Abstract
Background
Racial/ethnic disparities in treatment outcomes of peripheral arterial disease (PAD) are well documented. Compared to non-Hispanic (NH) whites, blacks and Hispanics are more likely to undergo amputation and less likely to undergo bypass surgery for limb salvage. Endovascular procedures are being increasingly performed as first line of therapy for PAD. In this study, we examined the outcomes of endovascular PAD treatments based on race/ethnicity in a contemporary large population-based study.
Methods
We used Patient Discharge Data (PDD) from California’s Office of Statewide Health Planning and Development (OSHPD) to identify all patients over the age of 35 who underwent a lower extremity arterial intervention from 2005 to 2009. A look-back period of five years was used to exclude all patients with prior lower extremity arterial revascularization procedures or major amputation. Cox proportional hazards regression was used to compare amputation-free survival and time to death within 365 days. Logistic regression was used for comparison of 1-month myocardial infarction (MI), 1-month major amputation, 1-month all-cause mortality, 12-month major amputation, 12-month reintervention, and 12-month all-cause mortality rates among NH white, black, and Hispanic patients. These analyses were adjusted for age, gender, insurance status, severity of PAD, comorbidities, history of coronary artery angioplasty or bypass surgery, or history of carotid endarterectomy.
Results
Between 2005 and 2009, a total of 41,507 individuals underwent PAD interventions, 25,635 (61.7%) of whom underwent endovascular procedures. There were 17,433 (68%) NH whites, 4,417 (17.2%) Hispanics, 1,979 (7.7%) blacks, 1,163 (4.5%) Asian/Native Hawaiians, and 643 (2.5%) others in this group. There was a statistically significant difference in the amputation-free survival within 365 days among the NH white, Hispanic and black groups (P < 0.0001); the hazard ratio for amputation within 365 days was 1.69 in Hispanics (95% CI 1.51–1.90; P <0.0001) and 1.68 in blacks (95% CI 1.44–1.96; P <0.001) compared to NH whites following endovascular procedures after adjusting for age, gender, insurance status, comorbidities, severity of PAD, history of coronary artery angioplasty or bypass surgery, or history of carotid endarterectomy. After adjusting for the aforementioned confounders, the first reintervention within 12 months was also significantly associated with race/ethnicity (P=0.002). Odds ratio for reintervention was 1.17 in blacks (95% CI 1.06–1.30, P=0.002) and 1.084 in Hispanics (95% CI 1.00–1.16, P=0.04) compared to NH whites.
Conclusions
In this contemporary large population-based study, we demonstrated that even among matched cohorts Hispanics and blacks have worse amputation-free survival than NH whites following endovascular therapy. Our study also found that Hispanics and blacks are more likely to undergo lower extremity arterial reinterventions than NH whites. Further research is crucial in understanding if higher reintervention rates in Hispanics and blacks are due to more severe disease and/or poor access to proper follow-up care and optimal medical management.
Introduction
In the last decade there has been a major paradigm shift in the management of peripheral arterial disease (PAD) with an increase in endovascular interventions and a decrease in open surgical bypass procedures with a subsequent decrease in lower extremity amputation rates (1–3). However, despite changes in practice patterns in lower extremity arterial interventions, significant racial/ethnic differences remain in outcomes of PAD treatment. According to recent analyses of Medicare and Nationwide Inpatient Sample data, blacks and Hispanics with PAD are more likely to undergo amputation and less likely to be offered lower extremity arterial revascularization or to undergo aggressive surgical therapy for limb salvage compared to NH whites (3–6). These disparities have been attributed to differences in socioeconomic status, access to care, insurance status, and/or severity of disease (5).
Epidemiologic studies of PAD have demonstrated significant differences in the prevalence of PAD based on race/ethnicity. Blacks have been demonstrated to have the highest prevalence of PAD across all age groups compared to NH whites and Hispanics (7). For instance, in men between the ages of 60–69, 5.4% of whites, 4.3% of Hispanics, and 13.2% of blacks have PAD. Among men over the age of 80, 59% of blacks have PAD compared to 22.6% of whites and 22.5% of Hispanics. The prevalence of PAD in Hispanics is similar to NH whites across all age groups (7). This racial/ethnic disparity in prevalence of PAD holds true among women as well. More severe arterial occlusive disease in every segment of the infragenicular arteries are found in blacks compared to whites (8). A recent evaluation of a large national database demonstrated that blacks and Hispanics undergo more open revascularization procedures involving tibial arteries compared to whites (9).
Previous studies on PAD management have reported a disparity in lower extremity revascularization procedures offered to black and Hispanic patients who present with complications of PAD (10, 11). Few studies have evaluated outcomes of endovascular PAD interventions based on race/ethnicity in matched cohorts. Even fewer large database studies have access to ambulatory surgery data or longitudinal follow up to evaluate reintervention rates. The aim of our study was to evaluate short and long-term outcomes of endovascular PAD interventions in patients based on race/ethnicity in a contemporary series using a large state-wide all-payer database.
