Abstract
PURPOSE
The present study was undertaken to evaluate osteoradionecrosis (ORN) in patients with salivary gland malignancies (SGM) after treatment with radiation therapy.
MATERIALS AND METHODS
The medical records of 172 patients treated with radiation therapy for SGM during a 12-year period (August 2001 to November 2013) were reviewed. Incidence, time to event, staging and management of ORN were analyzed.
RESULTS
Of the 172 patients, 7 patients (4%) developed ORN (median latency: 19 months, range: 4–72 months). Of those 7 patients, 4 required major surgery, 1 required hyperbaric oxygen therapy (HBO), one required minor debridement, and one required conservative management. Total prescribed radiation dose varied from 50 Gy (1 case) to 70 Gy (1 case) among those patients who developed ORN, and radiotherapy was delivered postoperatively after osseous resection in 4 of 7 cases. Three of the 7 cases of ORN occurred after traumatic injury to the bone. Of the 7 patients who developed ORN, 3 had SGM of the major glands, 3 had other sites of the oral cavity, and 1 had a sinonasal location.
CONCLUSION
While the rate of ORN after radiotherapy for SGM was somewhat lower (4%) than previously published data on patients with squamous cell carcinomas of the head and neck treated with radiation therapy (8% to 14%), ORN necessitating major surgery remains a rare, but clinically significant, possible late effect of radiotherapy in SGM survivors. Location is very important, with all cases that developed ORN having primary disease arising in the oral cavity.
Keywords: salivary gland neoplasm, osteoradionecrosis, hyperbaric oxygen, radiation therapy
Introduction
Salivary gland malignancies (SGM) are relatively rare but are rising in incidence [1]. SGM account for 3–5% of all head and neck cancers and can occur in major glands (parotid, submandibular, and sublingual) or minor glands within the mucosal lining of the oral cavity, oropharynx, and nasal cavity. The most common histological subtypes of SGM are adenoid cystic carcinoma, mucoepidermoid carcinoma, and acinic cell carcinoma [2]. Radiation therapy has been shown to be effective at increasing the survival of high-risk patients who have close or positive margins, lymph node metastasis, locally advanced disease, bone or nerve involvement, and/or recurrent disease [3], and therefore it is delivered in nearly 40% of SGM patients treated at our institution in the last decade.
Advancements have been made in radiation delivery and optimization of dose distribution, but many complications still cannot be avoided due to critical normal structures in close proximity to the radiation target volume. Among the most serious of these complications is osteoradionecrosis (ORN). ORN is linked to radiation-induced hypoxia, hypocellularity, hypovascularity and decreased wound healing [4]. Although most commonly a delayed complication, the onset of ORN has been shown to begin as early as 2 weeks after radiation concludes, with an irreversible and potentially progressive course [5, 6]. ORN has been well characterized after radiotherapy in mixed cohorts of patients with more common cancers of the head and neck, typically SCC, but not specifically for SGM patients whose radiation plans differ considerably from those for SCC patients [6, 7]. The present study was undertaken to evaluate the incidence, time to event, staging, and management of ORN after radiation therapy treatment for SGM.
Materials and Methods
Patients with newly diagnosed SGM and no prior history of cancer (with the exception of non-melanoma skin cancer) were prospectively recruited to an epidemiologic cohort at the University of Texas MD Anderson Cancer Center between 2001 and 2013. A total of 325 SGM patients were recruited for the study. A retrospective analysis was undertaken to evaluate ORN among the 181 patients who received radiation for malignant salivary gland tumors. Nine patients who did not complete the prescribed course of radiation or whose outside radiation records were incomplete were also excluded. Thus, 172 patients were included in this study (Figure 1).
Figure 1. Patient screening.
