Abstract
Aims
Smoking prevalence is higher among persons enrolled in addiction treatment as compared to the general population, and very high rates of smoking are associated with opiate drug use and receipt of opiate replacement therapy (ORT). We assessed whether these findings are observed internationally.
Methods
PubMed, PsycINFO and the Alcohol and Alcohol Problems Science Database were searched for papers reporting smoking prevalence among addiction treatment samples, published in English, from 1987 to 2013. Search terms included tobacco use, cessation, and substance use disorders using AND/OR Boolean connectors. For 4,549 papers identified, abstracts were reviewed by multiple raters. 239 abstracts met inclusion criteria and these full papers were reviewed for exclusion. 54 studies, collectively including 37,364 participants, were included. For each paper we extracted country, author, year, sample size and gender, treatment modality, primary drug treated, and smoking prevalence.
Results
The random-effect pooled estimate of smoking across persons in addiction treatment was 84% (CI 79%, 88%), while the pooled estimate of smoking prevalence across matched population samples was 31% (CI 29%, 33%). The difference in the pooled estimates was 52% (CI 48%, 57%, p < .0001). Smoking rates were higher in programs treating opiate use as compared to alcohol use (OR = 2.52, CI 2.00, 3.17), and higher in ORT compared to outpatient programs (OR = 1.42, CI 1.19, 1.68).
Conclusions
Smoking rates among people in addiction treatment are more than double those of people with similar demographic characteristics. Smoking rates are also higher in people being treated for opiate dependence compared with people being treated for alcohol use disorder.
Keywords: Addiction, Co-substance use, Global health, Surveillance and monitoring, Priority/special populations
INTRODUCTION
Each year, nearly 6 million people worldwide die from tobacco-related causes. Tobacco use accounts for about 18%, 11%, and 4% of deaths in high-, middle-, and low-income countries, respectively (1). Economic damages from global tobacco use are estimated at over one half trillion dollars per year (2). To address the global health and economic costs of tobacco the World Health Organization (WHO) approved the Framework Convention on Tobacco Control (FCTC), an international treaty that monitors global tobacco consumption and tobacco control policies and crafts measures to reduce tobacco supply and demand. The FCTC's six principal strategies, called “MPOWER,” include smoke-free environments, cessation programs, warning labels, mass anti-tobacco media, tobacco advertising bans, and taxation (2).
In concert with the FCTC, the Global Adult Tobacco Survey (GATS) has become an important tool for cross-national studies of smoking prevalence and tobacco policies. A recent analysis of GATS data demonstrated high variability in smoking rates across 14 low- and middle-income countries, with smoking prevalence ranging from 21.6% to 60.2% among men and from <1% to 24.4% among women (3). Similar to the GATS, European researchers developed the Tobacco Control Scale (TCS) to evaluate smoking prevalence across European Union nations (4-7). While both the GATS and TCS evaluate smoking prevalence cross-nationally, there are no systematic approaches to compare international smoking rates in subgroups where smoking is most prevalent. These subgroups include, but are not limited to, persons with mental health and substance abuse disorders (8, 9).
Concerning substance abuse specifically, studies in the United States (U.S.) indicate that smoking rates are 2 to 4 times higher in persons with substance use disorders than in the general public (9, 10). Smoking rates are highest, however, among those with substance use disorders who also enter addiction treatment, with smoking prevalence in this subgroup estimated at around 67% (11). Smokers with comorbid substance abuse are more likely to die from tobacco-related causes than from other substance-related causes (12, 13), and quitting smoking is associated with longer-term maintenance of recovery from other addictions (14).
We previously conducted a systematic review of smoking in addiction treatment in the U.S. from 1987 through 2009 (15). Focusing on 42 papers, and aggregating samples by year, we found annual smoking rates ranging from 65 to 87.2% with a median of 76.3%. This was consistent with National Survey on Drug Use and Health (NSDUH) data, where smoking prevalence among persons who received recent addiction treatment ranged from 68.9% in 2000 to 74.2% in 2011 (15, 16). The current study estimates smoking prevalence for persons entering addiction treatment internationally, using studies published between 1987 and 2013, and compares prevalence reported in treatment samples to national epidemiologic estimates. Such a review may be useful for directing tobacco control resources and policies to concentrations of smokers who seek treatment for other addictions.
METHODS
Article Identification and Selection
Search Procedures
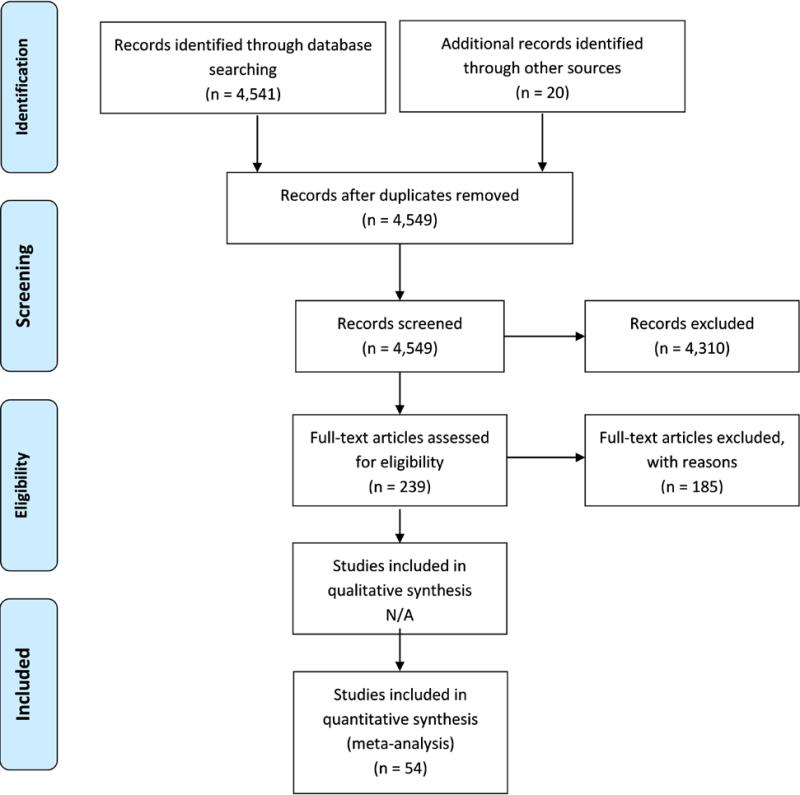
PubMed, PsycINFO and the ETOH Alcohol and Alcohol Problems Science Database (an archived database of alcohol-related research) were searched for published papers reporting smoking prevalence for addiction treatment samples. These sources contain the title and abstract for each paper in the database, so the electronic search was limited to titles and abstracts in each database. Specific search terms were used for each database, reflecting their respective search term mapping, and we identified the broadest search terms relevant to our goals. PubMed MeSH terms and Boolean connectors included “smoking OR tobacco use cessation OR tobacco use disorder OR tobacco OR nicotine” AND “substance-related disorders OR substance abuse treatment centers” AND “patients.” PsycINFO Thesaurus descriptors used included: “addiction OR drug usage” AND “client attitudes OR clients OR patients” AND “nicotine OR tobacco smoking OR smoking cessation.” The Alcohol and Other Drug Thesaurus (AOD) descriptors were used for the ETOH search and consisted of: “(tobacco in any form or smoking or nicotine)” AND “(survey or questionnaire or interview or self-report).” Search results were limited to articles published in English. Papers from all countries were included in the screening process. A total of 4,541 papers were identified electronically, and 20 additional papers were identified through bibliographic review of the final selected papers. After removing duplicates, abstracts for 4,549 articles were screened for inclusion. Systematic review procedures were conducted in accordance with PRISMA guidelines, and Figure 1 shows the PRISMA flow diagram (17).
Figure 1.
PRISMA Flow Diagram Summarizing Article Identification and Selection
Rating Abstracts for Inclusion
Abstracts were reviewed using three inclusion criteria: (a) the paper reported data gathered in an addiction treatment setting, (b) patient-level data were reported, and (c) tobacco use was mentioned in the abstract. Only abstracts meeting all three criteria were reviewed further. Abstract review procedures were conducted for two time periods, first for the years 1987-2009, and again for the period 2009 through June of 2013.
