Abstract
Introduction
Functional MRI (fMRI) can assess language lateralization in brain tumor patients; however, this can be limited if the primary language area—Broca's area (BA)—is affected by the tumor. We hypothesized that the middle frontal gyrus (MFG) can be used as a clinical indicator of hemispheric dominance for language during presurgical workup.
Methods
Fifty-two right-handed subjects with solitary left-hemispheric primary brain tumors were retrospectively studied. Subjects performed a verbal fluency task during fMRI. The MFG was compared to BA for fMRI voxel activation, language laterality index (LI), and the effect of tumor grade on the LI.
Results
Language fMRI (verbal fluency) activated more voxels in MFG than in BA (MFG = 315, BA = 216, p < 0.001). Voxel activations in the left-hemispheric MFG and BA were positively correlated (r = 0.69, p < 0.001). Mean LI in the MFG was comparable to that in BA (MFG = 0.48, BA = 0.39, p = 0.06). LIs in MFG and BA were positively correlated (r = 0.62, p < 0.001). Subjects with high-grade tumors demonstrate lower language lateralization than those with low-grade tumors in both BA and MFG (p = 0.02, p = 0.02, respectively).
Conclusion
MFG is comparable to BA in its ability to indicate hemispheric dominance for language using a measure of verbal fluency and may be an adjunct measure in the clinical determination of language laterality for presurgical planning.
Keywords: Functional imaging, Brain mapping, Language lateralization, Middle frontal gyrus, Hemispheric dominance
Introduction
Blood-oxygen-level-dependent functional MRI (fMRI) is increasingly used to map neuronal activation in the motor and language cortices prior to neurosurgery [1, 2]. For the purpose of language mapping, fMRI serves to both localize and lateralize speech. When the goal is to localize speech, fMRI provides functional language mapping of eloquent cortices that guides intraoperative direct cortical stimulation (DCS). For the purpose of lateralization, the noninvasive fMRI of language areas rivals the invasive WADA test in its ability to assess which hemisphere is dominant for speech [3–6]. However, the presence of abnormal tumor neovasculature and resultant neurovascular uncoupling often leads to a decrease in volume of fMRI activation ipsilateral to the tumor [7]. Because fMRI language lateralization is a volume-dependent ratio comparison of activated voxels between the left and right hemispheres, the muted blood-oxygen-level-dependent (BOLD) response ipsilateral to the tumor can lead to the inaccurate determination of hemispheric dominance, known as “pseudo-reorganization” [7, 8]. As a result, developing alternate strategies for using fMRI data to declare hemispheric dominance for language is essential to the presurgical workup.
One indicator of language laterality is activation in primary language areas like Broca's area (BA) and Wernicke's area, which can be evaluated using a fMRI region of interest (ROI) approach [3, 9, 10]. However, secondary areas (termed only in contrast to putative primary areas) like the supplementary motor area, supramarginal, and angular gyri are well-known participants in the dominant speech network as well. Currently, secondary language areas only factor insomuch as they contribute additively to the measure of hemispheric language dominance. As language mapping using fMRI matures, focus on a network of language areas including both primary and secondary regions is gaining favor [11].
The middle frontal gyrus (MFG), located just superior to BA, is a well-known secondary language area. Previous functional imaging studies have shown that the MFG is involved in expressive language processes including semantics [12], grammar and syntax [13], verbal fluency (e.g., generation of words beginning with a specific letter) [14], and verbal working memory [15]. Verbal working memory and language production are implicitly linked, as verbal working memory is necessary in the maintenance and sequential output of expressive language [16, 17]. The MFG is also widely attributed to a variety of other cognitive functions in part as a result of its broad anatomical span in the frontal lobe [18, 19]. Earlier studies had noted that the MFG consistently activates during fMRI language tasks [6, 20, 21]. We, too, noticed during our clinical practice that the MFG often activates along with or instead of BA. Because of this observation and the fact that working memory is inherent in most typical fMRI speech lateralization protocols, we hypothesized that MFG may be used as an indicator for language hemispheric dominance in brain tumor patients. We further hypothesized that in clinically difficult cases, where a tumor may possibly be causing false-negative activation in BA, the MFG may be used as surrogate to identify language lateralization when using a measure of verbal fluency.
Methods
Subjects
This anonymized retrospective study was reviewed and exempted by the Memorial Sloan-Kettering Cancer Center Institutional Review Board. Fifty-two subjects with solitary left-hemispheric primary neuroepithelial tumors were studied. All subjects were native English speakers, cognitively intact, and determined to be 100 % right handed by the Edinburgh Handedness Inventory [22]. Subjects were randomly selected from a database of patients referred for preoperative fMRI between April 2009 and July 2014 as necessary to meet the inclusion criteria. Subjects were selected before analysis. Subjects had no pre-existing language impairment per chart review. Subjects that were unable to perform fMRI language tasks without difficulty were excluded, as judged by their clinical status and task participation (see “Methods” section: fMRI Activation Task for information on task participation). WHO grade I and II tumors were classified as low grade (n=21), and WHO grade III and IV tumors were classified as high grade (n=31). Twenty-six subjects had tumors involving the frontal lobe. Patient demographics are shown in the Supplementary Table (online).
