Abstract
Metabolism is the set of biochemical reactions that allows cells to acquire and utilize nutrients needed to sustain life. Accessing nutrients and meeting metabolic demands are necessary for cells to survive, proliferate, and to properly perform their intended functions. During an immune response leukocytes undergo major changes in growth and function that are tightly coupled to dynamic shifts in metabolic processes. Immunometabolism is an emerging field that investigates the interplay between immunological and metabolic processes. Interest in this field stems from the realization that incorrect metabolic remodeling underlies many aberrant immune responses, and that manipulating cellular metabolism can beneficially enhance or temper immunity.
Graphical abstract
Introduction
T cells are crucial players in the immune response to cancer and infection, and the regulation of nutrient uptake and utilization in these cells is critically important for controlling their cell number and function 1. While manipulation of metabolic pathways in T cells can alter their function and longevity 2,3, the significance of why T cells remodel their metabolism in different settings is not fully understood. Although cell-based immunotherapy is in clinical trials and seen as an emerging area for next-generation therapy, reliably targeting T cell metabolism for disease treatment is still in the early stages of pre-clinical testing. Here we comment on up-to-date findings about the relationship between metabolism and T cell function and longevity. Further we discuss potential approaches and applications in which T cells might be manipulated by reprogramming metabolic pathways for therapeutic purposes.
Basic metabolism in a nutshell
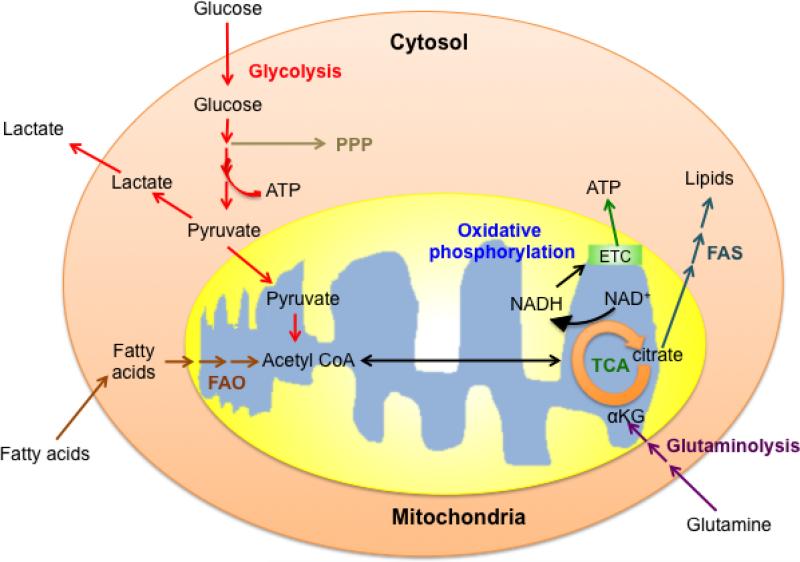
Adenosine triphosphate (ATP) transports chemical energy within cells and is generated by two major processes: Glycolysis and oxidative phosphorylation (OXPHOS). Depending on the needs of a cell, metabolism can be weighted toward either anabolic (construction of molecules required for growth and biosynthesis) or catabolic (breakdown of macromolecules into smaller units for energy production or use in anabolic pathways) reactions 1,3. A balance of these processes allows cells to maintain energy homeostasis. Glucose is one of the main nutrients from which cells extract energy. The glycolysis pathway converts glucose to pyruvate via a series of intermediate metabolites that can enter other pathways, such as the pentose phosphate pathway, and contribute to biosynthesis and cell growth. Glucose-derived pyruvate can be converted to acetyl-CoA in the mitochondria and enter the tricarboxylic acid (TCA) cycle, or to lactate in the cytoplasm, which is excreted from the cell. In addition to the generation of ATP and biosynthetic precursors, glycolysis also helps to maintain NAD+/NADH redox balance, which reflects the metabolic health of a cell. Other major substrates include amino acids such as glutamine, which is metabolized via glutaminolysis, and lipids, which are burned via fatty acid oxidation (FAO). The intermediates produced by these processes also enter the TCA cycle. Coenzymes NADH and FADH2 are generated by the oxidation of substrates in the TCA cycle, and donate electrons to the electron transport chain (ETC) for OXPHOS. OXPHOS is highly efficient, and compared to glycolysis alone, is able to produce more than 10 times the ATP per molecule of glucose. In addition to generating reducing equivalents for OXPHOS, the TCA cycle provides substrates for biosynthesis, such as citrate, which can be exported to the cytosol for fatty acid synthesis (FAS) (Fig. 1). A cell must balance its nutrient utilization and metabolism to meet demands for energy, biosynthesis, and redox balance.