Materials and Methods
With approval from the state of California institutional review board, we used Patient Discharge Data (PDD) from California’s Office of Statewide Health Planning and Development (OSHPD). PDD is a population-based all-payer dataset that allows longitudinal tracking within California of morbidity outcomes for both inpatient and ambulatory procedures performed at non-federal hospitals. Multiple admissions within California for each unique patient are linked by encrypted identifiers provided by the PDD to follow a patient over a specified period of time. This database is also linked to the state’s vital statistics records. Inpatient data using ICD-9-CM have been used in OSHPD since 1990. Since 2005, OSHPD has captured outpatient or ambulatory surgery (AS) data, where the more anatomically specific CPT procedure codes are recorded (Appendix 1).
All patients over the age of 35 who had an admission for a primary or secondary diagnosis of PAD or diabetes mellitus (DM) associated with diagnosis of lower extremity disease or tissue loss from 2005 to 2009 were evaluated for this study. We used ICD-9-CM codes 440.20–440.24 for the diagnosis of PAD specifying intermittent claudication, rest pain, ulceration, or ischemic gangrene to characterize the target population (Appendix 1). Subsequently we used the ICD-9-CM codes for inpatient or CPT codes for outpatient endovascular lower extremity revascularization procedures to link the cohorts to identify our study population (Appendix 1). A-five year look-back exclusion was then performed to remove any patients with previous bypass, endovascular treatment or major amputation from our cohort. This allowed selection of as many patients undergoing first-time lower extremity arterial revascularization procedures for this study.
OSHPD provides data on age, sex, race/ethnicity, insurance status, severity of PAD at presentation, chronic comorbid conditions, history of coronary artery angioplasty or bypass surgery, and history of carotid endarterectomy. We used history of prior coronary artery and/or carotid artery interventions as additional tools to identify patients at high risk for cardiovascular comorbidity. We used the Elixhauser comorbidity codes, which are derived from the ICD-9-CM codes for conditions present on admission (12). We categorized patients into non-Hispanic (NH) white, Hispanic, black, Asian/Native Hawaiian and others. We evaluated perioperative outcomes of 30-day myocardial infarction (MI) rates, major amputation rates, and all-cause mortality rates. Since PDD is linked to the vital statistics data, 12-month mortality data were also collected. OSHPD allows longitudinal follow up of patients within California, therefore reintervention rates within 12 months were captured. Reinterventions were categorized by using the endovascular and open procedure codes matched with PAD and associated diabetic foot ulcer ICD-9-CM codes for each admission. We used ICD-9-CM codes for amputation through the tibia and above, excluding any amputations below the ankle, to collect data on 30 day and 12-month major amputation rates (Appendix 1).
Statistical analysis
We used the Chi-square test to compare the distribution of each diagnosis for diabetes mellitus, renal failure, congestive heart failure, hypertension, and insurance status among the three racial/ethnic groups: NH white, Hispanic, and black groups. The Kruskal-Wallis test was used to compare age among the three racial/ethnic groups. We used logistic regression to compare short-term outcomes including 1-month MI rate, 1-month major amputation rate, and 1-month all-cause mortality rate, and long-term outcomes including 12-month MI rate, 12-month major amputation rate, 12-month reintervention rate, and 12-month all-cause mortality rate, respectively, among the three racial/ethnic groups. These analyses were adjusted for age, gender, insurance status (Medicare, Medi-Cal, County, Private, Workers Comp / Government), severity of PAD at presentation (gangrene, ulceration, rest pain, claudication, aortic atherosclerosis), comorbidities (diabetes mellitus, renal failure, congestive heart failure, hypertension), history of coronary artery angioplasty or bypass surgery, or history of carotid endarterectomy.
In the group of patients requiring reintervention within 12 months, logistic regression was used to compare the distributions of reinterventions that were performed endovascularly among the three racial/ethnic groups adjusting for the aforementioned confounders. Cox proportional hazards regression(13) was used to compare amputation-free survival time and time to death within 365 days, respectively, among the three racial/ethnic groups controlling for the confounders. A P < 0.05 was considered statistically significant. All statistical analyses were performed using SAS software, Version 9.2 (SAS institute, Cary, NC).
Results
Demographics and Patient Presentation
During 2005 to 2009, 41,507 individuals underwent open and endovascular PAD interventions in non-federal California hospitals. In this cohort, 25,635 patients (61.8%) had endovascular procedures, including both inpatient and outpatient procedures. The AS or outpatient procedures accounted for 47.0% (12,061) of all the endovascular procedures. In our complete endovascular cohort, 11,389 patients were women (44.4%) and 14,246 were men (55.6%) (Table 1). The endovascular group included 17,433 (68.0%) NH whites, 4,417 (17.2%) Hispanics, 1,979 (7.7%) blacks, 1,163 (4.5%) Asian/Native Hawaiians, and 643 (2.5%) others (Table 1). The age distribution of the study population is presented in Table 1. In our study population 70.8% of patients were over the age of 65. The mean age at discharge for NH whites was 71.8 years (median 73; range 35–105) whereas the mean age for blacks and Hispanics was 69.4 (median 70; range 36–103) and 70.7 (median 72; range 35–100) respectively. There was a statistically significant difference in age among the three racial/ethnic groups (P < 0.0001; Table 2).
