Summary
Patients with bilateral serous retinal detachments and panuveitis related to Vogt-Koyanagi-Harada disease are commonly managed with oral corticosteroids, immunosuppressive agents, and/or intravitreal injections. We present the case of a 56-year-old Hispanic man with Harada disease whose bilateral serous retinal detachments and panuveitis were treated with topical corticosteroid difluprednate alone. Functional and anatomical recoveries were assessed by fluorescein angiograms and optical coherence tomography studies over a period of 9 months. The patient’s serous retinal detachments resolved, and his vision and panuveitis improved dramatically over a period of 2 weeks, after which he was placed on a drop taper and maintenance therapy for the remainder of the 9 months.
Introduction
Vogt-Koyanagi-Harada (VKH) disease is an autoimmune disease characterized by bilateral, granulomatous panuveitis and serous retinal detachments. Systemic manifestation includes neurological and cutaneous abnormalities.1,2 The current therapeutic approach relies on systemic immunosuppressive therapy, beginning with high-dosage oral corticosteroids, administration of intravitreal injections,1,2 and, if necessary, subsequent immunosuppressant agents (ie, methotrexate).3,4 We report the first known case of VKH successfully managed solely with the strong topical corticosteroid difluprednate.
Case Report
A 56-year-old Hispanic man with a history of diabetes mellitus and hypertension presented at the Retina Macula Insitute with vitiligo of the forehead, headache, and blurry vision in both eyes of approximately 3 months’ duration. Visual acuities were hand motion in the right eye and counting fingers at 5 feet in the left eye. Examination revealed pseudophakia in each eye, with mild cells in the anterior chamber and vitreous cavity. Fundus examination and optical coherence tomography (OCT) revealed bilateral, serous retinal detachments in both eyes, with central foveal thickness of >600 μm in the right eye and 595 μm in the left eye (Figure 1A–B). Fluorescein angiography revealed diffuse retinal pigment epithelium (RPE) involvement with significant cystoid macular edema (CME) and optic disc leakage (Figure 1C–D). The patient was immediately started on topical difluprednate every hour, and a uveitis work-up completed with. chest x-ray, complete blood count, rapid plasma reagin, fluorescent treponemal antibody absorption, and tuberculosis skin test.
Figure 1.
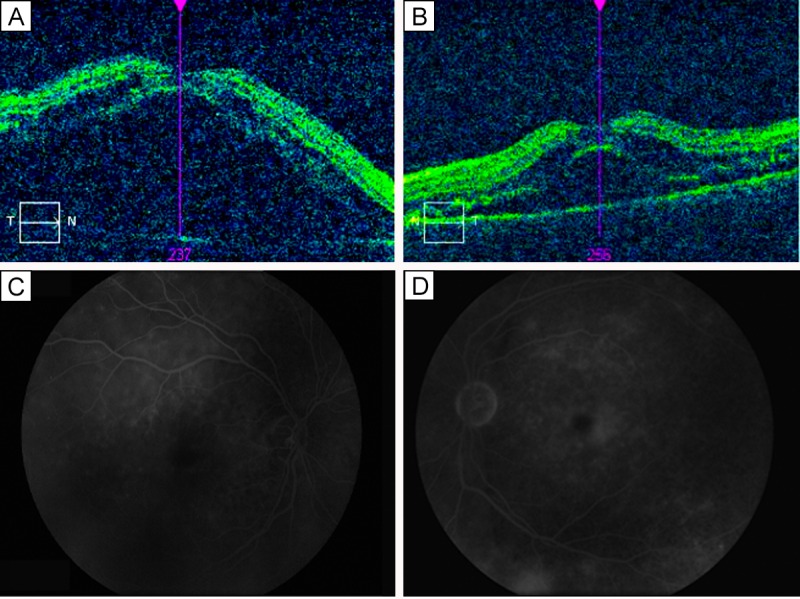
Initial presentation. On optical coherence tomography (OCT), foveal thickness of the right eye (A) was >600 μm; of the left eye (B), 595 μm. Fluorescein angiograms of the right eye (C) and left eye (D) showing classic findings consistent with Vogt-Koyanagi-Harada disease and serous retinal detachments.