To assess inter-rater reliability for the first period (1987-2009), 6 raters were trained on inclusion criteria and then rated the same set of 30 abstracts (ICC = 0.83, p < .001). Thereafter, each rater rated a unique set of 300 abstracts, with a final test of inter-rater reliability to assess potential rater drift during the rating process (ICC = 0.79, p< .001) (15). To assess inter-rater reliability for the second period (2009 - 2013), 4 raters (3 were the same and 1 different) were trained on inclusion criteria and then rated the same set of 20 abstracts (ICC = 1.0). Thereafter, each rater rated unique sets of abstracts. Last, inter-rater reliability was assessed using a single set of 20 abstracts, to assess potential rater drift (ICC = 1.0).
Reviewing Papers for Exclusion
A total of 239 met abstract inclusion criteria. Each of these papers was read by one of two reviewers, with attention to four exclusion criteria: (a) a review paper (not primary data); (b) smoking prevalence not reported for addiction treatment sample; (c) participant selection was based on smoking status; (d) participants were adolescents. Of the 61 papers remaining eligible, one was excluded due to a small sample size (defined for this review as less than 25 participants) (18), and one multinational paper was excluded because it did not report smoking prevalence of the sample by country where data were collected (19). Five papers were excluded due to overlapping sample with another paper included in the review (20-24). A total of 54 papers from 20 countries are included in the review. Search terms and the checklists used for rating abstracts and reviewing papers are available from the first author.
National Smoking Prevalence Data
Each study in the review provides smoking prevalence for a sample of persons enrolled in addiction treatment, in one country and in one year. We compared smoking prevalence in each study sample to national smoking prevalence for the same country and in the same year. Smoking prevalence differs widely by gender in many countries. When the addiction treatment sample included ≥ 70% women we used national prevalence estimates for women (5 studies), and when the sample included ≥ 70% men we used the national prevalence estimate for men (35 studies). When the sample included 31-69% of either women or men (10 studies) (25-34), or when the gender breakdown was unknown (4 studies) (35-38), we used a national prevalence estimate for men and women combined.
To obtain national smoking prevalence rates we first consulted the World Health Organization (WHO) Global Health Observatory Data Repository (http://apps.who.int/gho/data/view.main). Within the Repository we referred to the Tobacco Control section, and to the Prevalence – adult age-standardized by country dataset which reports current smoking for male, female and both sexes. However, these data refer only to 2006, 2009 and 2011. For national smoking prevalence from additional years we consulted the 2011 Country Profiles from the WHO Report on Global Tobacco Epidemic (http://www.who.int/tobacco/global_report/2011/en/). Where national prevalence rates were not available through these WHO sources, we consulted the web edition of the International Smoking Statistics (ISS) (http://www.pnlee.co.uk/ISS.htm). If rates were not available the WHO or ISS sources, we consulted official country websites. Finally, rates reported in published articles were used if rates could not be found through other sources. Smoking prevalence estimates were not available for all countries in all years. For 30 papers we found national smoking prevalence in the year the study was published. For 21 papers we found prevalence estimates within one year, and for 3 papers we found prevalence estimates within two years of the publication year.
To calculate Confidence Intervals (CIs) for national smoking prevalence, we found the report where each estimate originated. We used either the total N from the original survey, or the N for men or women, according to the gender matched prevalence for each study. For 13 papers where the original source was not found, was found behind a paywall, or was found in a language we could not translate using Google Translate, we used N = 4000 to calculate CIs. This is the lower bound of the Ns that were found and so represents a conservative approach to estimating the population Cis. When the sample size for the national survey was not reported by gender, we estimated the N for each gender by halving the total.
Data Analysis
Country, year of publication, author, sample size, proportion of women, and smoking prevalence were extracted for each paper. For papers reporting intervention studies, or for papers reporting repeated measures over time, we used prevalence from the baseline data. Treatment modality (inpatient, outpatient, opiate replacement therapy), and primary drug treated (alcohol, alcohol and other drugs, opiates) were also extracted. Opiate replacement therapy (ORT) is an outpatient modality distinguished by its focus on replacing illicit opiates with either methadone or buprenorphine. Where papers drew clients from two modalities, they were coded to whichever category represented more than half of the patients involved in the article (39, 40). Two papers reporting cocaine as the primary drug treated were grouped in the “alcohol and other drug” category (41, 42).
We extracted the smoking prevalence and calculated the 95% CI for each estimate. Using a Forest plot, we plotted the smoking prevalence and CI for each paper alongside the year and gender-matched national smoking prevalence for the country where the paper was reported. Using these estimates and CIs, we calculated the random-effect pooled estimates for smoking across addiction treatment samples, across national prevalence estimates, and the difference between the two (study – national).
Research in the U.S. has shown that opiate use as compared to alcohol use, and enrollment in opiate replacement therapy (ORT) as compared to non-opiate outpatient programs, were associated with higher smoking prevalence (16, 43). To assess whether these associations may also be observed internationally, we calculated smoking rates by treatment modality and primary drug treated combined across all papers. Treatment modality and primary drug treated were intercorrelated (r = .55, p < .0001), so each variable was evaluated in a separate model before both were included in the same model. Random-effect logistic regression models were used to assess univariate relationships between each predictor (treatment modality, primary drug, year of study) and smoking prevalence, and then to assess multivariate relationships of treatment modality and primary drug with smoking prevalence, controlling for year. In these analyses a random intercept model was used, with country as a random factor. Two papers were removed from analyses because they reported on patient samples that were aggregated over 20 years, confounding any relationship between time in those samples (39, 44). One paper from 1989 was removed (45) so that the analysis period would be 20 years in length (1994 – 2013) and most years would be represented by at least one study. We conducted these analyses with nesting by country.
RESULTS
Prevalence of Smoking in Addiction Treatment
Papers in the review are summarized in Table 1, listed alphabetically by country and, within country, by year published. Among the studies were 6 from Germany, 5 each from Australia and Italy, and 4 each from Brazil, France, Switzerland and the UK. The remaining 22 papers were from 13 different countries. Programs were identified in the reports as inpatient (41%), methadone or other opiate treatment (30%) or outpatient programs (26%). Type of program could not be determined for 2 studies (46, 47). Programs identified the primary drug treated as alcohol (48%), heroin or other opiates (35%), or alcohol and other (non-specified) drugs (17%). The final column shows the year and age matched national smoking prevalence for the country in which each study was reported. Smoking prevalence across all studies ranged from 41.1% (39) to 100% (46, 48).
Table 1.