Figure 1. Basic metabolic pathways in a T cell.
Glucose, glutamine, and fatty acids are main nutrients that support T cell bioenergetics and biosynthesis. Cells use nutrients to generate ATP via glycolysis (in the cytosol) or via oxidative phosphorylation (in the mitochondria). The intermediates generated in the glycolysis pathway and the tricarboxylic acid (TCA) cycle also serve as substrates for biosynthesis. FAO (fatty acid oxidation); FAS (fatty acid synthesis); PPP (pentose phosphate pathway).
Supply and demand - adjusting metabolism to T cell function
Compared to activated T cells, circulating naïve T cells are quiescent, have low metabolic demands, and predominantly use OXPHOS to generate ATP. Upon T-cell receptor (TCR)-mediated recognition of antigen and costimulatory signals, T cells become activated and adopt an anabolic metabolism. Nutrients are no longer used solely for survival and homeostasis, but also for the generation of building blocks for clonal expansion and for effector functions, such as the secretion of cytokines and cytolytic molecules important for fighting tumors and pathogens. After a tumor is controlled or an infection cleared, a small subset of long-lived memory T cells will persist and provide long-term protective immunity. Unlike effector T cells, memory T cells do not rapidly proliferate and thus do not require high rates of anabolic metabolism. Instead, they generate energy to support self-renewal 1,3. During each phase of a T cell's life, metabolism is tuned to match its function and fate.
T cell activation and effector function
Upon T cell activation, signals from the TCR, costimulatory molecules, and growth factor cytokines lead to the activation of signaling pathways that promote transcriptional programs important for effector functions. These signals also lead to the activation of mechanistic target of rapamycin (mTOR), which mediates the induction of glycolysis via multiple pathways to support cell growth, proliferation, and function 4. The activation of mTOR induces glucose transporter-1 (Glut1) 5, and transgenic expression of Glut1 enhances T cell proliferation and cytokine production 6. While it has been known for some time that increased glycolysis and glucose uptake are associated with the augmented effector functions that occur after T cell activation 7, we recently demonstrated that optimal IFN-γ production in T cells is also posttranscriptionally regulated by the glycolysis pathway via the bi-functional metabolic enzyme/ RNA binding protein glyceraldehyde 3-phosphate dehydrogenase (GAPDH) 8. When cells engage glycolysis to a high degree, GAPDH is occupied by its metabolic function. When T cells are unable to use glycolysis, GAPDH becomes disengaged from its metabolic function, and binds to IFN-γ mRNA and prevents its efficient translation. These data demonstrate that aside from providing fuel and biosynthetic precursors, metabolic pathways are able to modulate immune cell effector function by directly influencing the translation of specific mRNAs.
Activation of mTOR and engagement of glycolysis lead to the expression of downstream transcriptional regulators such as Hypoxia inducible factor-1α (Hif-1α), c-Myc, and estrogen-related receptor α (ERRα). These factors regulate metabolism in T cells and activate pathways involved in rapid cell proliferation and effector function 9. The HIF-1α–dependent transcriptional program mediates glycolysis in T cells, and is thought to favor the development of T helper 17 (Th17) cells while dampening regulatory T (Treg) cells 10. Immediately after activation, c-Myc induces the expression of glycolysis and glutaminolysis enzymes, the products of which contribute to the synthesis of lipids, amino acids, and nucleic acids for cell expansion 11. ERRα is known to promote expression of genes involved in mitochondrial biogenesis, fatty acid metabolism, and OXPHOS in metabolic tissues, such as muscle and adipose 12. ERRα is also an important metabolic regulator of effector CD4+ T-cell homeostasis and function, broadly affecting metabolic gene expression and glucose metabolism 13. In addition, interferon regulatory factor 4 (IRF4) is known to be a key regulator for the differentiation and function of effector T cells 14. IRF4 regulates the expression of key molecules required for glycolysis and ‘translates’ TCR affinity into the appropriate transcriptional programs that link metabolic function with the clonal selection and effector differentiation of T cells 15. Overall, a broad spectrum of signaling pathways and transcriptional target genes are involved in metabolism and metabolic transitions that affect T cell function.
