidney injury: an in-depth review of the literature. Nephrol Dial Transplant. Feb 2013;28(2):254–273. doi: 10.1093/ndt/gfs380. [PubMed PMID: ISI:000316119500007. English] [DOI] [PubMed] [Google Scholar]
- 31.Shao X., Tian L., Xu W., Zhang Z., Wang C., Qi C. Diagnostic value of urinary kidney injury molecule 1 for acute kidney injury: a meta-analysis. PLoS One. 2014;9(1):e84131. doi: 10.1371/journal.pone.0084131. [PubMed PMID: 24404151. Pubmed Central PMCID: 3880280] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary materials.