International Addiction Treatment Studies by Country, Comparing Sample Smoking Prevalence to National Rates. (N=54)
| Country | Source (Year) | Source (Author) | N | % Female | Modality | Primary Drug treated | Smoking Prevalence (95%CI) | National Smoking Prevalencea |
|---|---|---|---|---|---|---|---|---|
| Australia | 2007 | Burns et al. (66) | 1519 | 100% | ORTb | opiate | 70.8% (0.685, 0.731) | 18% |
| Australia | 2004 | Teichtahl et al. (32) | 50 | 50% | ORT | opiate | 92% (0.808, 0.978) | 23% |
| Australia | 2002 | Shakeshaft et al. (67), c | 1212 | 29.6% | outpatient | AODd | 74.1% (0.715, 0.765) | 27% |
| Australia | 1996 | Zador et al. (68) | 86 | 100% | ORT | opiate | 95% (0.885, 0.987) | 20.3% |
| Australia | 1994 | Darke et al. (25) | 222 | 40.1% | ORT | opiate | 93.7% (0.897, 0.965) | 24% |
| Austria | 2012 | Hoflich et al. (69), e | 37 | 100% | ORT | opiate | 97%† (0.858, 0.999) | 34.7% |
| Austria | 2009 | Malik et al. (28) | 57 | 35.1% | inpatient | alcohol | 88% (0.763, 0.949) | 47% |
| Austria | 2009 | Winklbaur et al. (70) | 139 | 100% | ORT | opiate | 95.7% (0.908, 0.984) | 45% |
| Brazil | 2013 | Diehl et al. (41), f | 105 | 100% | inpatient | AOD | 92.4%† (0.855, 0.967) | 13% |
| Brazil | 2009 | Baltieri et al. (71) | 155 | 0% | outpatient | alcohol | 66.5% (0.584, 0.738) | 22% |
| Brazil | 2009 | de Meneses-Gaya et al. (72), g | 40 | 10% | outpatient | AOD | 75% (0.588, 0.873) | 22% |
| Brazil | 1999 | Dunn & Laranjeira (40), h | 294 | 10% | outpatient | AOD | 81%† (0.756, 0.85) | 35.4% |
| Canada | 1999 | Ellingstad et al. (35) | 185 | unknown | outpatient | alcohol | 54.1%† (0.466, 0.614) | 25.2% |
| Canada | 1995 | Toneatto et al. (38) | 155 | unknown | outpatient | alcohol | 58% (0.499, 0.659) | 31% |
| Canada | 1989 | Kozlowski et al. (45) | 289 | 27% | outpatient | AOD | 86% (0.816, 0.899) | 33% |
| China | 2011 | Liao et al. (27) | 139 | 32.4% | ORT | opiate | 80.6% (0.73, 0.868) | 28.1% |
| Croatia | 2011 | Nenadic-Sviglin et al. (73) | 505 | 21.4% | inpatient | alcohol | 59% (0.55, 0.637) | 36% |
| France | 2009 | Lahmek et al. (26) | 414 | 47.1% | inpatient | alcohol | 82% (0.776, 0.853) | 31% |
| France | 1999 | Aubin et al. (74), i | 222 | 26% | inpatient | alcohol | 79% (0.729, 0.84) | 31% |
| France | 1995 | Batel et al. (75) | 325 | 25.2% | outpatient | alcohol | 88% (0.84, 0.913) | 40% |
| France | 1995 | Levy et al. (76) | 50 | 0% | inpatient | alcohol | 92% (0.808, 0.978) | 40% |
| Germany | 2009 | Donath et al. (77) | 1403 | 25% | inpatient | AOD | 84% (0.819, 0.859) | 33% |
| Germany | 2008 | Hillemacher et al. (78) | 168 | 19% | inpatient | alcohol | 80% (0.735, 0.861) | 28.3% |
| Germany | 2007 | Hintz & Mann (79) | 125 | 20% | inpatient | alcohol | 63.2% (0.541, 0.717) | 28.3% |
| Germany | 2007 | Ohlmeier et al. (29), j | 89 | 36% | inpatient | alcohol | 67.4%† (0.567, 0.77) | 23.3% |
| Germany | 2001 | Schmidt & Smolka (37) | 63 | unknown | outpatient | alcohol | 76.2%† (0.638, 0.86) | 34.5% |
| Germany | 1999 | Hüttner et al. (80) | 31 | 16.1% | outpatient | alcohol | 90.3% (0.743, 0.98) | 36% |
| India | 2012 | Basu et al. (39), k | 6608 | 0.1% | outpatient | alcohol | 41.1%† (0.399, 0.423) | 24.3% |
| India | 2011 | Mattoo et al. (81) | 110 | 0% | inpatient | AOD | 52% (0.421, 0.615) | 24.3% |
| India | 2009 | Rooban et al. (82), l | 500 | 0.2% | outpatient | alcohol | 72.2% (0.681, 0.761) | 26% |
| Israel | 2003 | Amit et al. (83) | 72 | 5.6% | inpatient | alcohol | 91.6% (0.827, 0.969) | 23% |
| Italy | 2012 | Pajusco et al. (84) | 305 | 17.7% | ORT | opiate | 97.2% (0.953, 0.991) | 29.5% |
| Italy | 2011 | Barbadoro et al. (85) | 58 | 27.6% | inpatient | alcohol | 91.4% (0.81, 0.971) | 29.5% |
| Italy | 2011 | Pajusco et al. (44), m | 10181 | 19.4% | inpatient | opiate | 99.2% (0.99, 0.993) | 29.5% |
| Italy | 2008 | Barbadoro et al. (86) | 76 | 23.7% | inpatient | alcohol | 81.6% (0.71, 0.896) | 33% |
| Italy | 2001 | Pastorelli et al. (47), n | 60 | 25% | unknown | alcohol | 81.7% (0.696, 0.905) | 32.4% |
| Japan | 2010 | Matsui et al. (87) | 138 | 0% | outpatient | alcohol | 82% (0.744, 0.879) | 42% |
| Japan | 2005 | Nishiyori et al. (88) | 153 | 0% | inpatient | alcohol | 81% (0.739, 0.869) | 39.3% |
| Japan | 2003 | Nakamura et al. (89), o | 132 | 0% | inpatient | alcohol | 91.7%† (0.856, 0.958) | 52.8% |
| Netherlands | 2002 | Buster et al. (90) | 100 | 16% | ORT | opiate | 97%† (0.915, 0.994) | 32.2% |
| Nigeria | 1998 | Lawal et al. (91) | 80 | 9% | inpatient | opiate | 97.5% (0.913, 0.997) | 15.4% |
| Poland | 2002 | Bogucka-Bonikowska et al. (92) | 28 | 0% | ORT | opiate | 93% (0.765, 0.991) | 43% |
| Russia | 2001 | Kampov-Polevoy et al. (48) | 32 | 0% | inpatient | alcohol | 100% (0.891, 1) | 63.2% |
| Spain | 2011 | Pérez de Los Cobos et al. (42), p | 125 | 19.5% | outpatient | AOD | 84%† (0.773, 0.906) | 35.4% |
| Spain | 2002 | Boto de los Bueis et al. (46), q | 62 | 9.7% | unknown | opiate | 100% (0.942, 1) | 42.1% |
| Switzerland | 2010 | Walter et al. (33), r | 38 | 36.8% | ORT | opiate | 89.5% (0.752, 0.971) | 26% |
| Switzerland | 2008 | Wapf et al. (93) | 103 | 25% | ORT | opiate | 93% (0.865, 0.972) | 31% |
| Switzerland | 2000 | Zullino et al. (34) | 88 | 35.2% | inpatient | alcohol | 80.7% (0.709, 0.883) | 33.5% |
| Switzerland | 1998 | Perneger et al. (31) | 48 | 43.8% | ORT | opiate | 96%† (0.858, 0.995) | 34.3% |
| Turkey | 2003 | Ercan et al. (36), s | 60 | unknown | inpatient | alcohol | 88.3%† (0.774, 0.952) | 33.8% |
| UK | 2012 | Palmer et al. (30), t | 9285 | 36% | ORT | opiate | 85.9% (0.852, 0.866) | 21% |
| UK | 2004 | Harris et al. (94), u | 693 | 22.5% | inpatient | AOD | 89.5% (0.869, 0.917) | 26% |
| UK | 2001 | Tacke et al. (95) | 50 | 26% | ORT | opiate | 98% (0.894, 1) | 27% |
| UK | 1998 | Best et al. (96) | 100 | 27% | ORT | opiate | 93% (0.861, 0.971) | 30% |
Calculated by reviewers
National smoking prevalence is adjusted to the gender of the sample (If ≥70% of sample was either gender, national smoking rate is reported for that gender only. If gender of sample was not reported, the combined national rate was used.)
Opiate replacement therapy
(Shakeshaft et al. 2002) Shakeshaft et al. 1998 sample overlap, not included in review (21).
Alcohol and other drugs
(Holfich et al. 2012) Smoking prevalence collected based on number of deliveries (40) vs. number of women (37); so three women are double counted to determine this calculation. Also, to determine the rate, we totaled smokers of both vaginal and cesarean deliveries and divided that number by the total number of deliveries.
(Diehl et al. 2013) Smoking prevalence based on scale measuring nicotine dependence from “very low” to “elevated.”
(de Meneses-Gaya et al. 2009) Setting is psychosocial care center for alcohol or drug users. 40 clients invited to participate, of the 40, 30 self-reported as smokers.
(Dunn & Laranjeira 1999) Mixed modalities: outpatient 133, inpatient 94, police 40, hospice 26 (from Table 1).
(Aubin et al. 1999) Aubin et al. 1995 was not included in review (24).
(Ohlmeier et al. 2007) Paper reports 52.7% were average to heavy smokers in paper but smoking prevalence was calculated from Table 3 (minimal, average, heavy).
(Basu et al. 2012) Basu et al. 2009 uses subset (n=312) of this sample and was not included in review (20). For modality, facility also has “20 inpatient beds” and clients who utilized beds over a 30 year period were included in the study.
(Rooban et al. 2009) Overlapping sample with Thavarajah et al. 2006 (22).