Table I.
Demographics of all patients who underwent endovascular peripheral arterial disease interventions in California hospitals during 2005–2009
| Number (%) |
|
|---|---|
| Gender | |
| Women | 11,389 (44.4%) |
| Men | 14,424 (55.6%) |
| Race / Ethnicity | |
| Non-Hispanic White | 17,433 (68.0%) |
| Hispanic | 4,417 (17.2%) |
| Black | 1,979 (7.7%) |
| Asian/Native Hawaiian | 1,163 (4.5%) |
| Others | 643 (2.5%) |
| Age | |
| 35–54 | 1,801 (7.0%) |
| 55–60 | 2,544 (9.9%) |
| 61–65 | 3,140 (12.3%) |
| 66–70 | 3,969 (15.5%) |
| 71–75 | 4,163 (16.2%) |
| 76–80 | 4,329 (16.9%) |
| > 80 | 5,689 (22.2%) |
| Total n = 25,635 |
Table II.
Demographic and baseline comorbid characteristics of patients who underwent endovascular peripheral arterial disease interventions in California hospitals during 2005–2009, by race/ethnicity
| Non-Hispanic White |
Black | Hispanic | Total Cohort | |
|---|---|---|---|---|
| Mean age, years (median, range) | 71.8 (73,35–105) | 69.4 (70,36–103) | 70.7 (72,35–100) | 71.5 (72, 35–105) |
| Diagnosis | ||||
| Critical Limb Ischemia | 4,665 (26.8%) | 810 (40.9%) | 2,063 (46.7%) | 8,220 (32.1%) |
| Gangrene | 1,471 (8.4%) | 413 (20.9%) | 1,021 (23.1%) | 3,212 (12.5%) |
| Ulceration | 2,044 (11.7%) | 210 (10.6%) | 650 (14.7%) | 3,135 (12.2%) |
| Rest pain | 1,150 (6.6%) | 187 (9.4%) | 392 (8.9%) | 1,873 (7.3%) |
| Claudication | 6,784 (38.9%) | 612 (30.9%) | 1,075‡ (24.3%) | 9,040 (35.3%) |
| Aortic atherosclerosis / Misc | 5,984 (34.3%) | 557 (28.1%) | 1,279‡ (29.0%) | 8,375 (32.7%) |
| Comorbidities | ||||
| Diabetes Mellitus | 4,746 (27.2%) | 853 (43.1%) | 2,565 (58.1%) | 9,057 (35.3%) |
| Renal failure | 1,690 (9.7%) | 417 (21.1%) | 1,093 (24.7%) | 3,607 (14.1%) |
| Congestive heart failure | 1,228 (7.0%) | 210 (10.6%) | 401 (9.1%) | 2,009 (7.8%) |
| Hypertension | 10,431 (59.8%) | 1,489 (75.2%) | 3,128 (70.8%) | 16,307 (63.6%) |
| Insurance Status | ||||
| County / Other Indigent Programs | 147 (0.8%) | 17 (0.9%) | 45 (1.0%) | 218 (0.9%) |
| Medicare | 11,010 (63.2%) | 1,298 (65.6%) | 3,003 (68.0%) | 16,533 (64.5%) |
| Medi-Cal | 604 (3.5%) | 194 (9.8%) | 430 (9.7%) | 1,378 (5.4%) |
| Private | 5,386 (30.9%) | 443 (22.4%) | 887 (20.1%) | 7,107 (27.7%) |
| Workers Comp / Other Government / Other | 285 (1.6%) | 27 (1.4%) | 51 (1.2%) | 397 (1.5%) |
In regards to the severity of PAD, a total of 8,220 (32.1%) patients presented with critical limb ischemia (CLI) defined by rest pain, ulceration, or gangrene, as presented in table 2. Claudication was the primary or secondary diagnosis in 9,040 (35.3%) patients. The remaining 8,375 (32.7%) patients were diagnosed with aortic or miscellaneous atherosclerosis. The distribution of PAD severity diagnosis was significant among the three groups (P<0.0001). Among blacks and Hispanics, more patients presented with CLI (40.9% and 46.7% respectively) compared to NH Whites (26.7%). More NH white claudicants were treated with endovascular interventions (38.9%) compared to blacks (30.9%) and Hispanics (35.5%).
A higher proportion of black and Hispanic patients had comorbidities including DM, renal failure, congestive heart failure, and hypertension when compared to NH white patients (P < 0.0001 for comparisons among the three groups) as presented in Table2. There was a statistically significant difference in distribution of insurance status among the three race groups (P < 0.0001; Table 2). Furthermore, there was a statistically significant difference in distribution of insurance status between the black and NH white groups and between the Hispanic and NH white groups (P < 0.0001; Table 2). More blacks and Hispanics were insured by Medicare, Medi-Cal or County insurance / indigent programs than NH white patients. Conversely, more NH whites had private insurance compared to blacks or Hispanics.