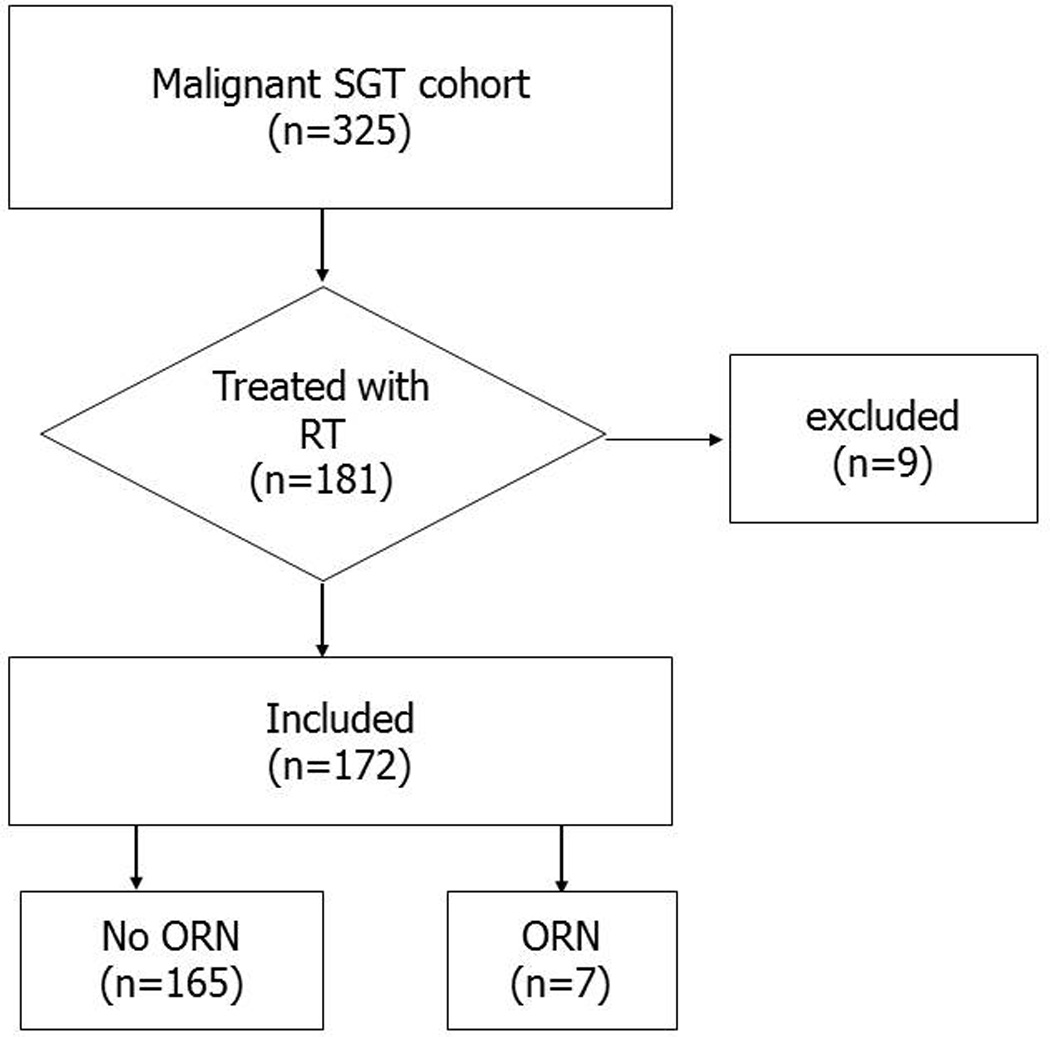
The medical records of the patients were reviewed. Age at diagnosis, sex, primary tumor site, histopathology, surgical history, and total radiation dosages were recorded. Mandible and maxillary specific dose-volume histograms (DVH) were reconstructed from radiation plans when available. Additionally, ORN subsite was contoured on post-ORN diagnostic CT and contours were propagated to original planning CT when available to calculate dose given to the respective subsite. The presence of ORN, site of ORN (mandibular or maxillary), time to occurrence, as well as grade was also recorded. ORN was graded according to the criteria in Table 1 [6]. The primary tumor location was identified and categorized as: major gland, oral cavity, sinonasal tract, and oro-hypopharynx/ larynx. SGMs were also grouped according to their pathology; adenoid cystic carcinoma, mucoepidermoid carcinoma, adenocarcinoma/salivary duct carcinoma, acinic cell carcinoma were the major pathologic categories. Pre-radiotherapy dental prophylaxis was routinely applied and has been detailed elsewhere along with surveillance schedules [6]. Descriptive statistics were calculated and graphically summarized. Cumulative incidence was estimated per the Kaplan Meier method. Statistical analyses were performed using Stata Data Analysis Software version 10.0 (College Station, TX, USA).
Table 1.