Availability of nutrients also greatly influences T cells. T cells rely on glucose for cytolytic activity and cytokine production, and in glucose-limiting conditions these functions are impaired.7,8,16. Extending this idea, two recent reports show that reduced availability of glucose within the tumor microenvironment dampens anti-tumor responses. We found that CD8+ T cells isolated from antigenic tumors with genetically enhanced rates of glycolysis have decreased IFN-γ expression and mTOR target activation in comparison to those T cells in control tumors, which was attributed to low concentrations of extracellular glucose in the tumor milieu 17. Control tumors and the tumors with enhanced glycolysis express the same major tumor rejection antigen, illustrating that even in situations where the immune system can recognize the cancer, that nutrient depletion, as a distinct mechanism, can dampen T cell effector function. Another study shows that in a glucose-limiting microenvironment, insufficient phosphoenolpyruvate (PEP), a glycolytic metabolite, promotes calcium re-uptake to the sarco/endoplasmic reticulum, and this leads to dampened anti-tumor T cell function 18. T cells with genetically increased PEP production had enhanced effector functions and restricted tumor growth in mice. Together these data suggest that substrate concentration in a local microenvironment can have a marked impact on immune cell function, and that altering tumor and/or T cell metabolism may effectively change nutrient availability and permit T cells to function more effectively in a particular niche.
AMP-activated protein kinase (AMPK) is an important metabolic sensor in T cells. AMPK phosphorylates the mTOR pathway components tuberous schlerosis complex 2 (TSC2) and Raptor, and generally leads to a reduction of mTOR activity 4. Accordingly, AMPKα-deficient CD8+ T cells have higher glycolytic activity in vitro 16. One recent study shows that AMPK also contributes to energy plasticity by promoting oxidative metabolism during metabolic stress due to low glucose conditions, and as such it is needed for durable effector T cell responses in vivo 19. Other studies have shown that AMPK can be dispensable for proliferation and effector function of CD8+ T cells in vivo 20, 34. These contrasting findings likely reflect the fact that even subtle differences in nutrient conditions, mTOR activity, and activation state of the cells can have profound effects on the role of AMPK in metabolic adaptations in T cells in vivo.
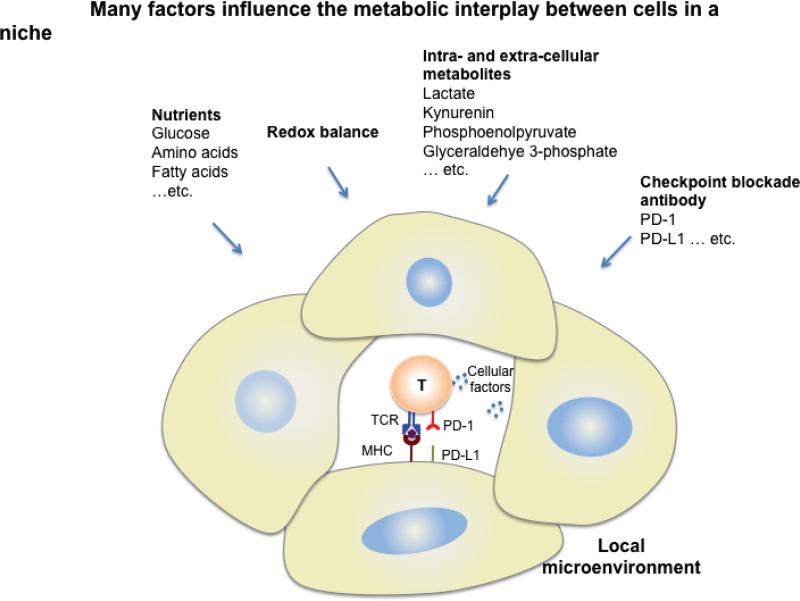
T cells not only rely on glucose, but also depend on amino acids for survival and function. Depletion of glutamine in culture medium blocks T cell proliferation and cytokine production 21, and it is likely that glutamine competition in the tumor microenvironment also influences their anti-tumor function. In T cells, branched chain aminotransferase (BCAT) negatively regulates mTOR and glycolysis. CD4+ T cells from cytosolic BCAT-deficient mice, exhibit lower leucine transamination and higher intracellular leucine concentrations, leading to mTOR activation and higher rates of glycolysis when compared to control T cells 22. Fatty acid synthesis (FAS) also plays an important role in T cell effector function. Inhibition of the FAS enzyme acetyl-Co A carboxylase I (ACC1) enhances Treg cell differentiation and limits Th1, Th2, and Th17 development 23. Although lack of ACC1 in CD8+ T cells does not affect effector T cell development, it does confer a shorter lifespan and limits expansion, suggesting that FAS is crucial for CD8+ T cell survival 24. Overall, fluctuations in concentrations of amino acids, as well as other nutrients and metabolites in the microenvironment, can considerably alter T cell function (Fig. 2). More research is needed to determine whether a given metabolic pathway is induced in particular cell types to provide biosynthetic precursors for growth, to fuel OXPHOS for ATP production, to maintain redox balance, or for other purposes.
Figure 2. Metabolic interplay in the local microenvironment.