Perioperative Outcomes
Myocardial infarction during admission or within 30 days of endovascular procedures occurred in 0.6% of NH whites compared to 0.7% of blacks and 0.8% of Hispanics. This was not statistically significantly different among the three groups (P = 0.23 without adjusting for any confounders). It remained statistically non-significant after adjusting for confounders: age, gender, insurance status, comorbidities, severity of PAD, and history of coronary artery angioplasty or bypass surgery, or history of carotid endarterectomy (P = 0.56 Table 3).
Table III.
Perioperative outcomes of patients who underwent endovascular peripheral arterial disease interventions in California hospitals during 2005–2009 within 30 days, by race/ethnicity
| Non-Hispanic White |
Black | Hispanic | Total Cohort | |
|---|---|---|---|---|
| Myocardial infarction within 30 days | 102 (0.6%) | 13 (0.7%) | 36 (0.8%) | 178 (0.7%) |
| Major amputation within 30 days | 294 (1.7%) | 123 (6.2%) | 236 (5.3%) | 715 (2.8%) |
| All-cause mortality within 30 days | 282 (1.6%) | 22 (1.1%) | 89 (2.0%) | 427 (1.7%) |
As demonstrated in Table 3, there were 123 black (6.2%), 236 Hispanic (5.3%), and 294 NH white (1.7%) patients who underwent major amputation within 30 days of admission. After adjusting for confounders: age, gender, insurance status, comorbidities, severity of PAD, and history of coronary artery angioplasty or bypass surgery or carotid endarterectomy, the differences in the distribution of major amputation within 30 days of admission were statistically significant (P < 0.0001; Table3). The odds ratio (OR) for major amputation within 30 days of admission for endovascular procedures was 1.50 in Hispanics (95% CI 1.23–1.83; P < 0.0001) and 1.99 in blacks (95% CI 1.56–2.55; P < 0.0001) compared to NH whites.
Finally, we found that the death distribution during the 30-day perioperative period among the racial/ethnic groups was statistically significantly different after adjusting for the aforementioned confounders: NH whites (1.6%), blacks (1.1%) and Hispanics (2.0%, P = 0.001; Table 3). The OR for death during the 30-day perioperative period was 0.86 in Hispanics (95% CI 0.66–1.13; P = 0.28) and 0.43 in blacks (95% CI 0.27–0.67; P = 0.0002) compared to NH whites.
Longitudinal Outcomes: Reintervention
Overall, 8,593 (33.5%) patients required at least one lower extremity arterial reintervention within 365 days. This included 5,729 NH white (32.9%), 725 black (36.6%), and 1,591 Hispanic (36.0%) patients (Table 4). After adjusting for confounders: age, gender, insurance status, comorbidities, severity of PAD, and history of coronary artery angioplasty or bypass surgery, or carotid endarterectomy, there was a statistically significant difference in the proportion of patients requiring reintervention among the three racial/ethnic groups (P = 0.002; Table 4). The OR for reintervention was 1.08 in Hispanics (95% CI 1.00–1.16; P = 0.04) and 1.17 in blacks (95% CI 1.06–1.30; P = 0.002) compared to NH whites following endovascular procedures.
Table IV.
Longitudinal outcomes of patients who underwent endovascular peripheral arterial disease interventions in California hospitals during 2005–2009 at one year, by race/ethnicity
| Non-Hispanic White |
Black | Hispanic | Total Cohort | |
|---|---|---|---|---|
| Total Number of patients requiring a reintervention within 365 days |
5,729 (32.9%) | 725 (36.6%) | 1,591 (36.0%) | 8,593 |
| Open or combined open and endovascular reinterventions |
1,817 (31.7%) | 189 (26.1%) | 389 (25.0%) | 2,551 (29.7%) |
| Endovascular reintervention | 3,912 (68.3%) | 536 (73.9%) | 1,193 (75.0%) | 6,042 (70.3%) |
| Major Amputation within 365 days | 720 (4.1%) | 237 (13.8%) | 623 (14.1%) | 1,717 (6.7%) |
| All-cause mortality within 365 days | 1,702 (9.8%) | 248 (12.5%) | 569 (12.9%) | 2,728 (10.6%) |
There was no statistically significant difference in the time to reintervention within 365 days of admission among the NH white, black, and Hispanic patients after adjusting for confounders (P = 0.07). Among the 8,593 reinterventions required within 365 days, 2,551 (29.7%) were performed open or with a combination of open and endovascular technique, while 6,042 (70.3%) were performed endovascularly (Table 4). The distribution of reinterventions that were performed endovascularly among the three racial/ethnic groups: black (73.9%), Hispanic (75.0%), and NH white patients (68.3%) were statistically significantly different after adjusting for confounders (P = 0.0003; Table 4). The OR for a reintervention performed endovascularly was 1.29 in Hispanics (95% CI 1.13–1.48; P = 0.0002) and 1.22 in blacks (95% CI 1.02–1.47; P = 0.03) compared to NH whites. This trend was also seen in the open group with the OR for reintervention in the open group being 1.16 (95% CI 1.047–1.29, P=0.035) in Hispanics vs NH whites and 1.06 (95% CI 0.939–1.208, P=0.035) in blacks vs NH whites.