Classification of ORN
| Grade 1: | Minimal bone exposure (conservative management only) |
| Grade 2: | Minor debridement needed |
| Grade 3: | Hyperbaric oxygen needed |
| Grade 4: | Major surgery required |
Results
SGM cohort
Demographic information of the 172 included patients is tabulated in Table 2. The mean age of the patients at diagnosis was 54, and 51% of the patients were male. 67% of SGM were in the major salivary glands or in the oral cavity. 92 (53%) were current smokers at diagnosis. Radiotherapy was delivered postoperatively in 156 (91%) of cases, 29 of whom received chemotherapy. 96% of the patients received a total dose of more than 60 Gy of radiation, and IMRT technique was used in 143 (83%) of patients.
Table 2.
Patient information
| All patients [%] |
Patients with ORN |
|
|---|---|---|
| Median Age (years) | 54 | 53 |
| Gender | ||
| Male | 87 [51] | 1 |
| Female | 85 [49] | 6 |
| Radiation Dose (Gy) | ||
| 50–59 | 7 [4] | 1 |
| 60–64 | 103 [60] | 4 |
| 65+ | 41 [24] | 1 |
| Outside* | 21 [12] | 1 |
| Radiation technique | ||
| IMRT | 143 [83] | 5 |
| Proton | 15 [9] | 1 |
| Unknown | 14 [8] | 1 |
| Tumor Site | ||
| Parotid/ Submandibular/ Sublingual | 74 [43] | 3 |
| Oral cavity | 41 [24] | 3 |
| Oro-hypopharynx/ larynx | 19 [11] | |
| Sinonasal tract | 23 [13] | 1 |
| Other | 15 [10] | |
| Histological Type | ||
| Adenoid Cystic Carcinoma | 93 [54] | 4 |
| Mucoepidermoid Carcinoma | 16 [9] | 1 |
| Adenocarcinoma / Salivary duct Carcinoma | 27 [16] | 1 |
| Acinic Cell Carcinoma | 10 [6] | |
| Other | 26 [15] | 1 |
| Smoking (at diagnosis) | ||
| Never | 92 [53] | 3 |
| Former | 56 [33] | 2 |
| Current | 24 [14] | 2 |
Osteoradionecrosis
At a median follow-up of 29 months, ORN developed in 7 of the 172 patients (4%), with a median latency of 19 months (range: 4 to 72 months). Case details are summarized in Table 3 and incidence plot of ORN is shown in Figure 2. Three patients developed maxillary ORN, 3 developed mandibular ORN, and one developed ORN of the maxilla, mandibular condyle, and temporomandibular joint. All 7 ORN cases had pre-treatment dental evaluation and prophylaxis including 1 planned dental extraction prior to cancer treatment. Three of the 7 cases of ORN developed following a traumatic insult to the bone after initial healing (two had dental extractions and one a motor vehicle accident). Among these ORN cases, 4 required major surgery, 1 required hyperbaric oxygen (HBO) therapy, 1 required minor debridement, and the final case was managed conservatively over a period of 48 months. Total radiation dose among ORN cases ranged from 50 Gy to 70 Gy.
Table 3.
Details of osteoradionecrosis (n=7)
| Case | Cancer site | Sex | Age | Radiation dose (total) |
Surgery | ORN time to onset (months) |
ORN grade |
Site | Mandible dose (mean Max V40 V50 V60) |
Mean dose to ORN sub- volume |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Major gland (submandibular) |
F | 42 | Out* >50 Gy |
Neck dissection (I-III) |
19 | 1 | Mandible | Not available |
Not available |
| 2 | Major gland (parotid) |
F | 56 | 60 Gy | Parotid, mandibulotomy, parapharyngeal space, neck dissection I-IV |
60 | 4 | Mandible | 29.9 64.7 29% 27% 19% |
61.8 |
| 3 | Oral cavity (maxilla) |
F | 52 | 60 Gy | Maxillectomy | 24 | 4 | Maxilla | Not available |
Not available |
| 4 | Oral cavity (maxilla/soft palate) |
F | 68 | 60 Gy | Maxillectomy | 6 | 3 | Maxilla | Not available |
Not available |
| 5 | Oral cavity (retromolar trigone) |
F | 52 | 60 Gy | Mandibulectomy with free flap, neck dissection |
5 | 4 | Mandible | 32.1 62.8 48% 46% 21% |
60.2 |
| 6 | Major gland (parotid) |
M | 54 | 40 Gy E*, 10 Gy P* |
Parotidectomy, neck dissection |
72 | 4 | Mandible, TMJ, Maxilla |
35.5 50.1 27.5% 0.01% 0% |
47.3 |
| 7 | Sinonasal tract (maxillary sinus) |
F | 44 | 70 Gy | None | 4 | 2 | Maxilla | Not available |
Not available |
Out denotes radiation received at an outside institution, E (electron), P (photon)
Figure 2. Incidence of ORN.