(Pajusco et al. 2011). The data collected from patients entering a single program over a period of 27 years (1980-2007).
(Pastorelli et al. 2001) Unable to determine modality.
(Nakamura et al. 2003) Calculated by reviewers (11 nonsmokers).
(Pérez de Los Cobos et al. 2011) Includes 205 cocaine users, reported smoking rate for subsample (n=125).
(Boto de los Bueis et al. 2002) Unable to determine modality.
(Walter et al. 2010) Sample evenly split between MMT and diacetylmorphine.
(Ercan et al. 2003) N is 15 (childhood ADHD) + 45 (no childhood ADHD).
(Palmer et al. 2012) Does not separate current and ex-smoker.
(Harris et al. 2004) Does not specify proportions of inpatient, outpatient, and detox.
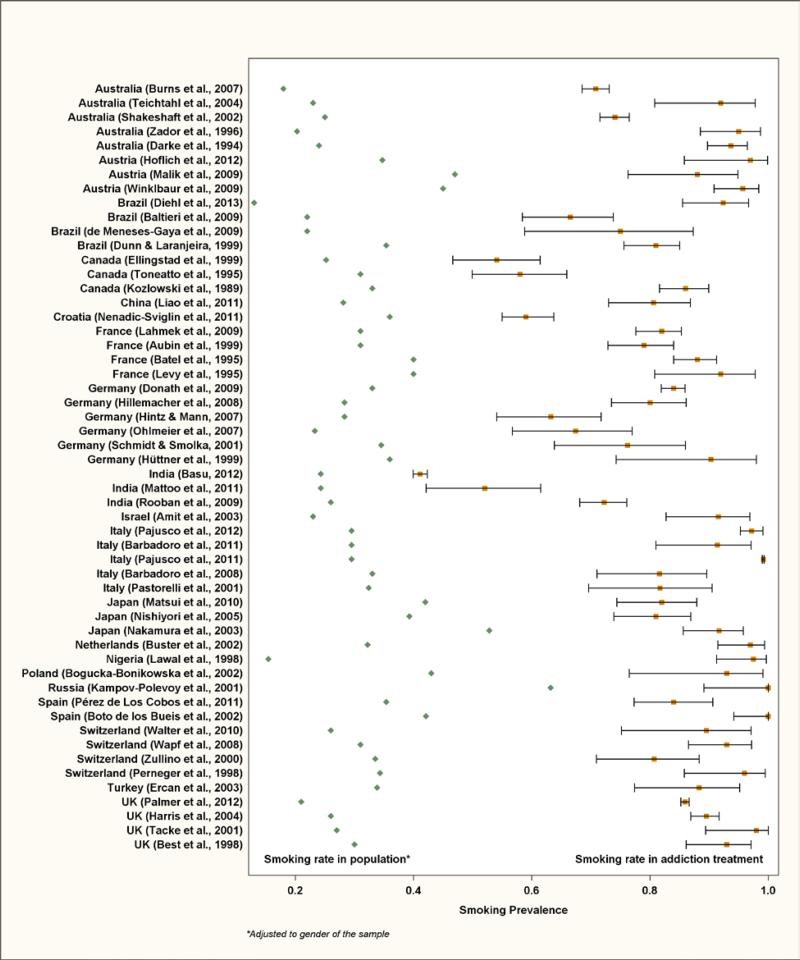
Figure 2 compares smoking prevalence visually in each sample to the corresponding national prevalence. National prevalence rates are shown on the left, while study prevalence rates and 95% CIs are on the right. For each study, the white space between the two estimates represents how much higher smoking is in the addiction treatment sample as compared to the general population. Scanning this white space from top to bottom shows that smoking rates are consistently higher in addiction treatment. Not shown in the Figure, the random-effect pooled estimate of smoking across persons in addiction treatment was 84% (CI 79%, 88%). The pooled estimate of smoking prevalence across the year and gender-matched population samples was 31% (CI 29%, 33%), and the difference in pooled estimates was 52% (CI 48%, 57%, p < .0001).
Figure 2.
Comparison of Smoking Prevalence in Addiction Treatment to Smoking Prevalence in the Population of 20 Countries
Association of Smoking with Treatment Modality and Primary Drug
Smoking rates were 85% in ORT, 80.9% in inpatient, and 74.5% in outpatient programs. By primary drug, smoking rates were 85.1% for opiates, 80.9% for alcohol and other drugs, and 75.2% for alcohol. Regression results are reported in Table 2. The unadjusted ORs in the first column show that treatment modality was not associated with differences in smoking rates. However, compared to programs treating alcohol, smoking rates were higher in programs treating both alcohol and other drug use (OR = 1.75, CI 1.45, 2.11) and in programs treating primarily opiate use (OR = 1.84, CI 1.49, 2.28). In Model 1, adjusting for year of study, odds of smoking were higher in ORT as compared to outpatient programs (OR = 1.42, CI 1.19, 1.68). In Model 2, compared to programs treating alcohol, smoking rates were higher in programs treating alcohol and other drug use (OR = 1.83, CI 1.52, 2.21) and in programs treating opiate use (OR = 2.52, CI 2.00, 3.17). In Model 3, only persons in programs treating alcohol and other drug use showed a higher rate of smoking, as compared those in programs treating alcohol use. Neither ORT modality nor opiate as primary drug was associated with smoking, and we believe this is because the two variables are confounded. In all adjusted models, year of study was inversely associated with smoking prevalence, such that the odds of smoking decreased by 6% per year in addiction treatment samples (OR = 0.94, CI = 0.93, 0.96). We discount this result because the 51 papers are spread across 20 years, and across countries with wide variation in population smoking rates. A large study reported in one year can affect the estimate of addiction treatment smoking prevalence for that year, influencing an estimate of linear change over time.
Table 2.
Treatment Modality, Primary Drug, and Year as Predictors of Smoking Prevalence in International Addiction Treatment Studies1
| Predictor | Unadjusted OR (95%CI)2 | Adjusted OR (95%CI)3 | ||
|---|---|---|---|---|
| Univariate | Multivariate- Model 1 (Modality and Year) | Multivariate- Model 2 (Primary Drug and Year) | Multivariate- Model 3 (Modality, Primary Drug and Year) | |
| Modality | ||||
| Outpatient | Reference | Reference | Reference | |
| Inpatient | 0.95 (0.80, 1.21) | 1.07 (0.89, 1.27) | 0.97 (0.81, 1.16) | |
| ORT | 1.03 (0.88, 1.19) | 1.42 (1.19, 1.68) | 0.38 (0.04, 3.54) | |
| Primary Drug | ||||
| Alcohol | Reference | Reference | Reference | |
| Alcohol or Drug | 1.75 (1.45, 2.11) | 1.83 (1.52, 2.21) | 1.79 (1.48, 2.17) | |
| Opiate | 1.84 (1.49, 2.28) | 2.52 (2.00, 3.17) | 6.21 (0.67, 57.72) | |
| Year | 0.96 (0.95, 0.97) | 0.94 (0.93, 0.96) | 0.94 (0.93, 0.96) | 0.94 (0.93, 0.96) |
Analyses are based studies shown in Table 1, excluding Kozolowski et al. 1989 (45), Pajusco et al. 2011 (44), and Basu et al. 2012 (39) (N = 51 studies).
Unadjusted analyses based on the same random-effects model as adjusted models which includes a random country accounts for nesting by country.
There are three adjusted models. One for the relationship of modality to smoking prevalence, and one for the relationship of primary drug to smoking prevalence. One for the relationship of modality and primary drug to smoking prevalence. Three models include a random country and account for nesting by country.
DISCUSSION
In every study reviewed, smoking prevalence among persons enrolled in addiction treatment was 2 to 4 times higher than that in the general population. This is consistent with results from the U.S., where NSDUH estimates of smoking among persons receiving addiction treatment (1987 – 2009) ranged across years from 66.9% to 75%(43). Considering both the prior U.S. review and the current international review, 96 papers reporting from 21 countries show that smoking prevalence is higher in addiction treatment as compared to the general population. World-wide, smoking among persons in addiction treatment programs contributes prominently to the tobacco epidemic, and to associated economic costs and morbidity and mortality.