Data acquisition
Each subject received an fMRI scan as part of the routine presurgical workup. Scanning was performed on 1.5- and 3-T scanners (GE Healthcare, Milwaukee, Wisconsin) using an eight-channel head coil. fMRI data were acquired with a single shot gradient echo echo-planar imaging (EPI) sequence (repetition time/echo time (TR/TE) = 4000/35 ms, field of view (FOV) = 240 × 240 mm, flip angle = 90°, matrix=128×128 voxels, thickness=4.5 mm, no skip, EPI voxel size=1.875×1.875×4.5 mm). Three-dimensional T1-weighted images were acquired with a spoiled gradient-recalled echo sequence (TR/TE = 22/4 ms, FOV = 240 × 240 mm, flip angle=30°, matrix=256×256 voxels, thickness=1.5 mm).
fMRI activation task
Subjects performed verbal fluency (letter) task [23] as part of the presurgical language task panel. During the letter task, subjects generated words that began with the presented letter (example: subject was presented with the letter “A” and may generate words such as “apple,” “apron,” or “ashtray”). A block design was used in accord with previous practices designed to evaluate the language system [24, 25]. There were 90 images in total, consisting of five images during activation (20 s) followed by ten images during rest (40 s) repeated six times (6 min total). Subjects were provided oral instruction before the commencement of the task as a whole and before each activation epoch. Subjects were monitored continuously while performing the task. Subject participation was confirmed using real-time imaging software, which provided real-time acquisition, processing, and display of functional results (Brainwave RT, GE Healthcare, Milwaukee, Wisconsin).
fMRI data analysis
Image processing and analysis were performed using Analysis of Functional Neuroimaging (AFNI, Cox, NIH). Head motion correction was performed using a 3D rigid-body registration. Spatial smoothing (Gaussian filter 4-mm full-width half-maximum) was applied to improve the signal to noise ratio. Additionally, linear trend and high-frequency noise were minimized where necessary. Statistical parametric maps were generated using a cross-correlation analysis, and signal changes over time were correlated with a mathematical model of the hemodynamic response to neural activation [26]. A modeled waveform corresponding to the task performance block was cross-correlated with all pixel time courses on a pixel-by-pixel basis to identify stimulus-locked responses [26]. Functional activation maps were generated at p < 0.001 (uncorrected). To reduce false-positive activity from large venous structures or head motion, voxels in which the standard deviation of the acquired time series exceeded 8 % of the mean signal intensity were set to zero [27].
Using high-resolution anatomical images, ROIs of BA and MFG for each subject were drawn. ROIs were drawn by a trained language fMRI specialist with >15 years of experience (NB). ROIs were then reviewed and approved by a board-certified neuroradiologist with >20 years of experience (AH). The BA ROI included pars triangularis and pars opercularis bounded by the inferior frontal sulcus superiorly. The MFG ROI was bounded by the precentral sulcus posteriorly, the inferior frontal sulcus latero-inferiorly, and the superior frontal sulcus medially and superiorly. The measured ROI data were imported into Matlab (The Mathworks Inc, Natick, Massachusetts) for analysis.
Voxel activation in MFG versus BA
In each subject, activated voxels in the left MFG and BAwere compared. The mean activated voxels in the MFG and BA were calculated, and significance testing was performed using the Wilcoxon signed-rank test. The possible relationship of voxel count between the MFG and the BA was displayed using a scatterplot, and correlation was tested using the Spearman correlation analysis. For all statistical analysis, a significance level of α = 0.05 was used.
Laterality index in MFG versus BA
The language laterality index (LI) was calculated using the standard LI [5, 28, 29] formula: LI=(L−R)/(L+R), where L and R are the numbers of voxels in given ROIs in the left and right hemispheres, respectively. The LI ranged from −1 (complete right dominance) to +1 (complete left dominance). Consistent with prior studies [29–31], we defined right hemisphere language laterality as −1≤LI<−0.2, bilaterality as −0.2≤LI≤0.2, and left hemisphere language laterality as 0.2<LI≤1.
Correlation between LIs in MFG and BA was investigated using the Spearman correlation analysis. A histogram of the LI distribution in MFG and BA was plotted, the mean LIs in the MFG and BA were calculated, and statistical significance was assessed using the signed-rank test.
Effects of tumor grades
Subjects were categorized into those with high-grade or low-grade tumors. The mean LIs in the high- versus low-grade groups were calculated in both ROIs. LI differences between high-grade and low-grade tumors in each ROI were assessed using rank-sum test. LI differences between ROIs in both high-grade and low-grade tumors were assessed using signed-rank test.