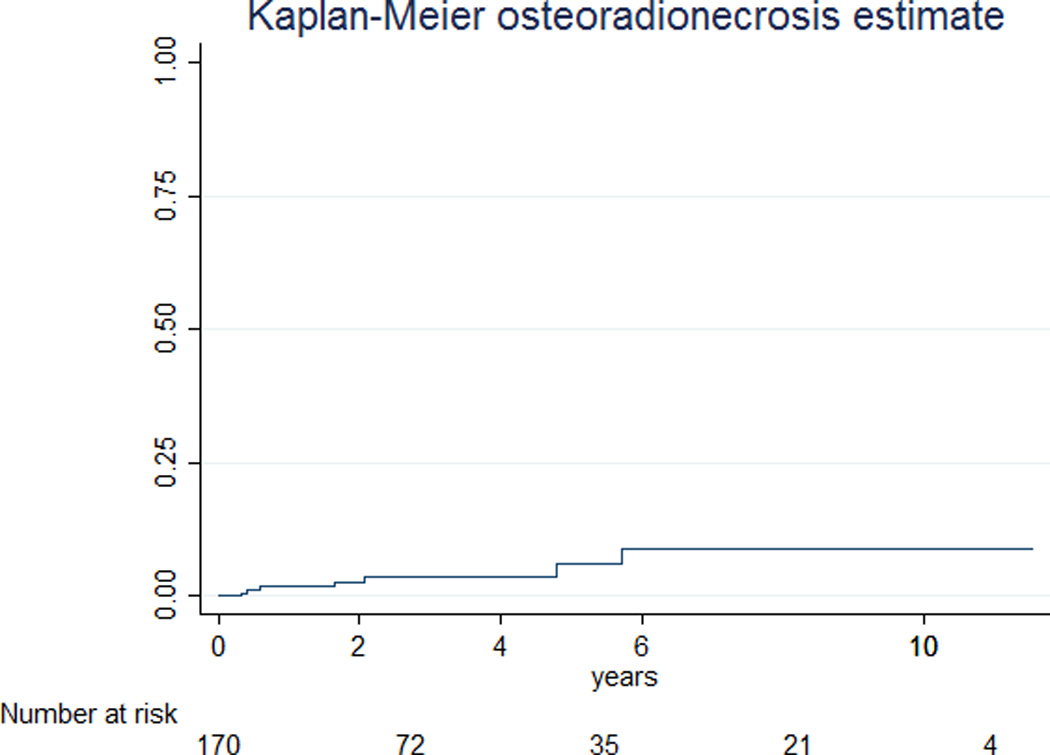
Kaplan-Meier estimate of time to ORN after end of radiotherapy.
Predisposing factors
ORN disproportionately occurred in females (p=0.045), and those with primary tumors in the oral cavity. Six of the 7 patients that developed ORN were female, and also 6 of the 7 patients had original tumors in the major glands or in the oral cavity with these tumor sites comprising roughly two-thirds of the SGM cohort. No ORN occurred in patients with minor salivary gland cancers arising from exclusively pharyngeal or laryngeal primary sites. 5 of 7 ORN cases had total radiotherapy doses over 60 Gy, and radiotherapy was delivered postoperatively after osseous resection in 6 of 7 cases. Mandible DVH were restored for just 3 of 4 mandibular ORN cases, detailed in Table 3. Total radiation dose among ORN cases ranged from 50 Gy to 70 Gy. For cases with retrievable radiation plans, mean dose to the subsite affected by ORN was comparatively higher than the respective whole organ mean dose (as shown in Table 3) suggesting a dose-ORN relationship in these cases. Type of radiation did not significantly predict ORN (p=0.835). The most common histology of the tumors encountered were adenoid cystic carcinoma (54%), mucoepidermoid carcinoma (9%), adenocystic (8%), and salivary duct carcinoma (8%), with similar distribution of ORN by histology. Neither smoking status at diagnosis (p=0.716) or pack-years (p=0.534) significantly predicted ORN.