Further, among persons enrolled in addiction treatment programs internationally, the highest rates of smoking are associated with opiate use and with participation in ORT programs. These findings are also consistent with prior research (16, 43), including findings that nicotine appears to potentiate the effect of methadone on opiate withdrawal (49), and that peak smoking rates are observed during methadone administration (50).
There are many potential reasons why smoking prevalence is higher in addiction treatment populations than in general populations. Like many drugs of abuse, smoking increases dopamine levels in reward regions of the brain, and dopamine receptor genes mediate smoking as well as other addiction-related behaviors (51). Smokers who also use other drugs are more heavily addicted to nicotine than smokers who do not (52, 53); and smokers who are more dependent on other drugs, and thus more likely to enter addiction treatment, are less successful in quitting smoking (53, 54). Importantly, Prochaska et al. also found that receipt of smoking cessation services was associated with improved outcomes for other addictions (55).
For patients in addiction treatment, other factors may support continued smoking or interfere with efforts to quit. These include elevated smoking prevalence among patients (43) and staff (56), and limited access to smoking cessation treatment (57-59). In a meta-analysis of smoking cessation trials among persons who received addiction treatment, Prochaska et al. found quit rates lower than those achieved in general population samples receiving similar treatments (55). Staff attitudes and beliefs about smoking may contribute to lower successful quit rates among persons in addiction treatment. Staff who smoke are less likely to address smoking with patients (60), and less likely to support smoking cessation in the context of addiction treatment (61). Both staff and directors sometimes express attitudes that quitting smoking hinders recovery from other addictions, that smoking cessation is a low priority, and that patients are not interested in quitting smoking (56). Importantly, many of the same attitudinal barriers to addressing smoking in addiction treatment programs are also reported in mental health programs (62, 63). Whether difficulty in quitting in addiction treatment populations is due to biological factors, features of the addiction treatment culture, or provider misconceptions, interventions specially tailored to this population may be needed to improve both motivation to quit smoking and successful quit rates.
Much of this research comes from the U.S. and some findings, particularly concerning the culture and beliefs within addiction treatment systems, may not apply internationally. At the same time the consistent finding of elevated smoking prevalence in addiction treatment internationally suggests that at least some of the contributing factors identified in the U.S. literature may apply in addiction treatment systems in other countries.
Limitations of the current study include reliance on English language publications, as this excludes an unknown number of reports in other languages which may meet inclusion criteria. Search procedures may have missed an unknown number of relevant papers, particularly if tobacco was not mentioned in the abstract but smoking prevalence was later reported in the paper itself. All smoking prevalence rates were provided in the papers or calculated from information provided in the papers. In one instance the prevalence estimate included former as well as current smokers (30). In another, smoking prevalence was inferred from a tobacco dependence scale (41). When developing national prevalence rates for comparison, a simple algorithm determined use of male, female, or combined national smoking prevalence. This may result in a national prevalence estimate that is either higher or lower than a national prevalence estimate based on the gender proportion in the sample. National smoking estimates do not consider differences in socioeconomic status (SES) between the general population and addiction treatment samples. If matched to SES of each treatment sample by year and by country, national estimates may be higher due to an association between lower SES and smoking in most countries (64). In that case, the gap between smoking in addiction treatment and smoking in national samples may be smaller. Each paper reported on a unique sample and, while we gathered all possible papers, we do not assert that any single sample or collection of papers represents smoking prevalence among all persons in addiction treatment in a single country.
Findings may inform tobacco control strategies in different countries. Addiction treatment programs offer a strategic point for tobacco intervention due to their high smoking prevalence and the potential to reach a large number of smokers. To better understand smoking prevalence in these populations, the WHO may wish to include a question in the GATS similar to that included in the NSDUH, which asks whether the respondent received any addiction treatment in the past year (65). When combined with current smoking status, this permits estimation of smoking prevalence in addiction treatment populations at the national level (43). The WHO, or individual FCTC signatories, may also consider adapting the MPOWER strategies, particularly smoke-free environments and cessation programs, for use in addiction treatment systems.
Even tobacco control efforts that are effective for the general public may have less success when applied to subpopulations such as addiction treatment clients. There is scant information, however, about strategies and success rates for addressing smoking in this population internationally. This paper is a first step toward encouraging dialogue among countries regarding ways to improve the efficacy of tobacco control for addiction treatment populations.
Acknowledgments
This work was supported by the NIDA Drug Abuse Treatment Research Center (P50 DA009253) and by the University of California Tobacco Related Disease Research Program (TRDRP 21XT-0088).
Footnotes
Competing Interests: None.
REFERENCES
- 1.World Health Organization . WHO global report: mortality attributable to tobacco. WHO; Geneva: 2012. [Google Scholar]
- 2.World Health Organization . WHO report on the global tobacco epidemic. WHO; Geneva: 2013. [Google Scholar]
- 3.Giovino GA, Mirza SA, Samet JM, Gupta PC, Jarvis MJ, Bhala N, et al. Tobacco use in 3 billion individuals from 16 countries: an analysis of nationally representative cross-sectional household surveys. The Lancet. 2012;380(9842):668–79. doi: 10.1016/S0140-6736(12)61085-X. [DOI] [PubMed] [Google Scholar]
- 4.Joossens L, Raw M. The Tobacco Control Scale: a new scale to measure country activity. Tob Control. 2006;15(3):247–53. doi: 10.1136/tc.2005.015347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martínez C, Martínez-Sánchez JM, Robinson G, Bethke C, Fernández E. Protection from secondhand smoke in countries belonging to the WHO European Region: an assessment of legislation. Tob Control. 2014;23(5):403–11. doi: 10.1136/tobaccocontrol-2012-050715. [DOI] [PubMed] [Google Scholar]
- 6.Ferketich AK, Lugo A, La Vecchia C, Fernandez E, Boffetta P, Clancy L, et al. Relation between national-level tobacco control policies and individual-level voluntary home smoking bans in Europe. Tob Control. 2014 doi: 10.1136/tobaccocontrol-2014-051819. [DOI] [PubMed] [Google Scholar]