Two weeks after the initial treatment with difluprednate emulsion once per hour in each eye, the patient returned with dramatically improved visual acuity (20/60 in both eyes) and resolved serous retinal detachments in each eye (central foveal thickness measured 205 μm in the right eye and 218 µm in the left eye; Figure 2) as well as resolved headaches and stabilized vitiligo. His intraocular pressures (IOPs) were measured at 13 in the right eye and 11 in the left eye. Gradual tapering of the medication to every 2 hours for 1 month, 4 times daily for 1 month, 3 times daily for 2 months, twice daily for 2 months, and once daily thereafter permitted further improvement of his vision to 20/30 in each eye. The central foveal thickness continued to improve with a measure of 192 µm in the right eye and 208 µm in the left eye; Figure 3). He had no elevation of IOP. His uveitis work-up, including all serological testing, was negative.
Figure 2.
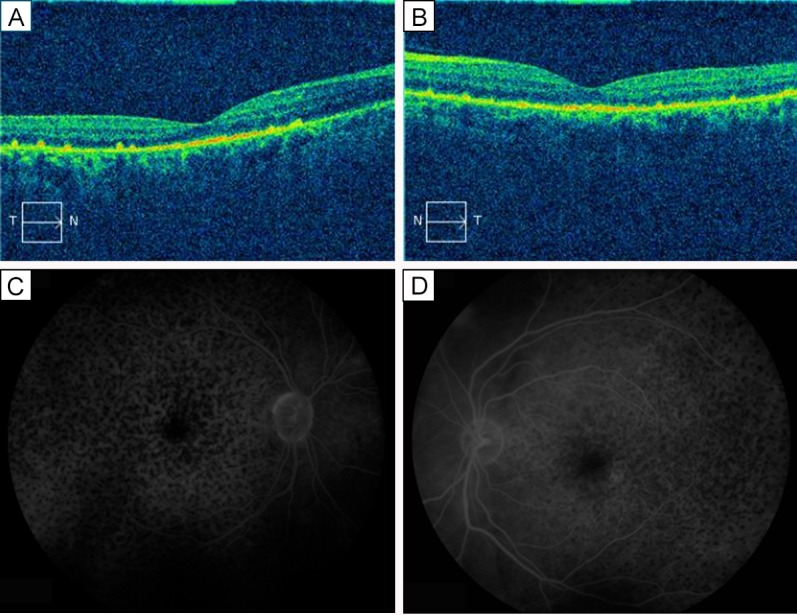
Two weeks after treatment. On OCT, foveal thickness of the right eye (A) was 205 μm; of the lefte eye (B), 218 μm. Fluorescein angiograms of the right eye (C) and left eye (D) show much improved leakage and cystoid macular edema.
Figure 3.
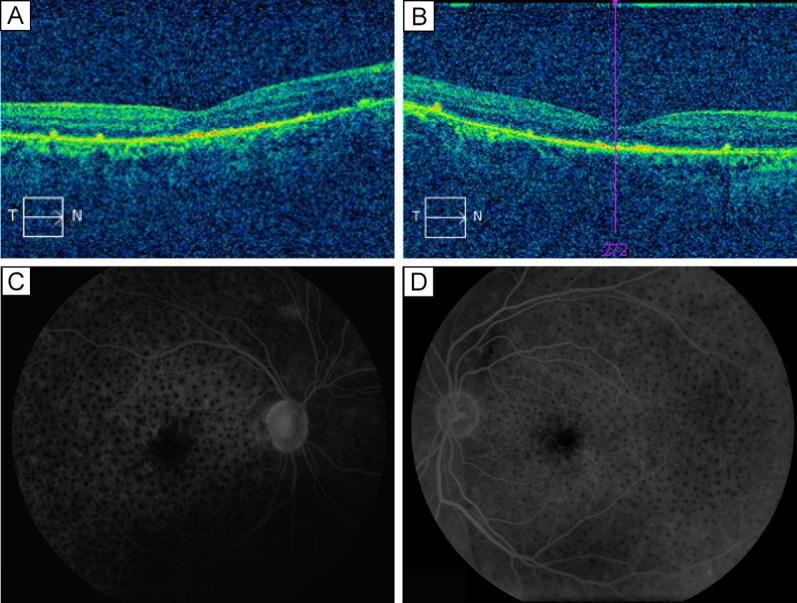
Nine months after treatment. On OCT, foveal thickness of the right eye (A) was 192 μm; of the left eye (B), 208 μm. Fluorescein angiograms of the right eye (C) and left eye (D) reveal further improvement of leakage and CME.