Discussion
ORN is a potentially significant sequelae of radiation therapy, which can range from largely asymptomatic lesions treated conservatively to significant, life-threatening fractures that require extensive composite resections and reconstructions. Late grades of ORN can adversely affect speech, swallowing, mastication, weight and self-image. When ORN progresses to a point necessitating radical mandibulectomy, the effects and treatment of ORN can be more debilitating than the index cancer and its therapy. The overall rate of ORN in SGM was found to be 4% (95% confidence interval: 1.6% to 8.2%) in this cohort during a median follow-up time of 29 months. This is less than the 6.6 to 8.2% ORN rates reported in clinical papers combining all head and neck malignancies. The observed rate in our SGM cohort is lower than a SEER-Medicare analysis suggesting 14% population-level risk of ORN of all grades after head and neck IMRT; however, in that series, only 3.8% of patients had a significant complication requiring an intervention (HBO or surgery) and the current study is more consistent with this [6–9].
The reported incidence of ORN varies greatly in the published literature, dependent largely on radiation technique, tumor subsite, surgical parameters, and dose/volume distributions of the irradiated bone. For instance, several notable IMRT series have reported zero event rates for ORN with more conformal radiotherapy methods. Notable distinctions in these IMRT papers (that are not SGM focused) include the coding only higher grade ORN events (grade≥2) in the Michigan [10] series and a short follow-up period in the UCLA [11] series. Diverse grading methodologies and follow-up schedules in part contribute further to differences in ORN rates in published series. SGM represents a complex and distinct subgroup of head and neck malignancies arising is numerous sites. Adding further complexity to characterizing toxicities such as ORN in SGM survivors, radiation is often delivered in the postoperative setting with fields that differ greatly from conventional head and neck radiotherapy. Despite these critical distinctions in SGM from conventional tobacco-related HNC, herein we find that ORN still afflicts a subset of SGM survivors, many of whom have excellent prognosis for long-term cure, making late effects of therapy of upmost importance.
SGM arises in diverse anatomic locations of the head and neck, and our subgroup analyses suggest that risk of ORN is dependent on site of the SGM. Among the SGM, ORN was only encountered in patients treated for cancers arising in the major glands and oral cavity. Oral cavity primary tumors are recognized as high risk sites for subsequent development of ORN in general head and neck cohorts comprised of mostly squamous cell histology [12]. Site-dependent differences in ORN rates likely reflect distinctions in the radiation dose distribution to the osseous structures of the head and neck. While the total dose to the tumor volume in ORN cases typically exceeded 60 Gy regardless of SGM site, the volume of mandibular and maxillary bone in the radiation fields varies dramatically based on the primary site of SGM. The major gland radiation fields are largely superficial relative to minor gland primary tumors, and associated radiation fields include smaller volumes of the angle of the mandible and the posterior mandible. Conversely, radiation fields for patients with SGM arising in the oral cavity or oropharynx, intraoral radiation fields likely cover larger volumes of the mandible and maxillary bones bilaterally. Figure 3 provides illustrative case examples comparing sample radiation plans in a patient with a parotid gland tumor to a patient with an oral cavity tumor.
Figure 3. Sample radiation plans.