Results
Voxel activation
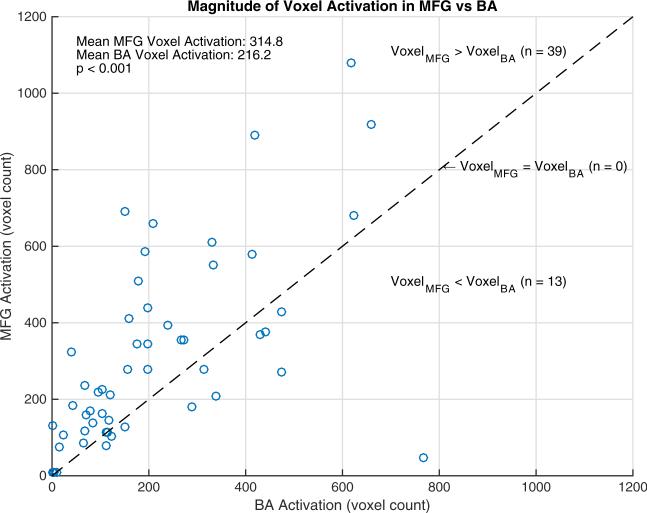
The relationship between activated voxel counts in the MFG and BA is presented in Fig. 1. There was a significant positive correlation between the voxel activations in the left MFG and BA (Spearman r = 0.69, p < 0.001).
Fig. 1.
Scatterplot of voxel activation in left MFG and BA. There is a positive correlation between voxel counts in the MFG and BA (Spearman r = 0.69, p < 0.001). In this cohort, 39 (75 %) had greater voxel counts in the MFG and 13 (25 %) had greater voxel counts in BA. The mean voxel count was 314.8 in MFG and 216.2 in BA (p < 0.001, signed-rank test)
Thirty-nine of the 52 subjects (75 %) had a greater number of voxels activated in the MFG than in BA compared to 13 (25 %) who had a greater number of voxels activated in BA. The mean voxel count was greater in the MFG compared to BA (314.8 vs 216.2, p < 0.001, signed-rank test).
Laterality indices
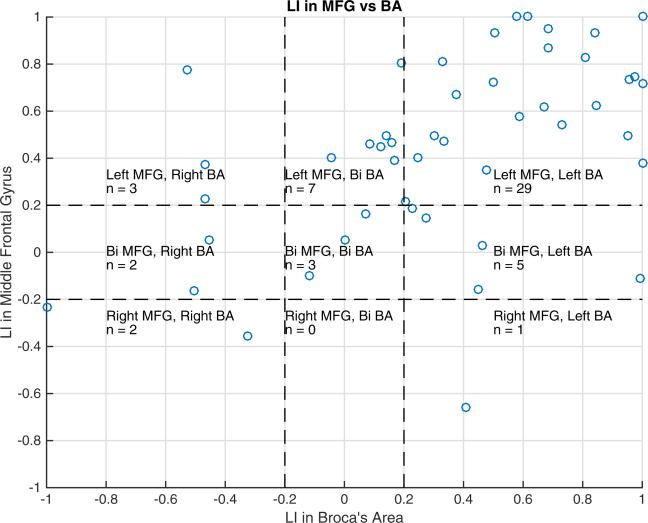
There was a significant positive correlation between the LIs in the MFG and BA (Spearman r = 0.62, p < 0.001). Of the 52 subjects, 29 (56 %) demonstrated left laterality in both MFG and BA, 7 (13 %) had left laterality for MFG and bilaterality for BA, and 5 (10 %) had bilaterality for MFG and left laterality for BA. These findings are summarized in Fig. 2. An example case of left laterality in MFG and bilaterality in BA is shown in Fig. 3.
Fig. 2.
Scatterplot of LIs in MFG versus BA. There is a positive correlation between LIs in MFG and BA (Spearman r = 0.62, p < 0.001). Of the 52 subjects, 29 (56 %) have left laterality in both MFG and BA, 7 (13 %) have left laterality in MFG and bilaterality in BA, while 5 (10 %) have bilaterality in MFG and left laterality in BA. The remainder of the subjects includes three (6 %) with bilaterality in MFG and BA, three (6 %) with left laterality in MFG and right laterality in BA, one (2 %) with right laterality in MFG and left laterality in BA, two (4 %) with bilaterality in MFG and right laterality in BA, and two (4 %) with right laterality in both MFG and BA.
Bi = bilateral
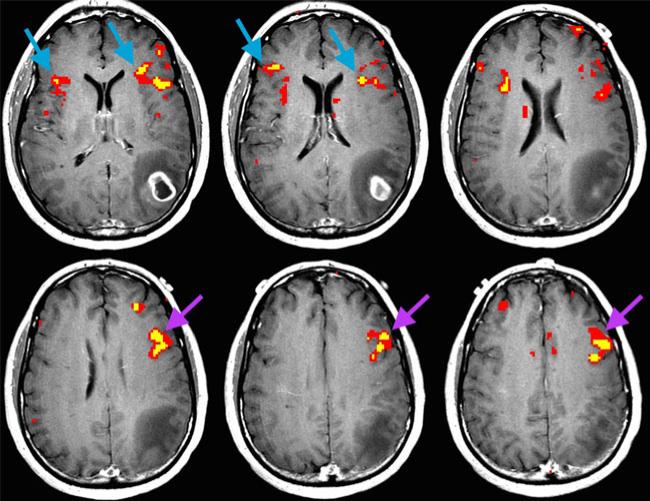
Fig. 3.