Longitudinal Outcomes: Amputation and Mortality
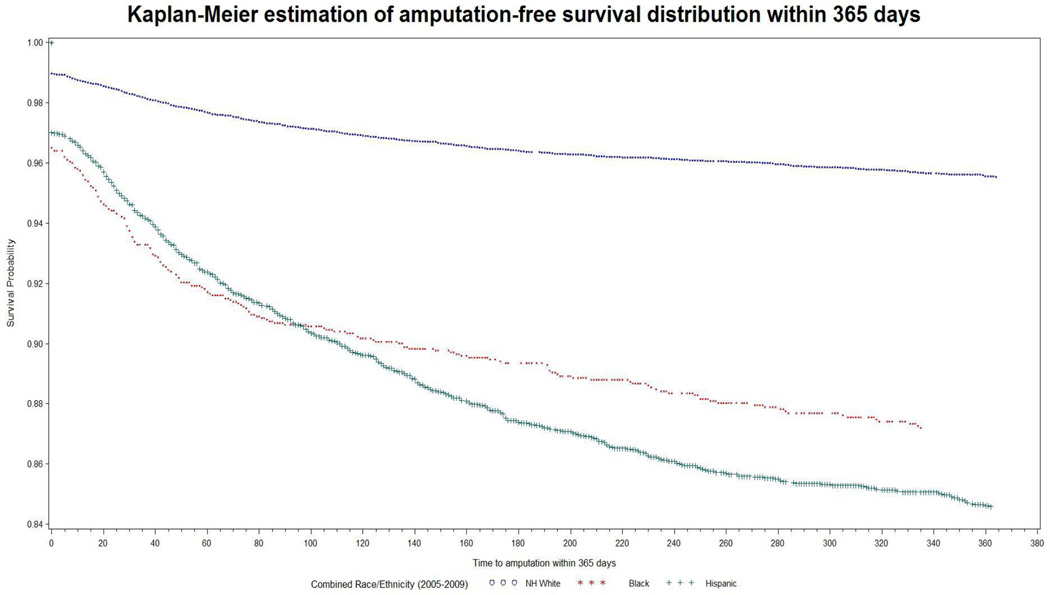
There were 237 black (13.8%), 623 Hispanic (14.1%), and 720 NH white (4.1%) patients who underwent major amputation within 365 days of admission (Table 4). After adjusting for the confounders the differences in the distribution of major amputation within 365 days of admission for endovascular procedures among the three racial/ethnic groups were statistically significant (P < 0.0001; Table 4). The OR for major amputation within 365 days of admission for endovascular procedures was 1.89 in Hispanics (95% CI 1.66–2.16; P < 0.0001) and 1.85 in blacks (95% CI 1.54–2.12; P < 0.0001) compared to NH whites. There was a statistically significant difference in the amputation-free survival within 365 days of admission among the NH white, black, and Hispanic groups after adjusting for confounders (P < 0.0001, Figure 1). Interestingly, we found similar findings following open procedures as well. The HR for amputation-free survival following open procedures was 1.41 (95% CI 1.24–1.601, P <0.0001) for Hispanics vs NH whites and 1.62 (95% CI 1.41–1.86, P <0.0001) for blacks vs NH whites.
Figure 1.
Amputation-free survival, by race/ethnicity
There were 248 black (12.5%), 569 Hispanic (12.9%), and 1,702 NH white (9.8%) patients who died within 365 days of admission, which was not statistically significant after adjusting for confounders (P =0.12). There was no statistically significant difference in the time to death within 365 days of admission among the NH white, black, and Hispanic groups after adjusting for confounders (P = 0.2).
Discussion
The primary goal of PAD treatment is to alleviate lower extremity symptoms of pain, improve wound healing, and prevent limb loss. Over the last decade the number of endovascular procedures have surpassed the number of open revascularization procedures. In our own cohort, we found that whereas endovascular procedures made up 53.2% of all procedures in 2005, by 2009, 65.9% of all procedures were performed via an endovascular approach. Even as an increasing trend in endovascular procedures and an “endovascular first” approach have decreased the number of major amputations (2), primary amputation is still more likely to be performed in patients with PAD who are non-white, have low income, and have no health insurance (4). Blacks are more likely to undergo lower extremity amputation and are less likely to undergo arterial revascularization for limb salvage compared to whites (10, 14, 15). An evaluation of Medicare claims data on 24,000 blacks and 65,881 NH white amputees found black amputees were less likely than NH whites to have undergone revascularization procedures, have limb related admissions, or wound debridement prior to the amputation (5). Furthermore, Hispanics have been shown to be almost 1.5 times more likely to undergo an amputation or die following lower extremity bypass procedures compared to whites (6). Therefore, race/ethnicity has remained an important correlate of PAD treatment outcomes. Many PAD intervention studies have addressed underutilization of open surgical procedures for non-white patients with PAD. Few studies have primarily addressed endovascular therapy or its long term outcomes based on race/ethnicity.