- 7.Martinez-Sanchez JM, Blanch C, Fu M, Gallus S, La Vecchia C, Fernandez E. Do smoke-free policies in work and public places increase smoking in private venues? Tob Control. 2014;23(3):204–7. doi: 10.1136/tobaccocontrol-2012-050877. [DOI] [PubMed] [Google Scholar]
- 8.Szatkowski L, McNeill A. The delivery of smoking cessation interventions to primary care patients with mental health problems. Addiction. 2013 doi: 10.1111/add.12163. [DOI] [PubMed] [Google Scholar]
- 9.Kalman D, Morissette SB, George TP. Co-morbidity of smoking in patients with psychiatric and substance use disorders. Am J Addict. 2005;14(2):106–23. doi: 10.1080/10550490590924728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Compton WM, Thomas YF, Stinson FS, Grant BF. Prevalence, correlates, disability, and comorbidity of dsm-iv drug abuse and dependence in the united states: Results from the national epidemiologic survey on alcohol and related conditions. Arch Gen Psychiatry. 2007;64(5):566–76. doi: 10.1001/archpsyc.64.5.566. [DOI] [PubMed] [Google Scholar]
- 11.Substance Abuse and Mental Health Services Administration . Results from the 2008 National Survey on Drug Use and Health: National findings. Office of Applied Studies; Rockville, MD: 2009. NSDUH Series H-36, HHS Publication No. SMA 09-4434. [Google Scholar]
- 12.Hser YI, McCarthy WJ, Anglin MD. Tobacco use as a distal predictor of mortality among long-term narcotics addicts. Prev Med. 1994;23(1):61–9. doi: 10.1006/pmed.1994.1009. [DOI] [PubMed] [Google Scholar]
- 13.Hurt RD, Offord KP, Croghan IT, Gomez-Dahl L, Kottke TE, Morse RM, et al. Mortality following inpatient addictions treatment. Role of tobacco use in a community-based cohort. JAMA. 1996;275(14):1097–103. doi: 10.1001/jama.275.14.1097. [DOI] [PubMed] [Google Scholar]
- 14.Tsoh JY, Chi FW, Mertens JR, Weisner CM. Stopping smoking during first year of substance use treatment predicted 9-year alcohol and drug treatment outcomes. Drug Alcohol Depend. 2011;114(2-3):110–8. doi: 10.1016/j.drugalcdep.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guydish J, Passalacqua E, Tajima B, Chan M, Chun J, Bostrom A. Smoking prevalence in addiction treatment: a review. Nicotine & Tobacco Research. 2011;13(6):401–11. doi: 10.1093/ntr/ntr048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guydish J, Yu J, Le T, Pagano A, Delucchi K. Predictors of Tobacco Use Among New York State Addiction Treatment Patients. Am J Public Health. 2015;105(1):e57–e64. doi: 10.2105/AJPH.2014.302096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS medicine. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teichtahl H, Prodromidis A, Miller B, Cherry G, Kronborg I. Sleep-disordered breathing in stable methadone programme patients: a pilot study. Addiction. 2001;96(3):395–403. doi: 10.1046/j.1360-0443.2001.9633954.x. [DOI] [PubMed] [Google Scholar]
- 19.Gossop M, Neto D, Radovanovic M, Batra A, Toteva S, Musalek M, et al. Physical health problems among patients seeking treatment for alcohol use disorders: a study in six European cities. Addiction biology. 2007;12(2):190–6. doi: 10.1111/j.1369-1600.2007.00066.x. [DOI] [PubMed] [Google Scholar]
- 20.Basu D, Banerjee A, Harish T, Mattoo SK. Disproportionately high rate of epileptic seizure in patients abusing dextropropoxyphene. Am J Addict. 2009;18(5):417–21. doi: 10.3109/10550490903077697. [DOI] [PubMed] [Google Scholar]
- 21.Shakeshaft AP, Bowman JA, Sanson-Fisher RW. Computers in community-based drug and alcohol clinical settings: are they acceptable to respondents? Drug Alcohol Depend. 1998;50(2):177–80. doi: 10.1016/s0376-8716(98)00019-2. [DOI] [PubMed] [Google Scholar]
- 22.Thavarajah R, Rao A, Raman U, Rajasekaran ST, Joshua E, R H, et al. Oral lesions of 500 habitual psychoactive substance users in Chennai, India. Arch Oral Biol. 2006;51(6):512–9. doi: 10.1016/j.archoralbio.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 23.Zullino DF, Besson J, Favrat B, Krenz S, Zimmermann G, Schnyder C, et al. Acceptance of an intended smoking ban in an alcohol dependence clinic. Eur Psychiatry. 2003;18(5):255–7. doi: 10.1016/s0924-9338(03)00067-1. [DOI] [PubMed] [Google Scholar]
- 24.Aubin H, Tilikete S, Laureaux C, Nguyen Hac H, Roullet-Volmi M, Troupel S, et al. Smoking and coffee intake following alcohol withdrawal in alcoholic inpatients. Eur Psychiatry. 1995;10(8):383–5. doi: 10.1016/0924-9338(96)80342-7. [DOI] [PubMed] [Google Scholar]
- 25.Darke S, Swift W, Hall W, Ross M. Predictors of injecting and injecting risk-taking behaviour among methadone-maintenance clients. Addiction. 1994;89(3):311–6. doi: 10.1111/j.1360-0443.1994.tb00897.x. [DOI] [PubMed] [Google Scholar]
- 26.Lahmek P, Berlin I, Michel L, Berghout C, Meunier N, Aubin HJ. Determinants of improvement in quality of life of alcohol-dependent patients during an inpatient withdrawal programme. International journal of medical sciences. 2009;6(4):160–7. doi: 10.7150/ijms.6.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liao Y, Tang J, Liu T, Chen X, Luo T, Hao W. Sleeping problems among Chinese heroin-dependent individuals. Am J Drug Alcohol Abuse. 2011;37(3):179–83. doi: 10.3109/00952990.2010.535580. [DOI] [PubMed] [Google Scholar]
- 28.Malik P, Gasser RW, Kemmler G, Moncayo R, Finkenstedt G, Kurz M, et al. Low bone mineral density and impaired bone metabolism in young alcoholic patients without liver cirrhosis: a cross-sectional study. Alcohol Clin Exp Res. 2009;33(2):375–81. doi: 10.1111/j.1530-0277.2008.00847.x. [DOI] [PubMed] [Google Scholar]
- 29.Ohlmeier MD, Peters K, Kordon A, Seifert J, Wildt BT, Wiese B, et al. Nicotine and alcohol dependence in patients with comorbid attention-deficit/hyperactivity disorder (ADHD). Alcohol Alcohol. 2007;42(6):539–43. doi: 10.1093/alcalc/agm069. [DOI] [PubMed] [Google Scholar]
- 30.Palmer F, Jaffray M, Moffat MA, Matheson C, McLernon DJ, Coutts A, et al. Prevalence of common chronic respiratory diseases in drug misusers: a cohort study. Prim Care Respir J. 2012;21(4):377–83. doi: 10.4104/pcrj.2012.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perneger TV, Giner F, del Rio M, Mino A. Randomised trial of heroin maintenance programme for addicts who fail in conventional drug treatments. Bmj. 1998;317(7150):13–8. doi: 10.1136/bmj.317.7150.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Teichtahl H, Wang D, Cunnington D, Kronborg I, Goodman C, Prodromidis A, et al. Cardiorespiratory function in stable methadone maintenance treatment (MMT) patients. Addiction biology. 2004;9(3-4):247–53. doi: 10.1080/13556210412331292578. [DOI] [PubMed] [Google Scholar]
- 33.Walter M, Wiesbeck GA, Degen B, Albrich J, Oppel M, Schulz A, et al. Heroin reduces startle and cortisol response in opioid-maintained heroin-dependent patients. Addiction Biology. 2010;16:145–51. doi: 10.1111/j.1369-1600.2010.00205.x. [DOI] [PubMed] [Google Scholar]
- 34.Zullino D, Besson J, Schnyder C. Stage of change of cigarette smoking in alcohol-dependent patients. European addiction research. 2000;6(2):84–90. doi: 10.1159/000019015. [DOI] [PubMed] [Google Scholar]
- 35.Ellingstad TP, Sobell LC, Sobell MB, Cleland PA, Agrawal S. Alcohol abusers who want to quit smoking: implications for clinical treatment. Drug and alcohol dependence. 1999;54(3):259–65. doi: 10.1016/s0376-8716(98)00180-x. [DOI] [PubMed] [Google Scholar]