Competition for nutrients and metabolites, as well as other factors, between immune cells and other cells in a microenvironment can mediate T cell differentiation and function. Blockade antibodies may also influence cellular metabolism and thus change the availability of local substrates. The balance of these factors may affect T cell function, hence altering the fate of the immune response and disease development.
Several by-products of metabolism can have negative effects on T cell function. Lactate from aerobic glycolysis dampens cytolytic function in CD8+ T cells 25 and promotes IL-17 production by Th17 cells 26. The metabolite kynurenine, generated from indoleamine-2,3-dioxygenase (IDO)–mediated tryptophan catabolism, is a potent and active suppressor of CD8+ T cell proliferation and effector function 27,28, and an inducer of Treg cell generation via an aryl hydrocarbon receptor (AhR)-dependent mechanism 29,30. Thus, the decreased availability of certain amino acids and the accumulation of metabolic waste products act in concert to alter the microenvironment and influence T cell function. Questions in this area remain, and current research is focused toward understanding how changes in available nutrients and metabolites in particular niches regulate immune cell differentiation and function.
Memory T cells
A successful immune response relies not only on the ability of T cells to extensively proliferate and attain effector function, but also to form long-lived memory T cells that can respond again to future antigen encounter. There is intense interest in understanding how long-lived cellular immunity is generated. We are now beginning to define the metabolic pathways that contribute to the generation of memory T cells following acute infection.
Several years ago we showed that engagement of FAO is critical for the generation of memory T cells 31, while a concurrent study shows that mTOR signals dampen memory T cell development 32. Together these papers illustrate that metabolic reprogramming in T cell populations after the induction of an immune response regulate the stable development of long-lived memory T cells. Underlying their capacity for FAO, we later found that memory T cells maintain substantial mitochondrial spare respiratory capacity (SRC) and have increased mitochondrial mass, in comparison to naïve and activated T cells 33. It was also shown that AMPK-α1 signals, which promote FAO, support memory CD8+ T cell development 31,34. These phenotypic attributes provide a metabolic advantage to these cells, equipping them for long-term survival, and the ability to rapidly recall upon antigen challenge 35. Supporting how the ‘metabolic signature’ of enhanced SRC and mitochondrial mass that is evident in memory T cells underlies their functional and phenotypic characteristics, it was later shown that both of these parameters increase even further in secondary and tertiary memory T cells, which are known to persist in even greater numbers and have a marked capacity to rapidly recall 36.
Delving into how this unique metabolic phenotype of memory T cells is supported, we more recently found that these cells acquire extracellular glucose to synthesize the lipids that are subsequently burned for FAO in the mitochondria 37. Other recent work shows that IL-7 signals support memory CD8+ T cell longevity by promoting extracellular glycerol import, which is used to synthesize the triacylglycerols that are used to fuel FAO 2. On the face of it, this is a rather complicated scheme for the cell, because rather than directly burning extracellular fatty acids, memory T cells synthesize fatty acids for mitochondrial FAO. At present, precisely why FAO is tied to SRC and enhanced mitochondrial mass in memory T cells is not clear. While less is known about memory CD4+ T cell metabolism, Notch signaling regulates glucose uptake in these cells, which is critical for their survival 38. How this glucose uptake relates to FAO, and whether CD4+ and CD8+ memory T cells have distinct metabolic machinery to support longevity, remains to be determined.
Integrating knowledge and keeping metabolism in mind when designing therapy
The generation of robust, stable populations of antigen-specific T cells is a goal of vaccination and cell-based therapies to prevent and treat various diseases. Targeting metabolic pathways to enhance T cell function and persistence has recently become a focus for scientists. While mTOR inhibition can promote memory T cells 31,32, administration of mTOR inhibitors during cancer has varying effects, promoting effector T cells in one model 39 and inhibiting effectors T cells in another 40. While varying results are likely caused by dose, timing, and/or characteristics of different tumors, it is clear that modulating metabolism in these cells via mTOR has extremely potent effects on T cells. Looking forward, careful consideration of how various treatments alter specific cellular metabolic pathways in disease settings is needed. Molecular targets in addition to mTOR within metabolic pathways that are related to memory T cell development, such as AMPK and glycogen synthase kinase 3 (GSK-3), are all potential candidates under clinical development 41. Continued investigation into the underlying metabolic features of these cells may reveal new therapeutic targets.