Axial fMRI cross section of a 50-year-old man with glioblastoma in the left parietal lobe (subject no. 40). In this case, language fMRI revealed bilaterality in BA (blue arrows) and left laterality in MFG (purple arrows). MFG may be helpful in such a case where BA is equivocal for language hemispheric dominance
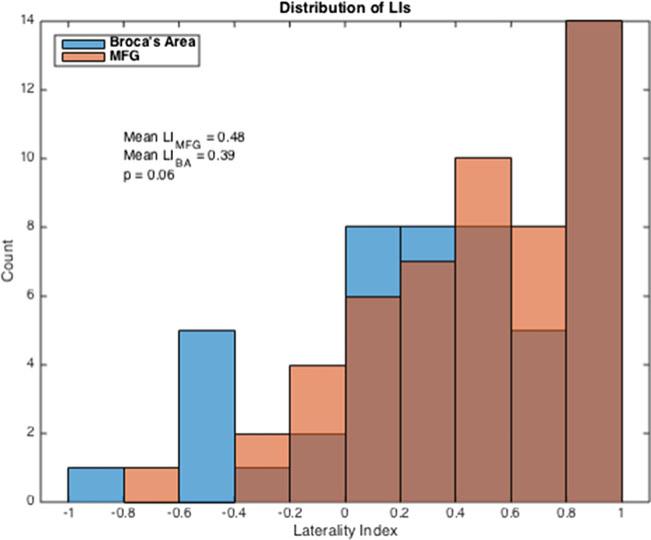
The distributions of LIs in MFG and BA are presented in a histogram in Fig. 4. Of the 52 subjects, 39 (75 %) demonstrated left laterality (LI >0.2) in the MFG, compared to 35 (67 %) in the BA. Ten subjects (19 %) showed bilaterality (−0.2≤LI≤0.2) in MFG, and ten (19 %) showed bilaterality in BA. Three (6 %) showed right laterality (LI <−0.2) in MFG, compared to seven (13 %) that showed right laterality in BA. There was no significant difference between the LIs of the two ROIs: the mean LI was 0.48±0.41 in MFG and 0.39±0.50 in BA (p = 0.06, signed-rank test).
Fig. 4.
Histogram of overall LI distribution in MFG and BA. There are 39/52 cases (75 %) in which MFG demonstrated left laterality and 35/52 (67 %) cases in which BA demonstrated left laterality. The mean LIs are 0.48 in MFG and 0.39 in BA (p = 0.06, signed-rank test)
Effect of tumor grade on LI
The mean LI in MFG was 0.63±0.35 for low-grade tumors and 0.37 ± 0.41 for high-grade tumors (p = 0.02, rank-sum test). The mean LI in BA was 0.58±0.39 for low-grade tumors and 0.26 ± 0.52 for high-grade tumors (p = 0.02, rank-sum test). High-grade tumors were associated with lower language lateralization than were low-grade tumors in both the MFG and BA. LI differences between the two ROIs were insignificant in both low-grade tumors (p = 0.33, signed-rank test) and high-grade tumors (p = 0.16, signed-rank test). Tumor location (frontal versus nonfrontal) did not contribute to differences in LI in the MFG or BA (p = 0.09 in MFG, p = 0.52 in BA, ANCOVA covariating for tumor grade).
Correlation of discrepant cases with intraoperative DCS
In the ten discrepant cases (where the MFG was left lateralized and BA was right or bilateralized), we correlated the fMRI findings with intraoperative DCS. Due to heterogeneity of tumor location, DCS was performed as clinically necessary in three of the ten discrepant cases. Of the three cases that underwent DCS in putative language areas, all of them elicited speech arrest in the left hemisphere. Such cases were subjects 34 (left-lateralized MFG, right-lateralized BA), 43 (left-lateralized MFG, bilateralized BA), and 50 (left-lateralized MFG, bilateralized BA), with anaplastic astrocytoma in the left frontal lobe, glioblastoma in the left temporal lobe, and diffuse astrocytoma in the left frontal lobe, respectively.
Conversely, in the one case of right-lateralized MFG and left-lateralized BA (subject no. 23), DCS did not elicit speech arrest. In the two cases of right-lateralized MFG and BA, DCS was done in one case (subject no. 13), which did not elicit speech arrest. DCS findings were concordant with MFG activation; in patients with right MFG activation, DCSs of the left hemisphere were negative for speech arrest.
Discussion
The purpose of this study was to test the hypothesis that the MFG can be used as an additional indicator in determining language hemispheric dominance in brain tumor patients. We found that voxel activation in the MFG correlated to that in BA and that the MFG had more activated voxels than BA did during language task. Similarly, we found that LIs in MFG correlated to LIs in BA. In other words, the greater the LI was in BA, the greater it was in the MFG. While the MFG had higher average LI than BA did, we found no significant differences in language lateralization between MFG and BA. In most cases, both MFG and BA showed robust left language lateralization as expected.