- 36.Ercan ES, Coskunol H, Varan A, Toksoz K. Childhood attention deficit/hyperactivity disorder and alcohol dependence: a 1-year follow-up. Alcohol and alcoholism. 2003;38(4):352–6. doi: 10.1093/alcalc/agg084. [DOI] [PubMed] [Google Scholar]
- 37.Schmidt LG, Smolka M. Relapse prevention in alcoholics by cigarette smoking? Involvement of nicotinic-dopaminergic mechanisms. Alcohol. 2001;24(2):111–5. doi: 10.1016/s0741-8329(01)00129-x. [DOI] [PubMed] [Google Scholar]
- 38.Toneatto A, Sobell LC, Sobell MB, Kozlowski LT. Effect of cigarette smoking on alcohol treatment outcome. Journal of substance abuse. 1995;7(2):245–52. doi: 10.1016/0899-3289(95)90008-x. [DOI] [PubMed] [Google Scholar]
- 39.Basu D, Aggarwal M, Das PP, Mattoo SK, Kulhara P, Varma VK. Changing pattern of substance abuse in patients attending a de-addiction centre in north India (1978-2008). Indian J Med Res. 2012;135(6):830–6. [PMC free article] [PubMed] [Google Scholar]
- 40.Dunn J, Laranjeira R. Cocaine--profiles, drug histories, and patterns of use of patients from Brazil. Substance use & misuse. 1999;34(11):1527–48. doi: 10.3109/10826089909039413. [DOI] [PubMed] [Google Scholar]
- 41.Diehl A, Silva RL, Laranjeira R. Female sexual dysfunction in patients with substance-related disorders. Clinics. 2013;68(2):205–12. doi: 10.6061/clinics/2013(02)OA14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perez de Los Cobos J, Sinol N, Puerta C, Cantillano V, Lopez Zurita C, Trujols J. Features and prevalence of patients with probable adult attention deficit hyperactivity disorder who request treatment for cocaine use disorders. Psychiatry Res. 2011;185(1-2):205–10. doi: 10.1016/j.psychres.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 43.Guydish J, Passalacqua E, Tajima B, Chan M, Chun J, Bostrom A. Smoking prevalence in addiction treatment: a review. Nicotine Tob Res. 2011;13(6):401–11. doi: 10.1093/ntr/ntr048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pajusco B, Boschini A, Chiamulera C, Benigni M, Smacchia C. F. L. Tobacco smoking prevalence in a large sample of heroin users accessing rehabilitation. Heroin Addict Relat Clin Probl. 2011;3:5–10. [Google Scholar]
- 45.Kozlowski LT, Skinner W, Kent C, Pope MA. Prospects for smoking treatment in individuals seeking treatment for alcohol and other drug problems. Addict Behav. 1989;14(3):273–8. doi: 10.1016/0306-4603(89)90058-0. [DOI] [PubMed] [Google Scholar]
- 46.Boto de los Bueis A, Pereira Vega A, Sanchez Ramos JL, Maldonado Perez JA, Ayerbe Garcia R, Garcia Jimenez D, et al. Bronchial hyperreactivity in patients who inhale heroin mixed with cocaine vaporized on aluminum foil. Chest. 2002;121(4):1223–30. doi: 10.1378/chest.121.4.1223. [DOI] [PubMed] [Google Scholar]
- 47.Pastorelli R, Bardazzi G, Saieva C, Cerri A, Gestri D, Allamani A, et al. Genetic determinants of alcohol addiction and metabolism: a survey in Italy. Alcoholism, clinical and experimental research. 2001;25(2):221–7. [PubMed] [Google Scholar]
- 48.Kampov-Polevoy AB, Tsoi MV, Zvartau EE, Neznanov NG, Khalitov E. Sweet liking and family history of alcoholism in hospitalized alcoholic and non-alcoholic patients. Alcohol Alcohol. 2001;36(2):165–70. doi: 10.1093/alcalc/36.2.165. [DOI] [PubMed] [Google Scholar]
- 49.Elkader AK, Brands B, Selby P, Sproule BA. Methadone-nicotine interactions in methadone maintenance treatment patients. J Clin Psychopharmacol. 2009;29(3):231–8. doi: 10.1097/JCP.0b013e3181a39113. [DOI] [PubMed] [Google Scholar]
- 50.Richter KP, Hamilton AK, Hall S, Catley D, Cox LS, Grobe J. Patterns of smoking and methadone dose in drug treatment patients. Exp Clin Psychopharmacol. 2007;15(2):144–53. doi: 10.1037/1064-1297.15.2.144. [DOI] [PubMed] [Google Scholar]
- 51.Vandenbergh DJ, O'Connor RJ, Grant MD, Jefferson AL, Vogler GP, Strasser AA, et al. Dopamine receptor genes (DRD2, DRD3 and DRD4) and gene-gene interactions associated with smoking-related behaviors. Addict Biol. 2007;12(1):106–16. doi: 10.1111/j.1369-1600.2007.00054.x. [DOI] [PubMed] [Google Scholar]
- 52.Hughes J. Do smokers with current or past alcoholism need different or more intensive treatment? Alcoholism, clinical and experimental research. 2002;26(12):1934–5. doi: 10.1097/01.ALC.0000041282.57396.30. [DOI] [PubMed] [Google Scholar]
- 53.Sobell LC, Sobell MB, Agrawal S. Self-change and dual recoveries among individuals with alcohol and tobacco problems: current knowledge and future directions. Alcoholism, clinical and experimental research. 2002;26(12):1936–8. doi: 10.1097/01.ALC.0000041001.11773.49. [DOI] [PubMed] [Google Scholar]
- 54.Drobes DJ. Cue reactivity in alcohol and tobacco dependence. Alcoholism, clinical and experimental research. 2002;26(12):1928–9. doi: 10.1097/01.ALC.0000040983.23182.3A. [DOI] [PubMed] [Google Scholar]
- 55.Prochaska JJ, Delucchi K, Hall SM. A meta-analysis of smoking cessation interventions with individuals in substance abuse treatment or recovery. Journal of consulting and clinical psychology. 2004;72(6):1144–56. doi: 10.1037/0022-006X.72.6.1144. [DOI] [PubMed] [Google Scholar]
- 56.Guydish J, Passalacqua E, Tajima B, Manser ST. Staff smoking and other barriers to nicotine dependence intervention in addiction treatment settings: a review. J Psychoactive Drugs. 2007;39(4):423–33. doi: 10.1080/02791072.2007.10399881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Richter KP, Choi WS, McCool RM, Harris KJ, Ahluwalia JS. Smoking cessation services in U.S. methadone maintenance facilities. Psychiatric services. 2004;55(11):1258–64. doi: 10.1176/appi.ps.55.11.1258. [DOI] [PubMed] [Google Scholar]
- 58.Fuller BE, Guydish J, Tsoh J, Reid MS, Resnick M, Zammarelli L, et al. Attitudes toward the integration of smoking cessation treatment into drug abuse clinics. Journal of substance abuse treatment. 2007;32(1):53–60. doi: 10.1016/j.jsat.2006.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Friedmann PD, Jiang L, Richter KP. Cigarette smoking cessation services in outpatient substance abuse treatment programs in the United States. Journal of substance abuse treatment. 2008;34(2):165–72. doi: 10.1016/j.jsat.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bobo JK, Gilchrist LD. Urging the alcoholic client to quit smoking cigarettes. Addict Behav. 1983;8(3):297–305. doi: 10.1016/0306-4603(83)90025-4. [DOI] [PubMed] [Google Scholar]
- 61.Gill BS, Bennett DL. Addiction professionals' attitudes regarding treatment of nicotine dependence. J Subst Abuse Treat. 2000;19(4):317–8. doi: 10.1016/s0740-5472(00)00106-9. [DOI] [PubMed] [Google Scholar]
- 62.Prochaska JJ, Fletcher L, Hall SE, Hall SM. Return to smoking following a smoke-free psychiatric hospitalization. The American Journal on Addictions / American Academy of Psychiatrists in Alcoholism and Addictions. 2006;15(1):15–22. doi: 10.1080/10550490500419011. [DOI] [PubMed] [Google Scholar]
- 63.Prochaska JJ, Reyes RS, Schroeder SA, Daniels AS, Doederlein A, Bergeson B. An online survey of tobacco use, intentions to quit, and cessation strategies among people living with bipolar disorder. Bipolar disorders. 2011;13(5 - 6):466–73. doi: 10.1111/j.1399-5618.2011.00944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.World Health Organization Systematic review of the link between tobacco and poverty. 2011 [Google Scholar]
- 65.U.S. Department of Health and Human Services, Substance Abuse and Mental Health Services Administration [2014 November 7];National Survey on Drug Use and Health. 2014 Available from: https://nsduhweb.rti.org/respweb/homepage.cfm.