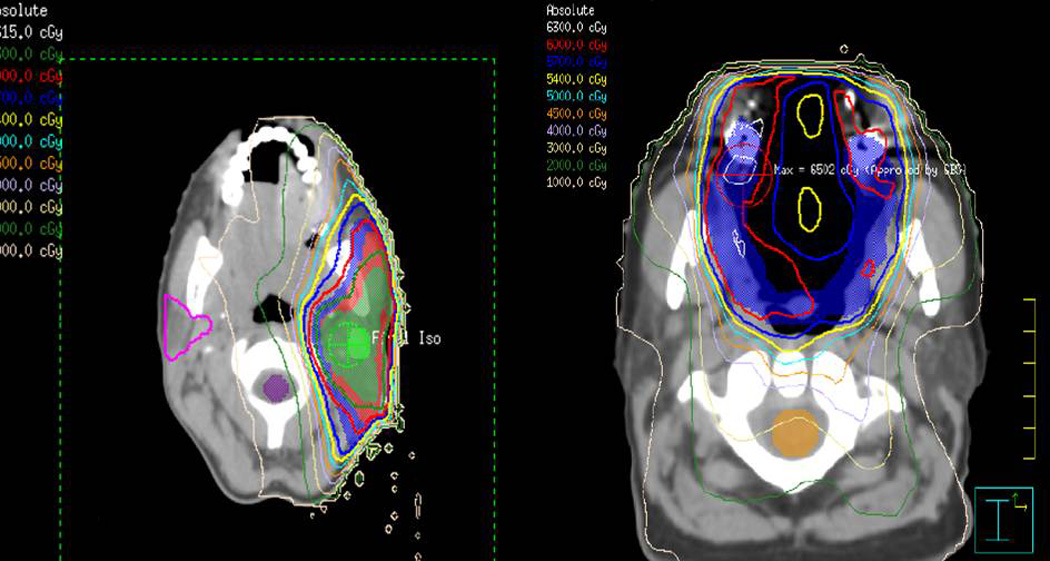
Case examples comparing radiation plans of a patient treated for a parotid salivary gland malignancy (left) and oral cavity salivary gland malignancy (right). Note difference in dose distributions of the mandibular bone.
ORN is a dose-volume dependent toxicity. Dose-response relationships of mandibular ORN have been described in patients with squamous cancers of the oral cavity and oropharynx, with 54 Gy maximum dose [9], V50 [6], and V44 [13] suggested as parameters that predict subsequent development of ORN. SGM cases were excluded or rare in these prior dose-response studies and almost all patients received definitive nonsurgical therapy with bilateral fields of irradiation. SGM specific dose-volume parameters related to ORN could not be evaluated in our SGM cohort. We could restore radiation plans to extract mandible specific DVH on only 3 cases yielding insufficient power for statistical comparisons in even a small nested case-control comparison. In the 3 cases for which mandible specific dose was available, Dmean and V50 ranging from 30–36 Gy and 0–46%, respectively, are notably lower than previously suggested threshold values found in other dose-response studies and 4 of 7 ORN cases had mandibular or maxillary resection preceding radiotherapy. Collectively, these observations suggest the need for SGM specific dose-response analysis as dose-volume thresholds for development of ORN are likely site dependent and lower when radiotherapy is delivered in the postoperative setting.
Trauma, tooth extractions, periodontal disease and dental decay/deterioration have long been considered predisposing factors to ORN. Similar to others [14], we found that a majority of patients who developed ORN had a traumatic event precede the process. These traumatic events should be avoided when possible, and patients who suffer mandibular trauma after radiotherapy should be monitored closely for any signs or ORN. Once the process of ORN begins, the management algorithm is the same regardless of the original malignancy. Over half of the patients who developed ORN required major surgical intervention/ reconstruction, again highlighting the clinical significance of this rare complication.
Herein, we provide an analysis of ORN in a large cohort of SGM survivors, notable given the rarity of this disease at the population level. Retrospective analysis of ORN events may be biased toward underreporting of the event, but comparisons are likely valid to ORN studies previously published using similar methodology in other patient cohorts with head and neck malignancy. Contribution of maxillary- and mandibular-specific radiation volume/dose distributions and the role of lymph node involvement on ORN risk warrants further study. Identification of high-risk subgroups and validation of organ-at-risk constraints relevant to ORN in SGM is the next extension of this early work in SGM specific ORN. Nonetheless, these data demonstrate a notable minority of even SGM patients treated with external beam radiotherapy are at risk for ORN, particularly those with major gland cancers and minor gland cancers arising in the oral cavity. The correlation is between the development of ORN and predisposing trauma; patients should be counseled carefully to reduce these risks and to follow-up immediately if they do occur in order to prevent subsequent ORN development.
Conclusion
While rare, ORN remains a potential, and at times severe, delayed sequela of multimodality therapy for SGM. This analysis suggests that SGM survivors treated with radiotherapy are at risk for ORN, despite comparatively lower rates of ORN (4%) relative to general head and neck cohorts with risk estimates ranging between 8% to 14%. This difference is likely accounted for by the distinct target delineation and dose distributions of the radiation therapy for SGM.