While the MFG has been shown to be involved in language processing [32–34], research characterizing its exact role is limited. Studies of patients with temporal lobe epilepsy showed increased activation in the superior and MFG [35, 36]. Another study of temporal lobe epilepsy patients found that asymmetric activation in the MFG correlated with WADA determination of language hemispheric dominance [6]. Other studies in brain tumor patients similarly found MFG activation during fMRI language tasks [20, 21]. This study provided a comparative measure of MFG's utility relative to BA in determining language lateralization. As a clinical tool for assessing language hemispheric dominance, BOLD fMRI activation of the MFG has a greater volume compared to that of BA. This confers an advantage to the clinician as the MFG's larger volume facilitates in visual identification of language activation without the need for brain standardization.
We examined the effects of tumor grade on language lateralization. We found that high-grade tumors were associated with lower language lateralization as compared to low-grade tumors. Additionally, we found that high-grade tumors exhibit higher variance in LI distribution. Previous studies have suggested that tumor neovasculature diminished fMRI activation in the tumor hemisphere [37–39], affecting the fMRI determination of true laterality for language. High-grade tumors can induce neurovascular decoupling, altering the hemodynamic relationship between neuronal activity and blood flow [8]. Abnormal blood flow may be the reason for the relatively higher variance in LIs in patients with high-grade tumors; such an uncoupling response may impair the fMRI measurement of true language lateralization in brain tumor patients. This is consistent with previous literature which demonstrated that right-handed patients with neoplasms affecting Broca's and Wernicke's areas in the left hemisphere had lowered LIs compared to normal controls [24].
BA was used as the primary metric, as word generation tasks more reliably activate BA than they activate Wernicke's area [6, 21, 40]. The decision to use one silent word generation language task instead of a panel of tasks was motivated by recent studies suggesting that a single silent word generation task is adequate to activate expressive (e.g., MFG and BA) language areas [41–43]. As this study does not investigate receptive language areas (e.g., Wernicke's area), the use of additional language tasks was felt to be unnecessary as they can introduce potential confounders. Additionally, Pillai et al. showed that receptive language paradigms were less effective than silent word generation task (such as the verbal fluency task) for language lateralization [43]. Therefore, to avoid potential loss of power, a single silent word generation task such as the verbal fluency task served the purpose of this study.
A major limitation of this study is the assumption of left brain dominance for language as ground truth. As previous studies have shown [6, 44], there is no standard criterion for assessing language hemispheric dominance. We selected our study population to be 100 % right-handed patients with one neuroepithelial neoplastic lesion in the left hemisphere because the vast majority (~96 %) of right-handed people are left brain dominant for language [45, 46]. Our selection criteria ensured the best possibility of attaining a population with true left-brain dominance for language. With that said, it is possible that a few subjects in our study were natively bilateral or even right brain dominant for language. We supplemented this limitation by correlating the ten discrepant cases (the cases in which MFG was left lateralized and BAwas right or bilateralized) with intraoperative DCS. Due to heterogeneity of tumor location, DCS was performed in three cases of the ten discrepant cases. Of those three cases that underwent DCS, all of them elicited speech arrest in the left hemisphere. This demonstrated that in the three cases where MFG showed left laterality and BA showed right or bilaterality, DCS confirmed left hemisphere language participation. Although bilaterality cannot be ruled out, this finding demonstrated that the MFG activations in the left hemisphere in these patients were not false positives. Conversely, in the two cases of right-lateralized MFG with confirmed DCS of the left brain, speech arrest was not elicited in either the left-lateralized BA case or the right-lateralized BA case. This demonstrated that nonactivation of the left-hemispheric MFG was not false negative. Taken together, DCS suggests preliminarily that MFG activation on a verbal fluency task shows sensitivity and specificity that are at least on par with BA activation, if not better.
It is important to note that results in analyses such as these vary markedly as a function of choices made in analyses. For instance, results can vary with the choice of threshold for laterality indices (as elsewhere, we elected to set values within 0.2 of 0 to represent bilateral) or with the thresholds used for excluding activation (we selected a cut value of 8 %, as arbitrarily used elsewhere) [27].
In assessing the anatomical relationship of an eloquent area adjacent to a brain tumor, intraoperative DCS remains the gold standard. However, DCS is imperfect. DCS assessment is limited to the cortical surface and can miss eloquent areas deep to the surface. Additionally, DCS of one hemisphere cannot rule out language participation in the contralateral hemisphere. As there is no perfect modality, the clinician should assess language hemispheric dominance in brain tumor patients individually, using multiple diagnostic indicators. For example, recent literature proposed that crossed cerebrocerebellar lateralization can be used as an additional diagnostic feature to determine language dominance in brain tumor patients [47]. When language lateralization based on BA is equivocal, additional indicators can be helpful. We propose that BOLD fMRI activation in MFG is one additional indicator to add to the growing clinical toolbox for noninvasive presurgical assessment of language hemispheric dominance in brain tumor patients.
Conclusion
MFG is comparable to BA in its ability to determine hemispheric dominance for language when using a verbal fluency task. High-grade tumors are associated with lower language lateralization indices than low-grade tumors in both MFG and BA. In cases where the results based on BA are equivocal, analysis of the MFG may provide an additional assessment of language lateralization. MFG may be an adjunct measure in the clinical determination of language laterality using fMRI in brain tumor patients.