In this study, we used an administrative database that allows all non-federal hospital admissions (in-patient procedures and outpatient or ambulatory procedures) to be captured and the patients tracked longitudinally though the state of California. This is unique as most national databases only capture inpatient admissions and procedures. Nearly half of all the endovascular procedures between 2005 and 2009 were performed in outpatient facilities in California.
We demonstrated that even after adjusting for confounders including age, gender, comorbidities, severity of PAD, and insurance status, blacks and Hispanics have worse short and long-term outcomes following endovascular interventions compared to NH whites. In our cohort, blacks and Hispanics presented more commonly with CLI than NH whites. This has been previously demonstrated by other investigators (9, 16). We found that Hispanics were 1.5 times and blacks were almost twice as likely as NH whites to undergo major amputation in the 30-day perioperative period following endovascular treatment. Furthermore, blacks and Hispanics were more likely to die in the 30-day perioperative period following endovascular procedures compared to NH whites.
Even after adjusting for age, gender, severity of PAD, comorbidities, and insurance status, blacks and Hispanics still had worse amputation-fee survival than NH whites among all patients who underwent an endovascular PAD intervention. However, blacks and Hispanics were found to be more likely to undergo reintervention following endovascular procedures than whites. Furthermore, NH whites were more likely to have an open procedure than an endovascular procedure for the reintervention than blacks or Hispanics. These findings have been infrequently addressed in prior studies.
Of the factors commonly attributed to disparities in outcomes based on race/ethnicity, access to care and proper follow up, patient non-compliance, suboptimal cardiovascular risk factor modifications, and physician bias are frequently sited (17). Based on the demonstration of under-utilization of lower extremity arterial revascularization procedures in non-white patients, one would expect the reintervention rates to be lower in this population as well. However, we found that blacks and Hispanics were more likely to undergo the first reintervention within twelve months of endovascular revascularization than NH whites. This may be secondary to poorer access to follow-up care to undergo surveillance Duplex ultrasound imaging and interventions to prevent failure of treatment or lower use of anti-platelet and lipid lowering therapy. Ethnic/racial disparities in the use of antiplatelet and statin therapy have been demonstrated among US adults who have significant cardiovascular disease or at risk for cardiovascular morbidity (18, 19). Unfortunately, large population databases using ICD-9-CM codes for diagnoses and procedures do not have data on imaging studies and medication use.
Another rationalization for the difference in outcomes of PAD based on race/ethnicity has focused on possible genetic differences in the behavior of PAD. This is difficult to demonstrate as there are few studies that have addressed this possible cause. Racial variations in the inflammatory markers, presence of metabolic syndrome, and endothelial dysfunction have all been demonstrated (20), but whether these are clinically significant differences to account for outcome differences are not completely understood and need further investigation.
Several studies have demonstrated lower rates of insurance coverage among blacks and Hispanics being a significant factor in treatments received. Amaranto et al. found that Caucasian race was an independent predictor of intervention (11). OSHPD has the ability to identify the insurance type for each patient, including Medicare, Medi-Cal, County, Private, and Workers Compensation/Government. We found that even after adjusting for the type of insurance a patient had, our data still identified a racial/ethnic disparity in outcomes. Factors such as physician bias are difficult to study in large data-bases but certainly hospital based factors such as volume and geographic location have been studied in relation to lower extremity interventions and amputations (21) (1). Recently, Durazzo et al. demonstrated that blacks with PAD have a greater odds for amputation compared to whites but that this disparity was even exaggerated in the circumstances where hospital revascularization capacity were the greatest (17).
It appears that there may be differences in arterial disease patterns among different races/ethnicities. Selvarajah and colleagues used data from the National Surgical Quality Improvement Program (NSQIP) to demonstrate that blacks and Hispanics undergo more bypass procedures involving tibial vessels compared to whites (9). In our own database of AS or outpatient procedures, which are defined by CPT codes, Hispanics had almost a third of all infragenicular interventions (angioplasty or atherectomy) compared to the other groups even though they only made up 5.28% of the total number of patients undergoing outpatient procedures (unpublished data). Determining the impact and/or contribution of biological mechanisms such as chronic inflammation and endothelial dysfunction on PAD level of disease and outcomes of PAD based on race/ethnicity is an area of importance and ongoing research (20).
There are several limitations to this study. Using administrative data for evaluation of treatment outcomes has inherent biases and limitations. As the OSHPD database is generated by coding and billing information, there are errors in how diagnoses are coded. Furthermore, data can be incomplete or missing. Administrative data do not provide information on laboratory values, medication use, imaging studies, or clinic visits. Finally, OSHPD does not include data from Veterans Administration hospitals, which historically perform 5–10% of all bypass procedures in men (22).
Conclusion
In this study we have demonstrated that even after adjusting for age, gender, comorbidities, PAD severity, and insurance status, there are still differences in the short and long-term outcomes of endovascular interventions based on race/ethnicity with blacks and Hispanics having worse amputation rates and survival rates than NH whites. Additional research is needed to understand factors that influence poor outcomes and higher reintervention rates in blacks and Hispanics compared to NH whites. These factors may be based on differences in biology, medical management, patient selection bias, or physician bias. Understanding the contribution of these factors may help bridge the disparity gap in outcomes of PAD intervention.