References
- 1.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2015, National Cancer Institute. Bethesda, MD: National Cancer Institute; 2015. [Accessed 11/1/2015]. http://seer.cancer.gov/csr/1975_2012/ [Google Scholar]
- 2.Adelstein DJ, Koyfman SA, El-Naggar AK, Hanna EY. Biology and management of salivary gland cancers. Semin Radiat Oncol. 2012;22:245–253. doi: 10.1016/j.semradonc.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 3.Zeidan YH, Pekelis L, An Y, et al. Survival benefit for adjuvant radiation therapy in minor salivary gland cancers. Oral Oncol. 2015;51:438–445. doi: 10.1016/j.oraloncology.2015.02.096. [DOI] [PubMed] [Google Scholar]
- 4.Marx RE. Osteoradionecrosis: a new concept of its pathophysiology. J Oral Maxillofac Surg. 1983;41:283–288. doi: 10.1016/0278-2391(83)90294-x. [DOI] [PubMed] [Google Scholar]
- 5.Li RH, Cai ZG, Mao C, Guo CB, Zhang JG, Zhang Y, et al. Retrospective study of 93 patients with jaw osteoradionecrosis. Zhonghua er bi yan hou tou jing wai ke za zhi = Chinese journal of otorhinolaryngology head and neck surgery. 2012;47:458–461. [PubMed] [Google Scholar]
- 6.Tsai CJ, Hofstede TM, Sturgis EM, et al. Osteoradionecrosis and radiation dose to the mandible in patients with oropharyngeal cancer. Int J Radiat Oncol Biol Phys. 2013;85:415–420. doi: 10.1016/j.ijrobp.2012.05.032. [DOI] [PubMed] [Google Scholar]
- 7.Reuther T, Schuster T, Mende U, Kubler A. Osteoradionecrosis of the jaws as a side effect of radiotherapy of head and neck tumour patients--a report of a thirty year retrospective review. Int J Oral Maxillofac Surg. 2003;32:289–295. doi: 10.1054/ijom.2002.0332. [DOI] [PubMed] [Google Scholar]
- 8.Beadle BM, Liao KP, Chambers MS, et al. Evaluating the impact of patient, tumor, and treatment characteristics on the development of jaw complications in patients treated for oral cancers: a SEER-Medicare analysis. Head Neck. 2013;35:1599–1605. doi: 10.1002/hed.23205. [DOI] [PubMed] [Google Scholar]
- 9.Lee IJ, Koom WS, Lee CG, et al. Risk factors and dose-effect relationship for mandibular osteoradionecrosis in oral and oropharyngeal cancer patients. Int J Radiat Oncol Biol Phys. 2009;75:1084–1091. doi: 10.1016/j.ijrobp.2008.12.052. [DOI] [PubMed] [Google Scholar]
- 10.Ben-David MA, Diamante M, Radawski JD, et al. Lack of osteoradionecrosis of the mandible after intensity-modulated radiotherapy for head and neck cancer: likely contributions of both dental care and improved dose distributions. Int J Radiat Oncol Biol Phys. 2007;68:396–402. doi: 10.1016/j.ijrobp.2006.11.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duarte VM, Liu YF, Rafizadeh S, Tajima T, Nabili V, Wang MB. Comparison of dental health of patients with head and neck cancer receiving IMRT vs conventional radiation. Otolaryngol Head Neck Surg. 2014;150:81–86. doi: 10.1177/0194599813509586. [DOI] [PubMed] [Google Scholar]
- 12.Gomez DR, Estilo CL, Wolden SL, et al. Correlation of osteoradionecrosis and dental events with dosimetric parameters in intensity-modulated radiation therapy for head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2011;81:e207–e213. doi: 10.1016/j.ijrobp.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 13.Fuller CD, Hutcheson KA, Mohamed AS, et al. Beam path dose-volume parameters associated with osteoradionecrosis of the mandible in head and neck patients receiving intensity-modulated radiotherapy: Results from an institutional oral toxicity registry study. unpublished: University of Texas MD Anderson Cancer Center. 2015 [Google Scholar]
- 14.Thorn JJ, Hansen HS, Specht L, Bastholt L. Osteoradionecrosis of the jaws: clinical characteristics and relation to the field of irradiation. J Oral Maxillofac Surg. 2000;58:1088–1093. doi: 10.1053/joms.2000.9562. [DOI] [PubMed] [Google Scholar]