Antibody blockade of inhibitory receptors is another way to target metabolism to regulate T cell function. For example, T cells receiving PD-1 signals decrease glycolysis and enhance FAO, which dampens their effector function while promoting longevity 42. These results may explain the longevity of T cells observed in some patients with chronic infections or cancer, and suggest that PD-1 blockade antibodies may reinvigorate T cell glycolysis, and hence function, in these patients. Although therapeutic compounds or antibodies that systemically target metabolic processes hold promise, the potential for off-target effects may also be high. Strategies to deliver drugs to specific cell types are needed. Some approaches, such as transporter-mediated drug uptake 43, bi-specific antibodies 44, and nanoparticle-mediated delivery 45, require further clinical evaluation.
While a lot of research has focused on the influence of metabolic signals during the generation of T cell responses, metabolic signals can also influence the phenotype of a lineage-committed T cell. There have been several studies attempting to manipulate T cell metabolism to skew cell function. For instance, dichloroacetate, an inhibitor of pyruvate dehydrogenase kinase 1 (PDK1), reduces Th17-mediated inflammation in models of inflammation bowel disease (IBD) and experimental autoimmune encephalomyelitis (EAE) by selectively limiting Th17 survival and proliferation 46. Blocking the amino acid transporter Slc7a5, which is important for the differentiation and function of Th1 and Th17 cells, with compounds such as JPH203 and 2-aminobicyclo-(2,2,1)-heptane-2-carboxylic acid (BCH), also constrains inflammatory T cells 39,47. Results such as these suggest that pharmacologically blocking immune cell metabolism to abrogate inflammation may be a therapeutic possibility in a variety of disease settings.
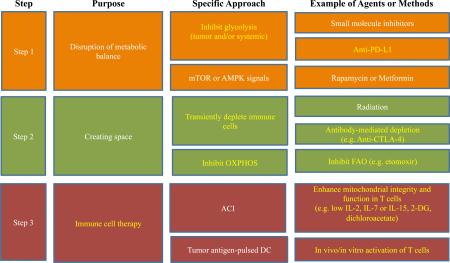
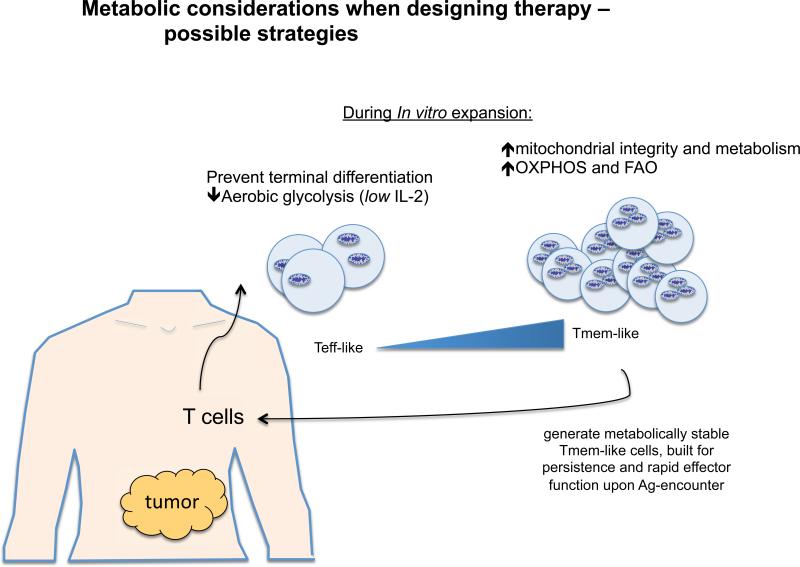
A broader understanding of the factors that control long-lived and stable T cell function is needed for improving T cell-based vaccination. Adoptive T cell immunotherapy (ACI) is an approach where T cells from a patient are expanded in vitro and then transferred back into the patient with the goal of improving their immune response to cancer or chronic viral infection 48. Cells generated for ACI are expected to maintain proliferative ability and effector function so that they sufficiently clear tumors or pathogens, as well as form stable memory T cells that can respond again in the future should the cancer or infection recur. Although we now know that in order to be effective, immune cells must undergo the correct metabolic reprogramming, relatively little research has focused on manipulating T cell metabolism to enhance vaccination or promote cell longevity after ACI. Exaggerated glycolysis in T cells can accelerate terminal differentiation, while inhibition of glycolysis leads to more stable CD8+ T cell memory development. Studies show that limiting glycolysis and promoting mitochondrial metabolism during priming allows more cells to enter the memory T cell pool, and these resulting cells maintain superior antitumor function and persistence after challenge 49,50. Since IL-2 strongly promotes glycolysis in T cells, culturing human cells destined for ACI in IL-2 may force their terminal differentiation. Furthermore, upon adoptive transfer, cells that are heavily relying on glycolysis will suffer from nutrient deprivation when entering the host, and as a result the vast majority will die because they cannot meet their energetic demands. Instead, providing signals that promote mitochondrial metabolism, such as could be achieved with IL-15 or IL-7, might better metabolically equip the cells for longevity in vivo. Given recent studies showing that there is a competition for glucose between tumors and T cells 17,18, researchers may want to carefully consider the timing of when activated T cells will enter a tumor microenvironment, and if nutrient availability to the T cells could be optimized prior to that time 3. Perhaps using inhibitors of glycolysis prior to inducing an immune response will allow newly activated immune cells to enter a tumor environment that is more nutrient replete. Although blocking glycolysis generally would presumably inhibit T cell glycolysis, it might not matter if this is administered in a step-wise fashion. Aside from creating new therapies, simply incorporating these ideas into current therapeutic strategies could yield much better results for patients (Fig. 3).