Acknowledgments
This project was supported by a comparative effectiveness research award from the Center for Healthcare Policy and Research at the University of California Davis Medical Center and the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health (NIH)- grant #UL1 TR000002.
Appendix 1
ICD-9-CM codes for Primary/secondary diagnosis of atherosclerosis of the lower extremities:
| 440.20 | Atherosclerosis of native arteries of the extremities, not specified |
| 440.21 | Atherosclerosis of native arteries of the extremities with intermittent claudication |
| 440.22 | Atherosclerosis of native arteries of the extremities with rest pain |
| 440.23 | Atherosclerosis of native arteries of the extremities with ulceration |
| 440.24 | Atherosclerosis of native arteries of the extremities with gangrene |
| 440.29 | Other atherosclerosis of native arteries of the extremities |
ICD-9-CM codes for endovascular procedures:
| 39.50 | Percutaneous transluminal angioplasty (PTA) of non-coronary vessels |
| 39.90 | Insertion of non-drug-eluting peripheral (non-coronary) vessel stent(s) |
| 00.55 | Insertion of drug-eluting peripheral stent (s) of other peripheral vessels(s) |
ICD-9-CM codes for open procedures:
| 39.29 | Other (peripheral) vascular shunt or bypass |
| 38.08 | Incision of vessel, embolectomy, thrombectomy |
| 38.18 | Endarterectomy |
| 38.38 | Resection of vessel with anastomosis |
| 38.48 | Resection of vessel with replacement |
CPT codes for outpatient percutaneous procedures:
| 35492 | Transluminal peripheral atherectomy, percutaneous; iliac |
| 35493 | Transluminal peripheral atherectomy, percutaneous; femoral-popliteal |
| 35495 | Transluminal peripheral atherectomy, percutaneous; tibioperoneal trunk and branches |
| 35473 | Transluminal balloon angioplasty,percutaneous; iliac |
| 35474 | Transluminal balloon angioplasty,percutaneous; femoral-popliteal |
| 35470 | Transluminal balloon angioplasty,percutaneous; tibioperoneal trunk and branches |
| 37205 | Transcatheter placement,intravascular stent, percutaneous; initial vessel |
| 37206 | Transcatheter placement,intravascular stent, percutaneous; additional vessel |
| 37220 | Revascularization, endovascular, open or percutaneous, iliac artery, unilateral, initial vessel; with transluminal angioplasty |
| 37221 | with transluminal stent placement |
| 37222 | each additional ipsilateral iliac vessel; with transluminal angioplasty |
| 37223 | with transluminal stent placement(s) |
| 37224 | Revascularization, endovascular, open or percutaneous, femoral/popliteal artery(s), unilateral; with transluminal angioplasty |
| 37225 | with atherectomy |
| 37226 | with transluminal stent placement |
| 37227 | with transluminal stent placement and atherectomy |
| 37228 | Revascularization, endovascular, open or percutaneous, tibial/peroneal artery, unilateral, initial vessel; with transluminal angioplasty |
| 37229 | with atherectomy |
| 37230 | with transluminal stent placement |
| 37231 | with transluminal stent placement and atherectomy |
| 37232 | Revascularization, endovascular, open or percutaneous, tibial/peroneal artery, unilateral, each additional vessel; with transluminal angioplasty |
| 37233 | with atherectomy |
| 37234 | with transluminal stent placement |
| 37235 | with transluminal stent placement and atherectomy |
ICD-9-CM codes for major amputations:
| 84.10 | Lower limb amputation, not otherwise specified |
| 84.15 | Amputation below knee |
| 84.16 | Disarticulation of knee |
| 84.17 | Amputation above knee |
| 84.18 | Disarticulation of hip |
| 84.19 | Abdominopelvic amputation |
References
- 1.Goodney PP, Beck AW, Nagle J, Welch HG, Zwolak RM. National trends in lower extremity bypass surgery, endovascular interventions, and major amputations. J Vasc Surg. 2009;50(1):54–60. doi: 10.1016/j.jvs.2009.01.035. [DOI] [PubMed] [Google Scholar]
- 2.Jones WS, Patel MR, Dai D, Subherwal S, Stafford J, Calhoun S, et al. Temporal trends and geographic variation of lower-extremity amputation in patients with peripheral artery disease: results from u.s. Medicare 2000–2008. J Am Coll Cardiol. 2012;60(21):2230–2236. doi: 10.1016/j.jacc.2012.08.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rowe VL, Weaver FA, Lane JS, Etzioni DA. Racial and ethnic differences in patterns of treatment for acute peripheral arterial disease in the United States, 1998–2006. J Vasc Surg. 51(4 Suppl):21S–26S. doi: 10.1016/j.jvs.2009.09.066. [DOI] [PubMed] [Google Scholar]
- 4.Eslami MH, Zayaruzny M, Fitzgerald GA. The adverse effects of race, insurance status, and low income on the rate of amputation in patients presenting with lower extremity ischemia. J Vasc Surg. 2007;45(1):55–59. doi: 10.1016/j.jvs.2006.09.044. [DOI] [PubMed] [Google Scholar]