Discussion
This case of VKH was diagnosed based on ocular findings (panuveitis, bilateral serous retinal detachments, optic disc leakage, and characteristic RPE changes), associated headache, and vitiligo around the forehead. The panuveitis and bilateral serous retinal detachments resolved quickly after an initial aggressive treatment with topical difluprednate alone while the headaches resolved and vitiligo stabilized. Previous studies have shown that serous retinal detachments in VKH can improve with corticosteroid-based anti-inflammatory medications,1,2 whether they are administered orally or injected intravitreally. In our case, treatment with topical difluprednate hourly resulted in dramatic improvement in 2 weeks, and subsequent low maintenance dosage prevented recurrence of serous retinal detachments. Difluprednate alone was used as the initial line of treatment because the patient had diabetes, and systemic steroids can cause severe blood sugar elevation and fluctuation.
Topical ocular therapy alone avoids possible side effects associated with systemic corticosteroids, including gastric ulcers, weight gain, and psychological disturbances.5 Intravitreal cortisteroid injections can trigger steroid-induced glaucoma,5 dramatically increase the rate of cataract development, and cause endophthalmitis.5 Treatment with immunosuppressants predispose patients to hepatotoxicity, renal toxicity, and development of malignancies.3
Although increased IOP and cataract formation5,6 are known side effects of topical difluprednate, studies have shown that IOP increases are generally mild and may resolve spontaneously or with IOP-lowering ophthalmic drops.6,7 Of note, most collected data are from normal control subects rather than from patients with uveitis. The unpredictable and potentially dramatic IOP response triggered by topical difluprednate provides limited data on steroid response in patients with uveitis. Thus, it is essential to closely monitor IOP in all patients treated with topical difluprednate.7
Topical difluprednate was an ideal agent in the present case because the patient did not have severe systemic manifestations. Furthermore, the patient’s pseudophakic status makes topical steroid an effective treatment for posterior segment uveitis and associated serous retinal detachments. Monitoring and maintenance treatment is required, and it was well tolerated in our case. However, this approach may not work in all cases, and it is very important to monitor patients’ responses to treatment regularly to prevent delay in effective treatment and possible vision loss.
Literature Search
PubMed was searched for English-language results on February 18, 2015, using the following terms: Vogt-Koyanai-Harada disease, difluprednate, topical corticosteroid treatment for Harada disease, uveitis.
References
- 1.Nazari H, Rao NA. Resolution of subretinal fluid with systemic corticosteroid treatment in acute Vogt-Koyanagi-Harada disease. Br J Ophthalmol. 2012;96:1410–4. doi: 10.1136/bjophthalmol-2012-301857. [DOI] [PubMed] [Google Scholar]
- 2.Maruko I, Iida T, Sugano Y, et al. Subfoveal choroidal thickness after treatment of Vogt-Koyanagi-Harada disease. Retina. 2011;31:510–7. doi: 10.1097/IAE.0b013e3181eef053. [DOI] [PubMed] [Google Scholar]
- 3.Bansal R, Gupta V, Gupta A. Current approach in the diagnosis and management of panuveitis. Indian J Ophthalmol. 2010;58:45–54. doi: 10.4103/0301-4738.58471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moorthy RS, Inomata H, Rao NA. Vogt-Koyanagi-Harada syndrome. Surv Ophthalmol. 1995;39:265–92. doi: 10.1016/s0039-6257(05)80105-5. [DOI] [PubMed] [Google Scholar]
- 5.Taylor S, Isa H, Joshi L, Lightman S. New developments in cortisteroid therapy for uveitis. Ophthalmologica. 2010;224(Suppl 1):46–53. doi: 10.1159/000318021. [DOI] [PubMed] [Google Scholar]
- 6.Foster CS, DaVanzo R, Flynn TE, McLeod K, Vogel R, Crockett RS, Difluprednate Ophthalmic Emulsion 0.05% (Durezol®) Study Group Durezol (difluprednate ophthalmic emulsion 0.05%) compared with Pred Forte 1% ophthalmic suspension in the treatment of endogenous anterior uveitis. J Ocul Pharmacol Ther. 2010;26:475–83. doi: 10.1089/jop.2010.0059. [DOI] [PubMed] [Google Scholar]
- 7.Birnbaum AD, Jiang Y, Tessler H, Goldstein D. Elevation of intraocular pressure in patients with uveitis treated with topical difluprednate. Arch Ophthalmol. 2011;129:667–8. doi: 10.1001/archophthalmol.2011.82. [DOI] [PubMed] [Google Scholar]