Figure 3. Targeting T cell metabolism for therapy.
The schematic shows potential strategies to design more effective therapies against cancer by altering cellular metabolism. T cells could be metabolically modulated in vitro to promote mitochondrial integrity and oxidative metabolism, which will better support their function and longevity. T cell efficacy could be enhanced in vivo by taking steps to ensure that anti-tumor T cells enter a more nutrient replete environment.
Future outlook
Every biological process is supported by intracellular metabolism. Metabolic pathways provide a common thread that links gene regulation, signaling, function, lifespan, and fitness. Integrating information from high-throughput technologies that are now available in metabolomics with proteomics, transcriptomics, and epigenetics may yield new information on how to manipulate metabolism to alter other diverse cellular processes. Armed with new information and a comprehensive understanding of how metabolism dictates immune cell fate, researchers may discover novel therapeutic strategies for treatment of disease.
Reference
- 1.Buck MD, O'Sullivan D, Pearce EL. T cell metabolism drives immunity. The Journal of experimental medicine. 2015;212:1345–1360. doi: 10.1084/jem.20151159. doi:10.1084/jem.20151159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cui G, et al. IL-7-Induced Glycerol Transport and TAG Synthesis Promotes Memory CD8+ T Cell Longevity. Cell. 2015;161:750–761. doi: 10.1016/j.cell.2015.03.021. doi:10.1016/j.cell.2015.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O'Sullivan D, Pearce EL. Targeting T cell metabolism for therapy. Trends in immunology. 2015;36:71–80. doi: 10.1016/j.it.2014.12.004. doi:10.1016/j.it.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Waickman AT, Powell JD. mTOR, metabolism, and the regulation of T-cell differentiation and function. Immunological reviews. 2012;249:43–58. doi: 10.1111/j.1600-065X.2012.01152.x. doi:10.1111/j.1600-065X.2012.01152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Michalek RD, et al. Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J Immunol. 2011;186:3299–3303. doi: 10.4049/jimmunol.1003613. doi:10.4049/jimmunol.1003613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jacobs SR, et al. Glucose uptake is limiting in T cell activation and requires CD28-mediated Akt-dependent and independent pathways. J Immunol. 2008;180:4476–4486. doi: 10.4049/jimmunol.180.7.4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cham CM, Gajewski TF. Glucose availability regulates IFN-gamma production and p70S6 kinase activation in CD8+ effector T cells. J Immunol. 2005;174:4670–4677. doi: 10.4049/jimmunol.174.8.4670. [DOI] [PubMed] [Google Scholar]
- 8.Chang CH, et al. Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell. 2013;153:1239–1251. doi: 10.1016/j.cell.2013.05.016. doi:10.1016/j.cell.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doedens AL, et al. Hypoxia-inducible factors enhance the effector responses of CD8(+) T cells to persistent antigen. Nature immunology. 2013;14:1173–1182. doi: 10.1038/ni.2714. doi:10.1038/ni.2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi LZ, et al. HIF1alpha-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. The Journal of experimental medicine. 2011;208:1367–1376. doi: 10.1084/jem.20110278. doi:10.1084/jem.20110278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang R, et al. The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation. Immunity. 2011;35:871–882. doi: 10.1016/j.immuni.2011.09.021. doi:10.1016/j.immuni.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Villena JA, Kralli A. ERRalpha: a metabolic function for the oldest orphan. Trends in endocrinology and metabolism: TEM. 2008;19:269–276. doi: 10.1016/j.tem.2008.07.005. doi:10.1016/j.tem.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Michalek RD, et al. Estrogen-related receptor-alpha is a metabolic regulator of effector T-cell activation and differentiation. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:18348–18353. doi: 10.1073/pnas.1108856108. doi:10.1073/pnas.1108856108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yao S, et al. Interferon regulatory factor 4 sustains CD8(+) T cell expansion and effector differentiation. Immunity. 2013;39:833–845. doi: 10.1016/j.immuni.2013.10.007. doi:10.1016/j.immuni.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Man K, et al. The transcription factor IRF4 is essential for TCR affinity-mediated metabolic programming and clonal expansion of T cells. Nature immunology. 2013;14:1155–1165. doi: 10.1038/ni.2710. doi:10.1038/ni.2710. [DOI] [PubMed] [Google Scholar]