- 5.Holman KH, Henke PK, Dimick JB, Birkmeyer JD. Racial disparities in the use of revascularization before leg amputation in Medicare patients. J Vasc Surg. 54(2):420–426. e1. doi: 10.1016/j.jvs.2011.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oh-Park M, McGinn A, Lipsitz E, Thomas M, Zonszein J. Racial disparity in amputation-free survival after infrainguinal bypass procedure: contribution of socioeconomic status. Am J Phys Med Rehabil. 2009;88(12):986–994. doi: 10.1097/PHM.0b013e3181bc0ae4. [DOI] [PubMed] [Google Scholar]
- 7.Allison MA, Ho E, Denenberg JO, Langer RD, Newman AB, Fabsitz RR, et al. Ethnic-specific prevalence of peripheral arterial disease in the United States. Am J Prev Med. 2007;32(4):328–333. doi: 10.1016/j.amepre.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 8.Sidawy AN, et al. Race as a risk factor in the severity of infragenicular occlusive disease: Study of an urban hospital patient population. J Vasc Surg. 1990;11:536–543. [PubMed] [Google Scholar]
- 9.Selvarajah S, Black JH, 3rd, Haider AH, Abularrage CJ. Racial disparity in early graft failure after infrainguinal bypass. The Journal of surgical research. 2014;190(1):335–343. doi: 10.1016/j.jss.2014.04.029. [DOI] [PubMed] [Google Scholar]
- 10.Huber TS, Wang JG, Wheeler KG, Cuddeback JK, Dame DA, Ozaki CK, et al. Impact of race on the treatment for peripheral arterial occlusive disease. J Vasc Surg. 1999;30(3):417–425. doi: 10.1016/s0741-5214(99)70068-6. [DOI] [PubMed] [Google Scholar]
- 11.Amaranto DJ, Abbas F, Krantz S, Pearce WH, Wang E, Kibbe MR. An evaluation of gender and racial disparity in the decision to treat surgically arterial disease. J Vasc Surg. 2009;50(6):1340–1347. doi: 10.1016/j.jvs.2009.07.089. [DOI] [PubMed] [Google Scholar]
- 12.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity Measures for Use with Administrative Data. Medical Care. 1998;36(1):8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Cox D. Regression Models and Life-Tables. Journal of the Royal Statistical Society. 1972;Series B 34(2):187–220. [Google Scholar]
- 14.Feinglass J, Kaushik S, Handel D, Kosifas A, Martin GJ, Pearce WH. Peripheral bypass surgery and amputation: northern Illinois demographics, 1993 to 1997. Arch Surg. 2000;135(1):75–80. doi: 10.1001/archsurg.135.1.75. [DOI] [PubMed] [Google Scholar]
- 15.Rucker-Whitaker C, Feinglass J, Pearce WH. Explaining racial variation in lower extremity amputation: a 5-year retrospective claims data and medical record review at an urban teaching hospital. Arch Surg. 2003;138(12):1347–1351. doi: 10.1001/archsurg.138.12.1347. [DOI] [PubMed] [Google Scholar]
- 16.Chong TJ, Rowe VL, Weaver FA, Katz SG. Impact of race on infrainguinal angioplasty and stenting. Annals of vascular surgery. 2011;25(2):204–209. doi: 10.1016/j.avsg.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 17.Durazzo TS, Frencher S, Gusberg R. Influence of Race on the Management of Lower Extremity Ischemia: Revascularization vs Amputation. JAMA surgery. 2013:1–6. doi: 10.1001/jamasurg.2013.1436. [DOI] [PubMed] [Google Scholar]
- 18.Sanchez DR, Diez Roux AV, Michos ED, Blumenthal RS, Schreiner PJ, Burke GL, et al. Comparison of the racial/ethnic prevalence of regular aspirin use for the primary prevention of coronary heart disease from the multi-ethnic study of atherosclerosis. The American journal of cardiology. 2011;107(1):41–46. doi: 10.1016/j.amjcard.2010.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qato DM, Lindau ST, Conti RM, Schumm LP, Alexander GC. Racial and ethnic disparities in cardiovascular medication use among older adults in the United States. Pharmacoepidem Dr S. 2010;19(8):834–842. doi: 10.1002/pds.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nguyen LL, Henry AJ. Disparities in vascular surgery: is it biology or environment? Journal of vascular surgery. 2010;51(4 Suppl):36S–41S. doi: 10.1016/j.jvs.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goodney PP, Holman K, Henke PK, Travis LL, Dimick JB, Stukel TA, et al. Regional intensity of vascular care and lower extremity amputation rates. J Vasc Surg. 2013;57(6):1471–1479. 80, e1–e3. doi: 10.1016/j.jvs.2012.11.068. discussion 9-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feinglass J, Sohn MW, Rodriguez H, Martin GJ, Pearce WH. Perioperative outcomes and amputation-free survival after lower extremity bypass surgery in California hospitals, 1996–1999, with follow-up through 2004. J Vasc Surg. 2009 doi: 10.1016/j.jvs.2009.05.050. [DOI] [PubMed] [Google Scholar]