Supplementary Material
Abbreviations
- BA
Broca's area
- LI
Laterality index
- MFG
Middle frontal gyrus
- ROI
Region of interest
- DCS
Direct cortical stimulation
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00234-016-1655-4) contains supplementary material, which is available to authorized users.
Compliance with ethical standards We declare that all human and animal studies have been approved by the Institutional Review Board and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. We declare that all patients gave informed consent prior to inclusion in this study.
Conflict of interest We declare that we have no conflict of interest.
References
- 1.Sunaert S. Presurgical planning for tumor resectioning. J Magn Reson Imaging. 2006;23:887–905. doi: 10.1002/jmri.20582. doi:10.1002/jmri.20582. [DOI] [PubMed] [Google Scholar]
- 2.Vlieger E-J, Majoie CB, Leenstra S, den Heeten GJ. Functional magnetic resonance imaging for neurosurgical planning in neurooncology. Eur Radiol. 2004;14:1143–1153. doi: 10.1007/s00330-004-2328-y. doi:10.1007/s00330-004-2328-y. [DOI] [PubMed] [Google Scholar]
- 3.Janecek JK, Swanson SJ, Sabsevitz DS, et al. Language lateralization by fMRI and Wada testing in 229 patients with epilepsy: rates and predictors of discordance. Epilepsia. 2013;54:314–322. doi: 10.1111/epi.12068. doi:10. 1111/epi.12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dym RJ, Burns J, Freeman K, Lipton ML. Is functional MR imaging assessment of hemispheric language dominance as good as the Wada test?: a meta-analysis. Radiology. 2011;261:446–455. doi: 10.1148/radiol.11101344. doi:10.1148/radiol.11101344. [DOI] [PubMed] [Google Scholar]
- 5.Desmond JE, Sum JM, Wagner AD, et al. Functional MRI measurement of language lateralization in Wada-tested patients. Brain. 1995;118:1411–1419. doi: 10.1093/brain/118.6.1411. doi:10.1093/brain/118.6.1411. [DOI] [PubMed] [Google Scholar]
- 6.Lehéricy S, Cohen L, Bazin B, et al. Functional MR evaluation of temporal and frontal language dominance compared with the Wada test. Neurology. 2000;54:1625–1633. doi: 10.1212/wnl.54.8.1625. doi:10.1212/WNL.54.8.1625. [DOI] [PubMed] [Google Scholar]
- 7.Ulmer JL, Hacein-Bey L, Mathews VP, et al. Lesion-induced pseudo-dominance at functional magnetic resonance imaging: implications for preoperative assessments. Neurosurgery. 2004;55:569–579. doi: 10.1227/01.neu.0000134384.94749.b2. discussion 580–581. [DOI] [PubMed] [Google Scholar]
- 8.Hou BL, Bradbury M, Peck KK, et al. Effect of brain tumor neovasculature defined by rCBVon BOLD fMRI activation volume in the primary motor cortex. Neuroimage. 2006;32:489–497. doi: 10.1016/j.neuroimage.2006.04.188. doi:10.1016/j.neuroimage.2006.04.188. [DOI] [PubMed] [Google Scholar]
- 9.Szaflarski JP, Binder JR, Possing ET, et al. Language lateralization in left-handed and ambidextrous people fMRI data. Neurology. 2002;59:238–244. doi: 10.1212/wnl.59.2.238. doi:10.1212/WNL.59.2.238. [DOI] [PubMed] [Google Scholar]
- 10.Frost JA, Binder JR, Springer JA, et al. Language processing is strongly left lateralized in both sexes. Brain. 1999;122:199–208. doi: 10.1093/brain/122.2.199. doi:10.1093/brain/122.2.199. [DOI] [PubMed] [Google Scholar]
- 11.Sanai N, Mirzadeh Z, Berger MS. Functional outcome after language mapping for glioma resection. N Engl J Med. 2008;358:18–27. doi: 10.1056/NEJMoa067819. [DOI] [PubMed] [Google Scholar]
- 12.Brown S, Martinez MJ, Parsons LM. Music and language side by side in the brain: a PET study of the generation of melodies and sentences. Eur J Neurosci. 2006;23:2791–2803. doi: 10.1111/j.1460-9568.2006.04785.x. doi:10.1111/j.1460-9568.2006.04785.x. [DOI] [PubMed] [Google Scholar]
- 13.Wang S, Zhu Z, Zhang JX, et al. Broca's area plays a role in syntactic processing during Chinese reading comprehension. Neuropsychologia. 2008;46:1371–1378. doi: 10.1016/j.neuropsychologia.2007.12.020. doi:10.1016/j.neuropsychologia.2007.12.020. [DOI] [PubMed] [Google Scholar]
- 14.Abrahams S, Goldstein LH, Simmons A, et al. Functional magnetic resonance imaging of verbal fluency and confrontation naming using compressed image acquisition to permit overt responses. Hum Brain Mapp. 2003;20:29–40. doi: 10.1002/hbm.10126. doi:10.1002/hbm.10126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leung H-C, Gore JC, Goldman-Rakic PS. Sustained mnemonic response in the human middle frontal gyrus during on-line storage of spatial memoranda. J Cogn Neurosci. 2002;14:659–671. doi: 10.1162/08989290260045882. doi: 10.1162/08989290260045882. [DOI] [PubMed] [Google Scholar]