- 16.MacIver NJ, et al. The liver kinase B1 is a central regulator of T cell development, activation, and metabolism. J Immunol. 2011;187:4187–4198. doi: 10.4049/jimmunol.1100367. doi:10.4049/jimmunol.1100367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang CH, et al. Metabolic Competition in the Tumor Microenvironment Is a Driver of Cancer Progression. Cell. 2015;162:1229–1241. doi: 10.1016/j.cell.2015.08.016. doi:10.1016/j.cell.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ho PC, et al. Phosphoenolpyruvate Is a Metabolic Checkpoint of Anti-tumor T Cell Responses. Cell. 2015;162:1217–1228. doi: 10.1016/j.cell.2015.08.012. doi:10.1016/j.cell.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blagih J, et al. The energy sensor AMPK regulates T cell metabolic adaptation and effector responses in vivo. Immunity. 2015;42:41–54. doi: 10.1016/j.immuni.2014.12.030. doi:10.1016/j.immuni.2014.12.030. [DOI] [PubMed] [Google Scholar]
- 20.Mayer A, Denanglaire S, Viollet B, Leo O, Andris F. AMP-activated protein kinase regulates lymphocyte responses to metabolic stress but is largely dispensable for immune cell development and function. European journal of immunology. 2008;38:948–956. doi: 10.1002/eji.200738045. doi:10.1002/eji.200738045. [DOI] [PubMed] [Google Scholar]
- 21.Carr EL, et al. Glutamine uptake and metabolism are coordinately regulated by ERK/MAPK during T lymphocyte activation. J Immunol. 2010;185:1037–1044. doi: 10.4049/jimmunol.0903586. doi:10.4049/jimmunol.0903586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ananieva EA, Patel CH, Drake CH, Powell JD, Hutson SM. Cytosolic branched chain aminotransferase (BCATc) regulates mTORC1 signaling and glycolytic metabolism in CD4+ T cells. The Journal of biological chemistry. 2014;289:18793–18804. doi: 10.1074/jbc.M114.554113. doi:10.1074/jbc.M114.554113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berod L, et al. De novo fatty acid synthesis controls the fate between regulatory T and T helper 17 cells. Nature medicine. 2014;20:1327–1333. doi: 10.1038/nm.3704. doi:10.1038/nm.3704. [DOI] [PubMed] [Google Scholar]
- 24.Lee J, et al. Regulator of fatty acid metabolism, acetyl coenzyme a carboxylase 1, controls T cell immunity. J Immunol. 2014;192:3190–3199. doi: 10.4049/jimmunol.1302985. doi:10.4049/jimmunol.1302985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fischer K, et al. Inhibitory effect of tumor cell-derived lactic acid on human T cells. Blood. 2007;109:3812–3819. doi: 10.1182/blood-2006-07-035972. doi:10.1182/blood-2006-07-035972. [DOI] [PubMed] [Google Scholar]
- 26.Haas R, et al. Lactate Regulates Metabolic and Pro-inflammatory Circuits in Control of T Cell Migration and Effector Functions. PLoS biology. 2015;13:e1002202. doi: 10.1371/journal.pbio.1002202. doi:10.1371/journal.pbio.1002202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Munn DH, et al. GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2,3-dioxygenase. Immunity. 2005;22:633–642. doi: 10.1016/j.immuni.2005.03.013. doi:10.1016/j.immuni.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 28.Opitz CA, et al. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature. 2011;478:197–203. doi: 10.1038/nature10491. doi:10.1038/nature10491. [DOI] [PubMed] [Google Scholar]
- 29.Mezrich JD, et al. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J Immunol. 2010;185:3190–3198. doi: 10.4049/jimmunol.0903670. doi:10.4049/jimmunol.0903670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharma MD, et al. Plasmacytoid dendritic cells from mouse tumor-draining lymph nodes directly activate mature Tregs via indoleamine 2,3-dioxygenase. The Journal of clinical investigation. 2007;117:2570–2582. doi: 10.1172/JCI31911. doi:10.1172/JCI31911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pearce EL, et al. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. 2009;460:103–107. doi: 10.1038/nature08097. doi:10.1038/nature08097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Araki K, et al. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460:108–112. doi: 10.1038/nature08155. doi:10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van der Windt GJ, et al. Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity. 2012;36:68–78. doi: 10.1016/j.immuni.2011.12.007. doi:10.1016/j.immuni.