- 66.Burns L, Mattick RP, Lim K, Wallace C. Methadone in pregnancy: treatment retention and neonatal outcomes. Addiction. 2007;102(2):264–70. doi: 10.1111/j.1360-0443.2006.01651.x. [DOI] [PubMed] [Google Scholar]
- 67.Shakeshaft AP, Bowman JA, Sanson-Fisher RW. Community-based drug and alcohol counselling: who attends and why? Drug and alcohol review. 2002;21(2):153–62. doi: 10.1080/09595230220139055. [DOI] [PubMed] [Google Scholar]
- 68.Zador D, Lyons Wall PM, Webster I. High sugar intake in a group of women on methadone maintenance in south western Sydney, Australia. Addiction. 1996;91(7):1053–61. [PubMed] [Google Scholar]
- 69.Hoflich AS, Langer M, Jagsch R, Bawert A, Winklbaur B, Fischer G, et al. Peripartum pain management in opioid dependent women. European journal of pain. 2012;16(4):574–84. doi: 10.1016/j.ejpain.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Winklbaur B, Baewert A, Jagsch R, Rohrmeister K, Metz V, Aeschbach Jachmann C, et al. Association between prenatal tobacco exposure and outcome of neonates born to opioid-maintained mothers. Implications for treatment. Eur Addict Res. 2009;15(3):150–6. doi: 10.1159/000216466. [DOI] [PubMed] [Google Scholar]
- 71.Baltieri DA, Daro FR, Ribeiro PL, Andrade AG. Effects of topiramate or naltrexone on tobacco use among male alcohol-dependent outpatients. Drug Alcohol Depend. 2009;105(1-2):33–41. doi: 10.1016/j.drugalcdep.2009.05.025. [DOI] [PubMed] [Google Scholar]
- 72.de Meneses-Gaya C, Zuardi AW, de Azevedo Marques JM, Souza RM, Loureiro SR, Crippa JA. Psychometric qualities of the Brazilian versions of the Fagerstrom Test for Nicotine Dependence and the Heaviness of Smoking Index. Nicotine Tob Res. 2009;11(10):1160–5. doi: 10.1093/ntr/ntp114. [DOI] [PubMed] [Google Scholar]
- 73.Nenadic-Sviglin K, Nedic G, Nikolac M, Kozaric-Kovacic D, Stipcevic T, Muck Seler D, et al. Suicide attempt, smoking, comorbid depression, and platelet serotonin in alcohol dependence. Alcohol. 2011;45(3):209–16. doi: 10.1016/j.alcohol.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 74.Aubin HJ, Laureaux C, Tilikete S, Barrucand D. Changes in cigarette smoking and coffee drinking after alcohol detoxification in alcoholics. Addiction. 1999;94(3):411–6. doi: 10.1046/j.1360-0443.1999.94341110.x. [DOI] [PubMed] [Google Scholar]
- 75.Batel P, Pessione F, Maitre C, Rueff B. Relationship between alcohol and tobacco dependencies among alcoholics who smoke. Addiction. 1995;90(7):977–80. doi: 10.1046/j.1360-0443.1995.90797711.x. [DOI] [PubMed] [Google Scholar]
- 76.Levy P, Mathurin P, Roqueplo A, Rueff B, Bernades P. A multidimensional case-control study of dietary, alcohol, and tobacco habits in alcoholic men with chronic pancreatitis. Pancreas. 1995;10(3):231–8. doi: 10.1097/00006676-199504000-00003. [DOI] [PubMed] [Google Scholar]
- 77.Donath C, Metz K, Chmitorz A, Gradl S, Piontek D, Floter S, et al. Prediction of alcohol addicted patients’ smoking status through hospital tobacco control policy: A multi-level-analysis. Drugs: education, prevention and policy. 2009;16(1):53–70. [Google Scholar]
- 78.Hillemacher T, Weinland C, Heberlein A, Wilhelm J, Bayerlein K, Kornhuber J, et al. Treatment with clomethiazole is associated with lower rates of premature discharge during alcohol withdrawal. Pharmacopsychiatry. 2008;41(4):134–7. doi: 10.1055/s-2008-1058106. [DOI] [PubMed] [Google Scholar]
- 79.Hintz T, Mann K. Long-term behavior in treated alcoholism: Evidence for beneficial carry-over effects of abstinence from smoking on alcohol use and vice versa. Addict Behav. 2007;32(12):3093–100. doi: 10.1016/j.addbeh.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 80.Huttner E, Matthies U, Nikolova T, Ehrenreich H. A follow-up study on chromosomal aberrations in lymphocytes of alcoholics during early, medium, and long-term abstinence. Alcoholism, clinical and experimental research. 1999;23(2):344–8. [PubMed] [Google Scholar]
- 81.Mattoo SK, Chakraborty K, Basu D, Ghosh A, Vijaya Kumar KG, Kulhara P. Prevalence & correlates of metabolic syndrome in alcohol & opioid dependent inpatients. Indian J Med Res. 2011;134:341–8. [PMC free article] [PubMed] [Google Scholar]
- 82.Rooban T, Rao A, Joshua E, Ranganathan K. The prevalence of oral mucosal lesions in alcohol misusers in Chennai, south India. Indian J Dent Res. 2009;20(1):41–6. doi: 10.4103/0970-9290.49064. [DOI] [PubMed] [Google Scholar]
- 83.Amit Z, Weiss S, Smith BR, Markevitch S. The affinity for sweet substances and cigarette smoking in chronic alcoholism. Isr J Psychiatry Relat Sci. 2003;40(2):96–102. [PubMed] [Google Scholar]
- 84.Pajusco B, Chiamulera C, Quaglio G, Moro L, Casari R, Amen G, et al. Tobacco addiction and smoking status in heroin addicts under methadone vs. buprenorphine therapy. International journal of environmental research and public health. 2012;9(3):932–42. doi: 10.3390/ijerph9030932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Barbadoro P, Ponzio E, Pertosa ME, Aliotta F, D'Errico MM, Prospero E, et al. The effects of educational intervention on nutritional behaviour in alcohol-dependent patients. Alcohol Alcohol. 2011;46(1):77–9. doi: 10.1093/alcalc/agq075. [DOI] [PubMed] [Google Scholar]
- 86.Barbadoro P, Lucrezi D, Prospero E, Annino I. Improvement of knowledge, attitude, and behavior about oral health in a population of alcohol addicted persons. Alcohol Alcohol. 2008;43(3):347–50. doi: 10.1093/alcalc/agn009. [DOI] [PubMed] [Google Scholar]
- 87.Matsui T, Yokoyama A, Matsushita S, Ogawa R, Mori S, Hayashi E, et al. Effect of a comprehensive lifestyle modification program on the bone density of male heavy drinkers. Alcohol Clin Exp Res. 2010;34(5):869–75. doi: 10.1111/j.1530-0277.2010.01159.x. [DOI] [PubMed] [Google Scholar]
- 88.Nishiyori A, Shibata A, Ogimoto I, Uchimura N, Egami H, Nakamura J, et al. Alcohol drinking frequency is more directly associated with alcohol use disorder than alcohol metabolizing enzymes among male Japanese. Psychiatry Clin Neurosci. 2005;59(1):38–44. doi: 10.1111/j.1440-1819.2005.01329.x. [DOI] [PubMed] [Google Scholar]
- 89.Nakamura Y, Ishikawa A, Sekiguchi S, Kuroda M, Imazeki H, Higuchi S. Spirits and gastrectomy increase risk for chronic pancreatitis in Japanese male alcoholics. Pancreas. 2003;26(2):e27–31. doi: 10.1097/00006676-200303000-00022. [DOI] [PubMed] [Google Scholar]
- 90.Buster M, Rook L, van Brussel GH, van Ree J, van den Brink W. Chasing the dragon, related to the impaired lung function among heroin users. Drug and alcohol dependence. 2002;68(2):221–8. doi: 10.1016/s0376-8716(02)00193-x. [DOI] [PubMed] [Google Scholar]
- 91.Lawal RA, Adelekan ML, Ohaeri JU, Orija OB. Rehabilitation of heroin and cocaine abusers managed in a Nigerian psychiatric hospital. East African medical journal. 1998;75(2):107–12. [PubMed] [Google Scholar]
- 92.Bogucka-Bonikowska A, Baran-Furga H, Chmielewska K, Habrat B, Scinska A, Kukwa A, et al. Taste function in methadone-maintained opioid-dependent men. Drug and alcohol dependence. 2002;68(1):113–7. doi: 10.1016/s0376-8716(02)00186-2. [DOI] [PubMed] [Google Scholar]
- 93.Wapf V, Schaub M, Klaeusler B, Boesch L, Stohler R, D. E. The barriers to smoking cessation in Swiss methadone and buprenorphine-maintained patients. Harm Reduct J. 2008;18:5–10. doi: 10.1186/1477-7517-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Harris CK, Warnakulasuriya KAAS, Cooper DJ, Peters TJ, Gelbier S. Prevalence of oral mucosal lesions in alcohol misusers in south London. J Oral Pathol Med. 2004;33(5):253–9. doi: 10.1111/j.0904-2512.2004.00142.x. [DOI] [PubMed] [Google Scholar]
- 95.Tacke U, Wolff K, Finch E, Strang J. The effect of tobacco smoking on subjective symptoms of inadequacy (“not holding”) of methadone dose among opiate addicts in methadone maintenance treatment. Addict Biol. 2001;6(2):137–45. doi: 10.1080/13556210020040217. [DOI] [PubMed] [Google Scholar]
- 96.Best D, Lehmann P, Gossop M, Harris J, Noble A, Strang J. Eating too little, smoking and drinking too much: Wider lifestyle problems among methadone maintenance patients. Addiction Research & Theory. 1998;6(6):489–98. [Google Scholar]