- 16.Acheson DJ, MacDonald MC. Verbal working memory and language production: common approaches to the serial ordering of verbal information. Psychol Bull. 2009;135:50–68. doi: 10.1037/a0014411. doi:10.1037/a0014411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baddeley A. Working memory and language: an overview. J Commun Disord. 2003;36:189–208. doi: 10.1016/s0021-9924(03)00019-4. doi:10.1016/S0021-9924(03)00019-4. [DOI] [PubMed] [Google Scholar]
- 18.Smolker HR, Depue BE, Reineberg AE, et al. Individual differences in regional prefrontal gray matter morphometry and fractional anisotropy are associated with different constructs of executive function. Brain Struct Funct. 2015;220:1291–1306. doi: 10.1007/s00429-014-0723-y. doi:10.1007/s00429-014-0723-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steinmann E, Schmalor A, Prehn-Kristensen A, et al. Developmental changes of neuronal networks associated with strategic social decision-making. Neuropsychologia. 2014;56:37–46. doi: 10.1016/j.neuropsychologia.2013.12.025. doi:10.1016/j.neuropsychologia.2013.12.025. [DOI] [PubMed] [Google Scholar]
- 20.Roux F-E, Boulanouar K, Lotterie J-A, et al. Language functional magnetic resonance imaging in preoperative assessment of language areas: correlation with direct cortical stimulation. Neurosurgery. 2003;52:1335–1347. doi: 10.1227/01.neu.0000064803.05077.40. doi:10.1227/01.NEU.0000064803.05077.40. [DOI] [PubMed] [Google Scholar]
- 21.Kamada K, Sawamura Y, Takeuchi F, et al. Expressive and receptive language areas determined by a non-invasive reliable method using functional magnetic resonance imaging and magnetoencephalography. Neurosurgery. 2007;60:296–305. doi: 10.1227/01.NEU.0000249262.03451.0E. doi:10.1227/01.NEU.0000249262.03451.0E. [DOI] [PubMed] [Google Scholar]
- 22.Veale JF. Edinburgh handedness inventory—short form: a revised version based on confirmatory factor analysis. Laterality Asymmetr Body Brain Cogn. 2014;19:164–177. doi: 10.1080/1357650X.2013.783045. doi:10.1080/1357650X.2013.783045. [DOI] [PubMed] [Google Scholar]
- 23.Benton AL, Hamsher KD, Sivan AB. Multilingual aphasia examination: manual of instructions. AJA Assoc; Iowa City: 1994. [Google Scholar]
- 24.Partovi S, Jacobi B, Rapps N, et al. Clinical standardized fMRI reveals altered language lateralization in patients with brain tumor. Am J Neuroradiol. 2012;33:2151–2157. doi: 10.3174/ajnr.A3137. doi:10.3174/ajnr.A3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stippich C, Rapps N, Dreyhaupt J, et al. Localizing and lateralizing language in patients with brain tumors: feasibility of routine preoperative functional MR imaging in 81 consecutive patients. Radiology. 2007;243:828–836. doi: 10.1148/radiol.2433060068. doi:10.1148/radiol.2433060068. [DOI] [PubMed] [Google Scholar]
- 26.Bandettini PA, Jesmanowicz A, Wong EC, Hyde JS. Processing strategies for time-course data sets in functional MRI of the human brain. Magn Reson Med. 1993;30:161–173. doi: 10.1002/mrm.1910300204. [DOI] [PubMed] [Google Scholar]
- 27.Haupage S, Peck KK, Branski RC, et al. Functional MRI of tongue motor tasks in patients with tongue cancer: observations before and after partial glossectomy. Neuroradiology. 2010;52:1185–1191. doi: 10.1007/s00234-010-0748-8. doi:10.1007/s00234-010-0748-8. [DOI] [PubMed] [Google Scholar]
- 28.Binder JR, Swanson SJ, Hammeke TA, et al. Determination of language dominance using functional MRI a comparison with the Wada test. Neurology. 1996;46:978–984. doi: 10.1212/wnl.46.4.978. doi:10.1212/WNL.46.4.978. [DOI] [PubMed] [Google Scholar]
- 29.Seghier ML. Laterality index in functional MRI: methodological issues. Magn Reson Imaging. 2008;26:594–601. doi: 10.1016/j.mri.2007.10.010. doi:10.1016/j.mri.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deblaere K, Boon PA, Vandemaele P, et al. MRI language dominance assessment in epilepsy patients at 1.0 T: region of interest analysis and comparison with intracarotid amytal testing. Neuroradiology. 2004;46:413–420. doi: 10.1007/s00234-004-1196-0. doi:10.1007/s00234-004-1196-0. [DOI] [PubMed] [Google Scholar]
- 31.Springer JA, Binder JR, Hammeke TA, et al. Language dominance in neurologically normal and epilepsy subjects a functional MRI study. Brain. 1999;122:2033–2046. doi: 10.1093/brain/122.11.2033. doi:10.1093/brain/122.11.2033. [DOI] [PubMed] [Google Scholar]