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rolf J, et al. AMPKalpha1: a glucose sensor that controls CD8 T-cell memory. European journal of immunology. 2013;43:889–896. doi: 10.1002/eji.201243008. doi:10.1002/eji.201243008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van der Windt GJ, et al. CD8 memory T cells have a bioenergetic advantage that underlies their rapid recall ability. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:14336–14341. doi: 10.1073/pnas.1221740110. doi:10.1073/pnas.1221740110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fraser KA, Schenkel JM, Jameson SC, Vezys V, Masopust D. Preexisting high frequencies of memory CD8+ T cells favor rapid memory differentiation and preservation of proliferative potential upon boosting. Immunity. 2013;39:171–183. doi: 10.1016/j.immuni.2013.07.003. doi:10.1016/j.immuni.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O'Sullivan D, et al. Memory CD8(+) T cells use cell-intrinsic lipolysis to support the metabolic programming necessary for development. Immunity. 2014;41:75–88. doi: 10.1016/j.immuni.2014.06.005. doi:10.1016/j.immuni.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maekawa Y, et al. Notch controls the survival of memory CD4+ T cells by regulating glucose uptake. Nature medicine. 2015;21:55–61. doi: 10.1038/nm.3758. doi:10.1038/nm.3758. [DOI] [PubMed] [Google Scholar]
- 39.Wang Y, Wang XY, Subjeck JR, Shrikant PA, Kim HL. Temsirolimus, an mTOR inhibitor, enhances anti-tumour effects of heat shock protein cancer vaccines. British journal of cancer. 2011;104:643–652. doi: 10.1038/bjc.2011.15. doi:10.1038/bjc.2011.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chaoul N, et al. Rapamycin Impairs Antitumor CD8+ T-cell Responses and Vaccine-Induced Tumor Eradication. Cancer research. 2015;75:3279–3291. doi: 10.1158/0008-5472.CAN-15-0454. doi:10.1158/0008-5472.CAN-15-0454. [DOI] [PubMed] [Google Scholar]
- 41.Gattinoni L, Klebanoff CA, Restifo NP. Pharmacologic induction of CD8+ T cell memory: better living through chemistry. Science translational medicine 1. 2009:11ps12. doi: 10.1126/scitranslmed.3000302. doi:10.1126/scitranslmed.3000302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patsoukis N, et al. PD-1 alters T-cell metabolic reprogramming by inhibiting glycolysis and promoting lipolysis and fatty acid oxidation. Nature communications. 2015;6:6692. doi: 10.1038/ncomms7692. doi:10.1038/ncomms7692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Calvaresi EC, et al. Dual targeting of the Warburg effect with a glucose-conjugated lactate dehydrogenase inhibitor. Chembiochem : a European journal of chemical biology. 2013;14:2263–2267. doi: 10.1002/cbic.201300562. doi:10.1002/cbic.201300562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lameris R, et al. Bispecific antibody platforms for cancer immunotherapy. Critical reviews in oncology/hematology. 2014;92:153–165. doi: 10.1016/j.critrevonc.2014.08.003. doi:10.1016/j.critrevonc.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 45.Mundra V, Li W, Mahato RI. Nanoparticle-mediated drug delivery for treating melanoma. Nanomedicine (Lond) 2015;10:2613–2633. doi: 10.2217/nnm.15.111. doi:10.2217/nnm.15.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gerriets VA, et al. Metabolic programming and PDHK1 control CD4+ T cell subsets and inflammation. The Journal of clinical investigation. 2015;125:194–207. doi: 10.1172/JCI76012. doi:10.1172/JCI76012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sinclair LV, et al. Control of amino-acid transport by antigen receptors coordinates the metabolic reprogramming essential for T cell differentiation. Nature immunology. 2013;14:500–508. doi: 10.1038/ni.2556. doi:10.1038/ni.2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maus MV, et al. Adoptive immunotherapy for cancer or viruses. Annual review of immunology. 2014;32:189–225. doi: 10.1146/annurev-immunol-032713-120136. doi:10.1146/annurev-immunol-032713-120136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sukumar M, et al. Inhibiting glycolytic metabolism enhances CD8+ T cell memory and antitumor function. The Journal of clinical investigation. 2013;123:4479–4488. doi: 10.1172/JCI69589. doi:10.1172/JCI69589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sukumar M, et al. Mitochondrial Membrane Potential Identifies Cells with Enhanced Stemness for Cellular Therapy. Cell metabolism. 2015 doi: 10.1016/j.cmet.2015.11.002. doi:10.1016/j.cmet.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]