- 32.Rogalski E, Cobia D, Harrison TM, et al. Anatomy of language impairments in primary progressive aphasia. J Neurosci Off J Soc Neurosci. 2011;31:3344–3350. doi: 10.1523/JNEUROSCI.5544-10.2011. doi:10.1523/JNEUROSCI.5544-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fertonani A, Rosini S, Cotelli M, et al. Naming facilitation induced by transcranial direct current stimulation. Behav Brain Res. 2010;208:311–318. doi: 10.1016/j.bbr.2009.10.030. doi:10.1016/j.bbr.2009.10.030. [DOI] [PubMed] [Google Scholar]
- 34.Grossman M, Cooke A, DeVita C, et al. Age-related changes in working memory during sentence comprehension: an fMRI study. Neuroimage. 2002;15:302–317. doi: 10.1006/nimg.2001.0971. doi:10.1006/nimg.2001.0971. [DOI] [PubMed] [Google Scholar]
- 35.Adcock J, Wise R, Oxbury J, et al. Quantitative fMRI assessment of the differences in lateralization of language-related brain activation in patients with temporal lobe epilepsy. Neuroimage. 2003;18:423–438. doi: 10.1016/s1053-8119(02)00013-7. doi:10.1016/S1053-8119(02)00013-7. [DOI] [PubMed] [Google Scholar]
- 36.Wong SWH, Jong L, Bandur D, et al. Cortical reorganization following anterior temporal lobectomy in patients with temporal lobe epilepsy. Neurology. 2009;73:518–525. doi: 10.1212/WNL.0b013e3181b2a48e. doi:10.1212/WNL.0b013e3181b2a48e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holodny AI, Schulder M, Liu W-C, et al. The effect of brain tumors on BOLD functional MR imaging activation in the adjacent motor cortex: implications for image-guided neurosurgery. Am J Neuroradiol. 2000;21:1415–1422. [PMC free article] [PubMed] [Google Scholar]
- 38.Schreiber A, Hubbe U, Ziyeh S, Hennig J. The influence of gliomas and nonglial space-occupying lesions on blood-oxygen-level–dependent contrast enhancement. Am J Neuroradiol. 2000;21:1055–1063. [PMC free article] [PubMed] [Google Scholar]
- 39.Ulmer JL, Krouwer HG, Mueller WM, et al. Pseudo-reorganization of language cortical function at fMR imaging: a consequence of tumor-induced neurovascular uncoupling. Am J Neuroradiol. 2003;24:213–217. [PMC free article] [PubMed] [Google Scholar]
- 40.Brannen JH, Badie B, Moritz CH, et al. Reliability of functional MR imaging with word-generation tasks for mapping Broca's area. Am J Neuroradiol. 2001;22:1711–1718. [PMC free article] [PubMed] [Google Scholar]
- 41.Zacà D, Jarso S, Pillai JJ. Role of semantic paradigms for optimization of language mapping in clinical fMRI studies. Am J Neuroradiol. 2013;34:1966–1971. doi: 10.3174/ajnr.A3628. doi:10.3174/ajnr.A3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zacà D, Nickerson JP, Deib G, Pillai JJ. Effectiveness of four different clinical fMRI paradigms for preoperative regional determination of language lateralization in patients with brain tumors. Neuroradiology. 2012;54:1015–1025. doi: 10.1007/s00234-012-1056-2. doi:10.1007/s00234-012-1056-2. [DOI] [PubMed] [Google Scholar]
- 43.Pillai JJ, Zaca D. Relative utility for hemispheric lateralization of different clinical fMRI activation tasks within a comprehensive language paradigm battery in brain tumor patients as assessed by both threshold-dependent and threshold-independent analysis methods. Neuroimage. 2011;54(Suppl 1):S136–145. doi: 10.1016/j.neuroimage.2010.03.082. doi:10.1016/j.neuroimage.2010.03.082. [DOI] [PubMed] [Google Scholar]
- 44.FitzGerald DB, Cosgrove GR, Ronner S, et al. Location of language in the cortex: a comparison between functional MR imaging and electrocortical stimulation. Am J Neuroradiol. 1997;18:1529–1539. [PMC free article] [PubMed] [Google Scholar]
- 45.Knecht S, Drager B, Bobe L, et al. Handedness and hemispheric language dominance in healthy humans. Brain. 2000;123:2512–2518. doi: 10.1093/brain/123.12.2512. [DOI] [PubMed] [Google Scholar]
- 46.Vikingstad EM, George KP, Johnson AF, Cao Y. Cortical language lateralization in right handed normal subjects using functional magnetic resonance imaging. J Neurol Sci. 2000;175:17–27. doi: 10.1016/s0022-510x(00)00269-0. doi: 10.1016/S0022-510X(00)00269-0. [DOI] [PubMed] [Google Scholar]
- 47.Orellana CM, Visch-Brink E, Vernooij M, et al. Crossed cerebrocerebellar language lateralization: an additional diagnostic feature for assessing atypical language representation in presurgical functional MR imaging. Am J Neuroradiol. 2015;36:518–524. doi: 10.3174/ajnr.A4147. doi:10.3174/ajnr.A4147. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.